Introduction
Preoperative neoadjuvant chemotherapy (NAC) is
considered the standard treatment for locally-advanced breast
carcinomas (1–6). Several large randomized clinical
studies have shown that NAC is effective as an adjuvant
chemotherapy and permits breast-conserving surgery (1–6). The
current roles of NAC include control and downstaging of primary
breast tumors, as well as reducing micrometastatic diseases.
Therefore, it is extremely important to obtain precise information
regarding the extent and distribution of residual breast carcinomas
following NAC by imaging modalities to assess the success of
breast-conserving surgery.
Findings of previous studies have demonstrated the
usefulness of imaging modalities for determining the tumor
distribution of residual breast carcinoma following NAC (7–12). A
study by Tozaki et al (9)
analyzed computed tomography (CT) findings of tumor distribution
patterns of locally-advanced breast carcinomas prior and subsequent
to NAC. The distribution patterns of the enhancing lesions of
breast carcinomas prior to NAC were classified into five
categories: Solitary, grouped, separated, mixed and replaced
lesions; and the shrinkage patterns subsequent to NAC were
classified into three categories: Concentric shrinkage with or
without surrounding lesions and shrinkage with residual
multinodular lesions (9). The
study concluded that CT classification of tumor distribution prior
to NAC and shrinkage patterns subsequent to NAC is important for
the evaluation of the residual lesions undergoing breast-conserving
surgery (9). In addition, Kim
et al (12) recently
reported magnetic resonance imaging (MRI) patterns of breast
carcinomas prior and subsequent to NAC, and analyzed the
correlation with pathological response grading. The shrinkage
patterns of breast carcinomas following NAC were classified into
five categories: Type I (concentric shrinkage without any
surrounding lesion); type II (concentric shrinkage with surrounding
lesions); type III (shrinkage with residual multinodular lesions);
type IV (diffuse contrast enhancement in whole quadrant); and
non-visualization (12). The study
concluded that the type I pattern was the most frequently observed
in the pathological responder group and type IV was more frequently
found in the non-responder group (12).
However, thus far, the analysis of the detailed
radiopathological correlation of MRI prior and subsequent to NAC
for breast carcinomas has not been reported. In the present study,
a detailed analysis of the radiopathological correlation of
residual breast carcinomas following NAC is reported, and the
usefulness of MRI subsequent to NAC for breast-conserving surgery
is discussed.
Materials and methods
Case selection
The study included 27 consecutive cases of breast
carcinomas. The subjects who underwent NAC at Department of
Surgery, Shiga University of Medical Science (Otsu, Shiga, Japan).
In 20 cases, MRI was performed prior and subsequent to NAC, and in
the remaining seven cases, MRI was performed only following NAC and
CT was performed prior to NAC.
The mean age of the patients was 48.4 years (range,
29–66 years). All the patients in the study were female. Table I shows the clinical features of the
27 cases.
 | Table IClinical characteristics of breast
carcinomas. |
Table I
Clinical characteristics of breast
carcinomas.
| Characteristics | No. of cases (%) |
|---|
| Initial clinical
stage |
| I | 1 (4) |
| IIA | 7 (26) |
| IIB | 17 (63) |
| IIIA | 2 (7) |
| IIIB | 0 (0) |
| IIIC | 0 (0) |
| IV | 0 (0) |
| Surgical method |
| Radical
mastectomy | 9 (33) |
| Partial
mastectomy | 18 (67) |
| Neoadjuvant
chemotherapy regimen |
| Taxane | 5 (18) |
| Taxane +
anthracycline | 18 (67) |
| Taxane +
anthracycline + trastuzumab | 4 (15) |
The NAC regimens used were: taxane in five, taxane +
anthracycline in 18 and taxane + anthracycline + trastuzumab in
four cases. Mastectomy was performed in 9 cases and
breast-conserving surgery was performed in 18 cases.
