Introduction
Lung cancer is the most common cause of
cancer-related mortality worldwide, with non-small-cell lung cancer
(NSCLC) accounting for ~85% of all lung cancer cases (1). Overall survival (OS) is considered
the most reliable endpoint in cancer studies and is generally the
preferred endpoint for survival studies (2). OS is precise, easily measured and
documented by the date of death. Surrogate endpoints, such as tumor
response and progression-free survival (PFS), are also valuable in
oncology clinical trials, since they can be measured earlier, are
easier to assess compared to ‘true’ endpoints and the events are
more frequent.
With the growing number of drugs and combination
therapies available for the treatment of NSCLC, the effect of
first-line chemotherapy on OS may be confounded by subsequent
therapies (3). Indeed, in a recent
randomized trial on NSCLC patients, an improvement in PFS did not
necessarily result in improved OS (4). A number of compounds are currently
available for second- and third-line chemotherapy for diseases such
as breast, ovarian and colorectal cancer (5–7), as
well as advanced NSCLC. Although PFS following first-line
chemotherapy is not a validated surrogate endpoint for OS,
post-progression survival (PPS) has been shown to be strongly
associated with OS after first-line chemotherapy for advanced NSCLC
(8,9). PPS has also become strongly
associated with OS over the last decade (2002–2012), in which
molecularly targeted agents, such as gefitinib and erlotinib, have
been used to treat advanced NSCLC (8,9). PPS
is calculated as follows: OS=PFS+PPS (2).
The effect on survival of therapies administered
after disease progression is of interest at the individual patient
level. Past analysis of individual-level data suggested that PPS
was a surrogate for OS in patients with advanced non-squamous NSCLC
with unknown oncogenic driver mutations and, therefore, limited
options for subsequent chemotherapy (10). However, it is unknown whether this
applies to advanced NSCLC patients with epidermal growth factor
receptor gene (EGFR) mutations sensitive to targeted
therapy. Therefore, examination of individual-level data to
determine whether PFS, PPS and tumor response are valid surrogate
endpoints for OS after first-line therapy in these patients may be
of clinical value.
Previous clinical trials have identified gefitinib,
an EGFR tyrosine kinase inhibitor (EGFR-TKI), as a first-line
treatment option for patients with NSCLC with sensitive EGFR
mutations (11–13). Although several patients achieve
initial clinical remission or disease control with first-line
chemotherapy, the majority experience subsequent disease
progression and death. We examined first-line gefitinib
chemotherapy, as it is the standard first-line chemotherapy for
advanced NSCLC with EGFR mutations. Patients with NSCLC
harboring an EGFR mutation who were treated with gefitinib,
platinum and pemetrexed or docetaxel exhibited a median survival of
~3 years (14). For advanced NSCLC
patients with sensitive EGFR mutations, the OS time is
longer and there are additional options for subsequent
chemotherapy.
In this study, we aimed to determine the
associations of PFS, PPS and tumor response with OS in patients
with advanced NSCLC harboring sensitive EGFR mutations. We
also assessed the prognostic value of baseline and tumor
characteristics for PPS.
Patients and methods
Patients
This study included 46 patients with advanced NSCLC
harboring sensitive EGFR mutations who were treated with
first-line gefitinib between January, 2006 and June, 2012. The
eligibility criteria were histologically or cytologically proven
NSCLC, unresectable stage IIIB/IV disease, a tumor with a
drug-sensitive EGFR mutation (exon 18 G719X, exon 19
deletion, or exon 21 L858R) and continuous gefitinib treatment.