MRI technique
MRI was performed using 1.5T- and 3T-scanners (Signa
HD; GE Healthcare, Milwaukee, WI, USA) with the use of a dedicated
breast coil. Patients underwent imaging in the prone position with
breasts immobilized. Contrast material was injected in all the
patients (0.1 mmol/kg gadopentetate dimeglumine). The
contrast-enhancement pattern was analyzed in the early phase.
CT technique
CT was obtained on a 64- (Aquilion CX; Toshiba
Medical Systems, Otawara, Japan) or a 320-multidetector CT scanner
(Aquilion ONE; Toshiba Medical Systems). Contrast material was
injected in all the patients (100 ml of non-ionic contrast
material).
Interpretation of MRI or CT findings
Four breast surgeons performed a consensus review of
the breast MRI or CT examinations in all the cases. The initial
contrast-enhancement pattern of breast carcinoma by MRI or CT prior
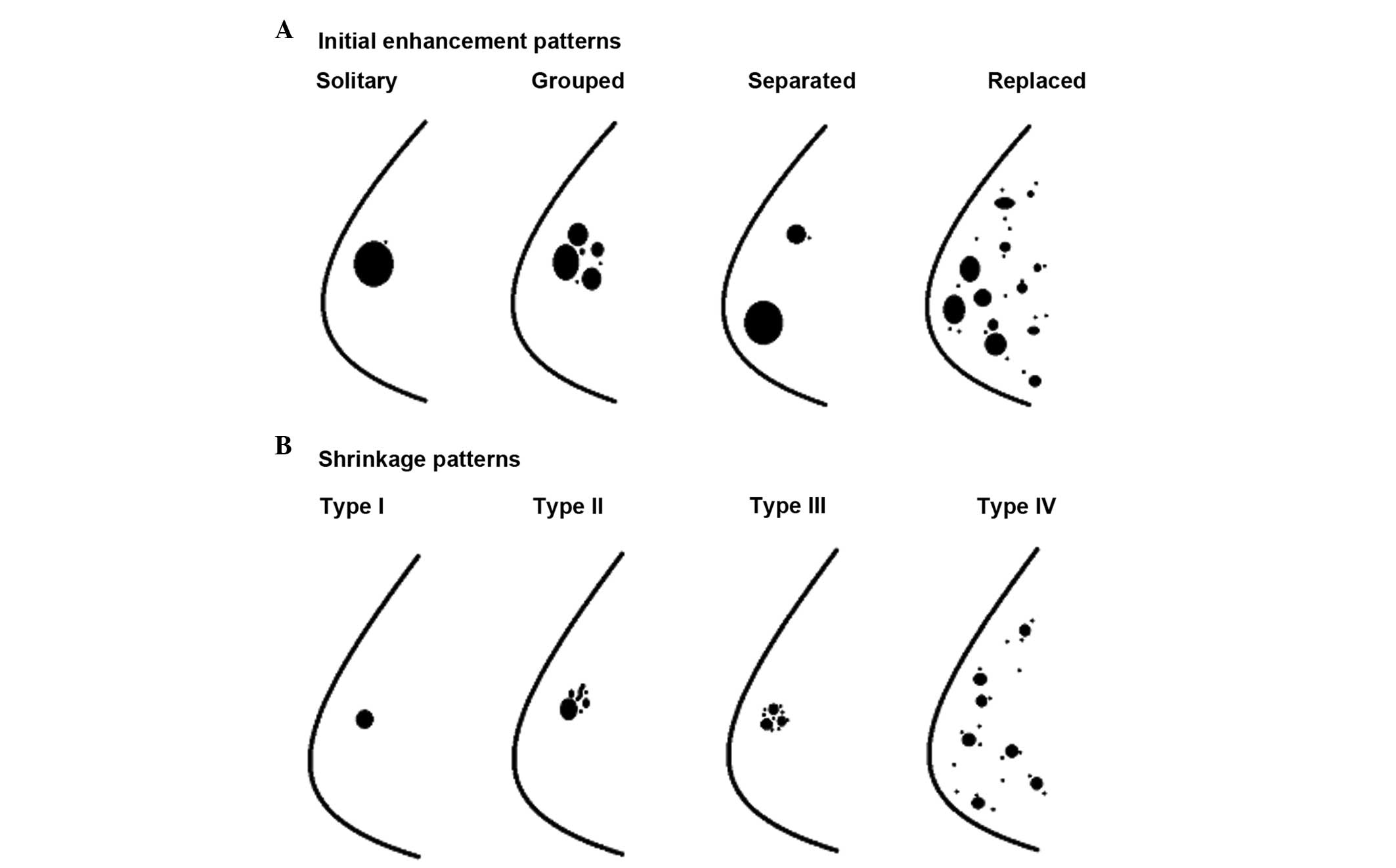
to NAC was classified into five categories as suggested by Tozaki
et al (9), i.e., solitary;
grouped (localized lesion with linear, spotty, or linear and spotty
enhancement); separated (multiple foci of contrast enhancement);
mixed (grouped lesion with multiple foci); and replaced lesions
(diffuse contrast enhancement in whole quadrant) (Fig. 1A).
The shrinkage pattern of breast carcinoma by MRI
following NAC was classified into five categories as suggested by
Kim et al (12): Type I
(concentric shrinkage without any surrounding lesion); type II
(concentric shrinkage with surrounding lesions); type III
(shrinkage with residual multinodular lesions); type IV (diffuse
contrast enhancement in whole quadrant); and non-visualization
(Fig. 1B).
Histopathological evaluation
The histopathological diagnosis of breast carcinomas
prior and subsequent to NAC was independently performed by two
diagnostic pathologists. The histopathological tumor regression
pattern was semi-quantitatively evaluated using the Miller-Payne
grading system as follows (13):
Grade 1, no change or particular alteration to individual malignant
cells and no reduction in overall cellularity; grade 2, minor loss
of tumor cells (≤30%) but overall cellularity remained high; grade
3, between an estimated 30–90% reduction in tumor cells; grade 4, a
marked disappearance of tumor cells such that only small clusters
or widely dispersed individual cells remained with a >90% loss
of tumor cells; and grade 5, no malignant cells identified in
sections from the tumor site and only vascular fibroelastic stroma
that often contains macrophages remains. However, intraductal
components may be present (13).
Patients showing Miller-Payne grades 3, 4 or 5 were classified as
responders and patients showing grades 1 or 2 were classified as
non-responders.
The histopathological pattern of the residual
carcinoma was also classified by the same method as the MRI
shrinkage pattern as suggested by Kim et al (12) (Fig.
1B).
Results
MRI or CT findings prior to NAC
Table II shows the
initial enhancement patterns of MRI or CT of 27 cases of breast
carcinomas prior to NAC. The most common initial
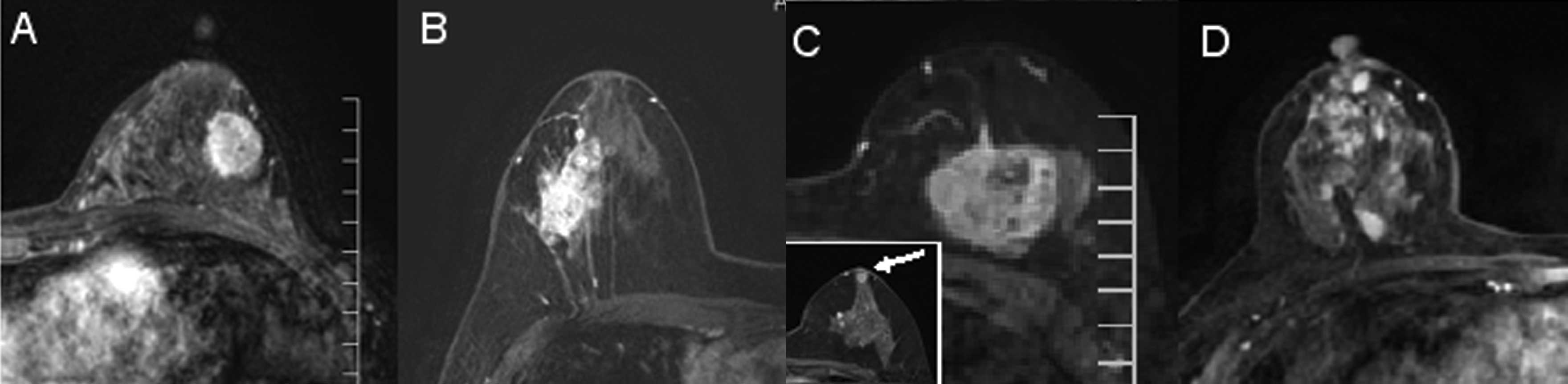
contrast-enhancement pattern was solitary (18 cases) (Fig. 2A). The second most common pattern
was grouped (five cases) (Fig.
2B), followed by separated (three cases) (Fig. 2C) and replaced patterns (one case)
(Fig. 2D). There was no case
showing a mixed pattern in the present study.
 | Table IICorrelation between initial
contrast-enhancement patterns prior to neoadjuvant chemotherapy and
shrinkage patterns. |
Table II
Correlation between initial
contrast-enhancement patterns prior to neoadjuvant chemotherapy and
shrinkage patterns.
| Initial enhancement
patterns |
|---|
|
|
|---|
| Shrinkage
patterns | Solitary | Grouped | Separated | Replaced |
|---|
|
Non-visualization | 2 | 3 | 1 | 0 |
| Type I | 9 | 1 | 1 | 0 |
| Type II | 5 | 1 | 0 | 0 |
| Type III | 2 | 0 | 1 | 0 |
| Type IV | 0 | 0 | 0 | 1 |
Histopathological findings of the biopsy
specimen prior to NAC
All the cases were diagnosed as invasive ductal
carcinoma of no special type by the biopsy specimen. No special
type of invasive breast carcinoma, such as invasive lobular or
mucinous carcinomas, was included in the present study.
MRI findings following NAC
Table II
summarizes the MRI findings of the shrinkage patterns of breast
carcinomas following NAC and the correlation between the initial
contrast-enhancement patterns prior to NAC and the shrinkage
patterns following NAC. The most common shrinkage pattern
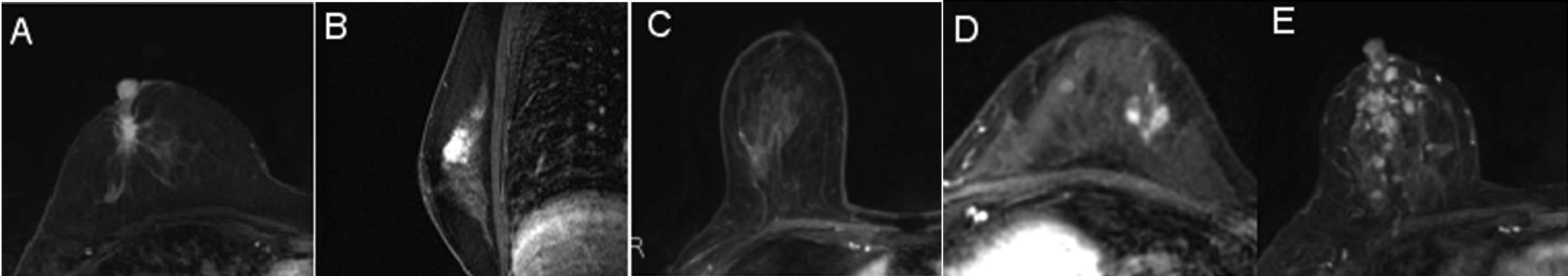
identified was type I (11/27 cases) (Fig. 3A). The second most common patterns
identified were type II and non-visualization (6 cases each)
(Fig. 3B and C). Three cases
showed a type III pattern (Fig.
3D), while only one case showed type IV (Fig. 3E).
The most common shrinkage pattern of solitary
lesions prior to NAC was type I (9/18 cases), in contrast to
grouped lesions, which demonstrated that the non-visualization
pattern (3/5 cases) was the most frequent. The replaced lesion
showed the type IV shrinkage pattern.
Histopathological findings following
NAC
Table III
summarizes the Miller-Payne grading system of the present study.
The most common histopathological regression grade was grade 3
(10/27 cases). In four cases showing grade 5, no viable carcinoma
cells and no residual intraductal component were observed in these
cases. The rate of responder cases was 70.4% (19/27 cases) and that
of non-responders was 29.6% (8/27 cases).
 | Table IIIMiller-Payne grading of the present
study. |
Table III
Miller-Payne grading of the present
study.
| Grade | No. of cases |
|---|
| 1 | 1 |
| 2 | 7 |
| 3 | 10 |
| 4 | 5 |
| 5 | 4 |
Table IV shows the
radiopathological correlation of the shrinkage patterns of breast
carcinomas following NAC in the study. The most common pathological
shrinkage pattern of the residual carcinoma was type II (11/27
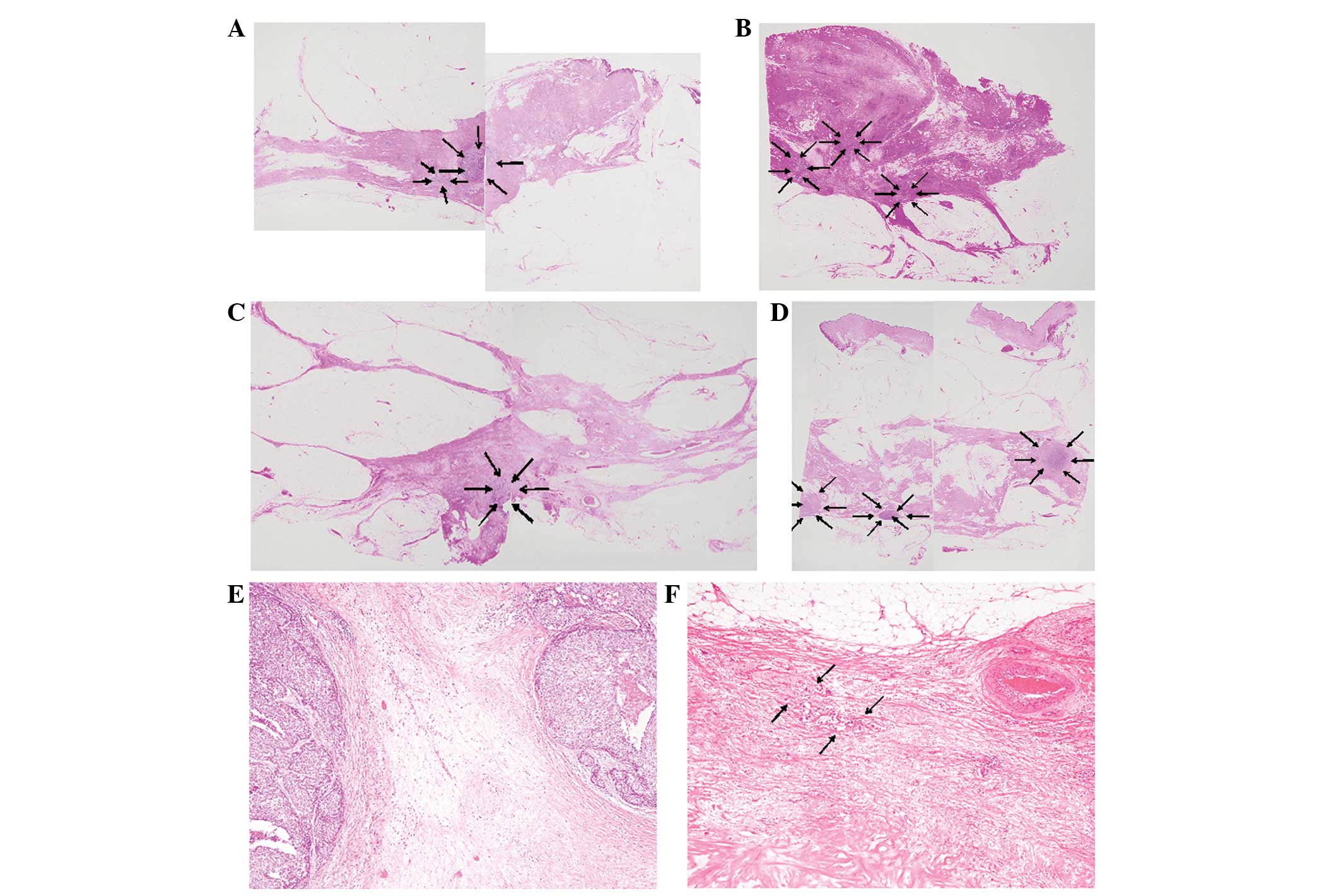
cases) (Fig. 4A), followed by type
III (8 cases) (Fig. 4B). The
concordance rate between the MRI pattern following NAC and
pathological pattern was 48%: Non-visualization, three cases; type
I, three (Fig. 4C); type II, four;
type III, two; and type IV, one case (Fig. 4D). The worst concordance MRI
pattern was type I as only three of the 11 cases corresponded to
the pathological pattern, while four cases showed pathological type
II and three cases showed pathological type III. In the residual
carcinoma nodules, scar, accumulation of macrophages and
hemosiderin deposition were observed in the cases showing MRI
pattern type I and pathological pattern type II or III (Fig. 4E).
 | Table IVCorrelation between magnetic resonance
imaging (MRI) shrinkage patterns and pathological patterns
following neoadjuvant chemotherapy. |
Table IV
Correlation between magnetic resonance
imaging (MRI) shrinkage patterns and pathological patterns
following neoadjuvant chemotherapy.
| Pathological
patterns |
|---|
|
|
|---|
| MRI patterns | Nonvisualization | Type I | Type II | Type III | Type IV |
|---|
|
Non-visualization | 3 | 0 | 2 | 1 | 0 |
| Type I | 1 | 3 | 4 | 3 | 0 |
| Type II | 0 | 0 | 4 | 2 | 0 |
| Type III | 0 | 0 | 1 | 2 | 0 |
| Type IV | 0 | 0 | 0 | 0 | 1 |
In the cases showing non-visualization by MRI
following NAC, three of the six cases had no residual carcinoma by
pathological examination and the remaining cases had only a few
residual carcinomas by pathological examination, which showed
pathological types II (two cases) and III (one case) (Fig. 4F).
Follow-up information subsequent to
surgery
No local recurrence was observed in any of the 27
patients (median follow-up period, 32.9 months; range, 6–88
months).
Discussion
NAC is widely performed for patients with
locally-advanced breast carcinomas in order to control and
downstage the primary breast carcinomas. Therefore, accurate
assessment regarding the extent and distribution of the residual
breast carcinomas following NAC by imaging modalities is extremely
important to assess the success of breast-conserving surgery.
However, it has been reported that MRI and CT often over- or
underestimate the extent and distribution of residual carcinomas
following NAC (7–12).
A study by Tozaki et al (9) analyzed the usefulness of determining
the breast carcinoma distribution prior to NAC and the shrinkage
patterns following NAC by multidetector CT. The study evaluated 47
consecutive locally-advanced breast carcinomas from 46 patients and
classified the distribution patterns of contrast enhancement prior
to NAC by CT into 5 categories: Solitary; grouped; separated;
mixed; and replaced lesions (9).
Solitary lesions were observed in 15 cases, grouped in 12,
separated in four, mixed in five and replaced in 11 cases in the
series (9). The rates of grouped
and replaced lesions in the present study were low compared to the
series reported by Tozaki et al (9) [18.5% were grouped lesions in the
present study vs. 25.5% in the study by Tozaki et al
(9) and 3.7% were replaced lesion
in the present study vs. 23.4% in the study by Tozaki et al
(9)]. However, the rates of the
initial enhancement patterns as reported by Kim et al
(12), who also classified the
initial enhancement patterns of MRI using the same methods
suggested by Tozaki et al (9), fundamentally corresponded to the
results of the present study. This difference may be due to a
relatively high percentage of inflammatory carcinomas in the study
reported by Tozaki et al (9).
Tozaki et al (9) also classified the shrinkage patterns
with non-replaced lesions following NAC as concentric shrinkage
with or without surrounding lesions, and shrinkage with residual
multinodular lesions. The most common shrinkage pattern was
concentric shrinkage without any surrounding lesions (16/28 cases
showing complete or partial response by CT), followed by shrinkage
with residual multinodular lesions (five cases), complete response
(four cases) and concentric shrinkage with surrounding lesions
(three cases) (9). Furthermore,
the study also reported that the assessment of the tumor extent
following NAC by CT and histopathological examination of the
residual carcinoma revealed that a deviation of <2 cm in
diameter was found in 88% of cases (14/16), showing a pattern of
concentric shrinkage without any surrounding lesions; 100% of cases
(3/3), who had a pattern of concentric shrinkage with surrounding
lesions; and none of the cases (0/5) demonstrating a pattern of
shrinkage with residual multinodular lesions (9). The cases showing a difference of
>2 cm in length from the histopathological examination were all
underestimated, while no cases of overestimation were present in
the study. Therefore, Tozaki et al (9) concluded that CT classification of
shrinkage patterns following NAC is useful for evaluation of the
residual lesions for undergoing breast-conserving surgery,
particularly in the cases showing concentric shrinkage with or
without surrounding lesions (9).
However, CT underestimated the residual tumor extent by >2 cm in
the cases showing shrinkage with residual multinodular lesions
(9). Therefore, a residual
carcinoma in the surgical margins from the breast-conserving
surgery is an extremely critical issue in these cases.
Recently, Kim et al (12) analyzed the MRI patterns of 56
consecutive breast carcinomas from 55 patients prior and subsequent
to NAC and evaluated the correlation with pathological response
grading. The shrinkage patterns of breast carcinomas following NAC
were classified into five categories: Type I (concentric shrinkage
without any surrounding lesion); type II (concentric shrinkage with
surrounding lesions); type III (shrinkage with residual
multinodular lesions); type IV (diffuse contrast enhancement in
whole quadrant); and non-visualization (12). In that study, the most common
shrinkage pattern was type I (29/56 cases), followed by type II (13
cases), type III (5 cases), type IV (4 cases) and non-visualization
(3 cases) (12). The study
concluded that type I was more frequently observed in the
pathological responder group and type IV was more frequently noted
in the non-responder group (12).
Although the ratio of non-visualization was relatively high in the
present study (22%), the shrinkage patterns fundamentally
corresponded to the results in the study by Kim et al
(12).
To the best of our knowledge, the present study
reports the first analysis regarding the radiopathological
correlation between MRI shrinkage patterns subsequent to NAC and
pathological residual tumor patterns. The histopathological
patterns of the residual carcinoma following NAC were classified by
the same method as the MRI shrinkage patterns suggested by Kim
et al (12), as shown in
Fig. 1B (13). The notable findings of the present
study were: i) The most common histopathological shrinkage pattern
was type II (11/27 cases), followed by type III (eight cases); ii)
the concordance rate between MRI patterns following NAC and
pathological patterns was 48%; and iii) the worst concordance MRI
pattern was type I (3/11 cases).
Of the eight cases showing type I MRI and non-type I
pathological patterns, four cases showed pathological type II and
three cases showed pathological type III. This indicates that the
residual tumor following NAC appeared as a concentric tumorous
lesion without any surrounding lesions by MRI. However,
histopathologically it did not form a tumorous lesion, but it was
surrounded by a few or multinodular residual lesions and harboured
fibrous scar with hemosiderin deposition and the accumulation of
macrophages among the multinodular residual carcinoma nests, as
shown in Fig. 4E. This may reflect
a multifocal tumor shrinkage but not a concentric shrinkage pattern
by NAC. In addition, of the six cases showing non-visualization by
MRI following NAC, three cases had no residual carcinoma by
pathological examination and the remaining cases had residual
carcinoma, which were pathological types II (two cases) and III
(one case). This may be due to the small amounts of residual
carcinoma, as shown in Fig. 4F,
which could not be detected by MRI.
In conclusion, the radiopathological correlation of
breast carcinomas was analyzed prior and subsequent to NAC. MRI is
considered to be a useful method for evaluating residual carcinomas
following NAC (the concordance rate was 48%). However, in the cases
with MRI pattern I, the concordance rate was low and multinodular
residual carcinomas were frequently present. Additionally, tiny
foci of residual carcinoma were present in half of the
non-visualization cases by MRI. Therefore, the tumor extent prior
to NAC must be completely resected for patients who undergo NAC,
particularly those with MRI patterns of type I, and
non-visualization and postoperative radiation therapy may be
important for preventing local recurrence of breast carcinomas.
References
|
1
|
Fisher B, Brown A, Mamounas E, et al:
Effect of preoperative chemotherapy on local-regional disease in
women with operable breast cancer: findings from National Surgical
Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 15:2483–2493.
1997.
|
|
2
|
Fisher B, Bryant J, Wolmark N, et al:
Effect of preoperative chemotherapy on the outcome of women with
operable breast cancer. J Clin Oncol. 16:2672–2685. 1998.PubMed/NCBI
|
|
3
|
Mamounas EP and Fisher B: Preoperative
(neoadjuvant) chemotherapy in patients with breast cancer. Semin
Oncol. 28:389–399. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS and Senn HJ: Meeting highlights: International Consensus Panel
on the Treatment of Primary Breast Cancer. Seventh International
Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin
Oncol. 19:3817–3827. 2001.
|
|
5
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: a
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mieog JS, van der Hage JA and van de Velde
CJ: Preoperative chemotherapy for women with operable breast
cancer. Cochrane Database Syst Rev. 2:CD0050022007.PubMed/NCBI
|
|
7
|
Rieber A, Zeitler H, Rosenthal H, et al:
MRI of breast cancer: influence of chemotherapy on sensitivity. Br
J Radiol. 70:452–458. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wasser K, Sinn HP, Fink C, et al: Accuracy
of tumor size measurement in breast cancer using MRI is influenced
by histological regression induced by neoadjuvant chemotherapy. Eur
Radiol. 13:1213–1223. 2003.
|
|
9
|
Tozaki M, Kobayashi T, Uno S, et al:
Breast-conserving surgery after chemotherapy: value of MDCT for
determining tumor distribution and shrinkage pattern. AJR Am J
Roentogenol. 186:431–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bahri S, Chen JH, Mehta RS, et al:
Residual breast cancer diagnosed by MRI in patients receiving
neoadjuvant chemotherapy with and without bevacizumab. Ann Surg
Oncol. 16:1619–1628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woodhams R, Kakita S, Hata H, et al:
Identification of residual breast carcinoma following neoadjuvant
chemotherapy: diffusion-weighted imaging - comparison with
contrast-enhanced MR imaging and pathological findings. Radiology.
254:357–366. 2010. View Article : Google Scholar
|
|
12
|
Kim TH, Kang DK, Yim H, Jung YS, Kim KS
and Kang SY: Magnetic resonance imaging patterns of tumor
regression after neoadjuvant chemotherapy in breast cancer
patients: correlation with pathological response grading system
based on tumor cellularity. J Comput Assist Tomogr. 36:200–206.
2012. View Article : Google Scholar
|
|
13
|
Ogston KN, Miller ID, Payne S, et al: A
new histological grading system to assess response of breast
cancers to primary chemotherapy: prognostic significance and
survival. Breast. 12:320–327. 2003. View Article : Google Scholar : PubMed/NCBI
|