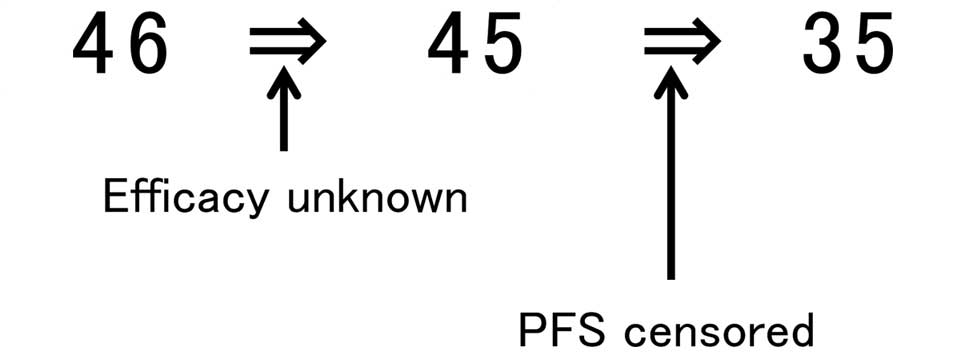
Tumor response was not evaluated in 1 patient and PFS data were
censored in 10 patients. To ensure patient background uniformity,
these 11 patients were excluded from the analysis. Therefore, 35
patients were retrospectively analyzed (Fig. 1). Genomic DNA was extracted from
tumor samples and EGFR mutations in exons 18–21 were
analyzed as previously described (15,16).
The study protocol was approved by the Institutional
Review Board of the National Hospital Organization Nishigunma
Hospital.
Response to treatment
The patients received first-line gefitinib (250 mg
per os, once daily), which continued until disease
progression, development of intolerable toxicity, or withdrawal of
consent. All the patients were EGFR-TKI naïve.
The best overall response and maximum tumor
shrinkage were recorded as tumor responses. Radiographic tumor
responses were evaluated according to the Response Evaluation
Criteria in Solid Tumors, version 1.1 (17) as follows: complete response (CR),
disappearance of all target lesions; partial response (PR),
decrease in the sum of the target lesion diameters by ≥30% compared
to baseline diameters; progressive disease (PD), increase of ≥20%
in the sum of the target lesion diameters compared to the smallest
sum during the study; and stable disease (SD), insufficient
shrinkage or expansion to qualify as PR or PD. PFS was calculated
from the start of treatment until PD or death from any cause. OS
was recorded from the first day of treatment until death or was
censored on the date of the last follow-up. PPS was recorded as the
time from tumor progression until death or was censored on the date
of the last follow-up.
Statistical analyses
To determine whether PFS, PPS and tumor shrinkage
were correlated with OS, we used Spearman’s rank correlation
analysis and linear regression analysis. To identify the prognostic
factors for PPS, we applied the proportional hazards model with a
stepwise regression procedure. Hazard ratios (HRs) and 95%
confidence intervals (95% CIs) were estimated. As the HR is defined
for a 1-unit difference, certain factors were converted to an
appropriate scale. PPS values were compared using the log-rank
test. P≤0.05 was considered to indicate statistically significant
differences. The two-tailed significance level was set at 0.05. All
the statistical analyses were performed using JMP software for
Windows, version 9.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and treatment
efficacy
Of the 35 patients included in the analysis, 25
succumbed to their disease; the median follow-up was 21.0 months
(range, 6.0–56.0 months). The patients’ median age was 67 years
(range, 45–88 years). The patient characteristics are summarized in
Table I. Target lesions were not
evaluated in 1 patient.
 | Table IBaseline patient characteristics. |
Table I
Baseline patient characteristics.
| Characteristics | Patient no.
(n=35) |
|---|
| Gender |
| Male/female | 11/24 |
| Age at treatment,
years |
| Median (range) | 67 (45–88) |
| Performance
status |
| 0/1/≥2 | 15/17/3 |
| Histology |
|
Adenocarcinoma/others | 35/0 |
| Stage |
| IIIB/IV | 3/32 |
| Mutation status |
| Exon 19 del/exon 21
L858R/others | 20/15/0 |
| Administration
period, years |
| <1/≥1 | 20/15 |
| Number of regimens
after progression |
| 0/1/2/3/4/5/≥6 | 11/6/6/3/5/1/3 |
| Median (range) | 2 (0–10) |
| Sum of target lesion
diameters, mm |
| Median (range) | 25 (10–85) |
Of the 35 patients, 2, 20, 12 and 1 exhibited CR,
PR, SD and PD, respectively. The response rate was 62.8% and the
disease control rate was 97.1%. After disease progression following
first-line chemotherapy, 11 of the 35 patients did not receive
additional chemotherapy, whereas the remaining 24 did. Among the 35
patients, the median number of follow-up therapeutic regimens was 2
(range, 0–10 regimens). The chemotherapeutic regimens employed
after disease progression are listed in Table II. Platinum combination
chemotherapy was the most common second-line treatment and
docetaxel was the most common treatment following second-line
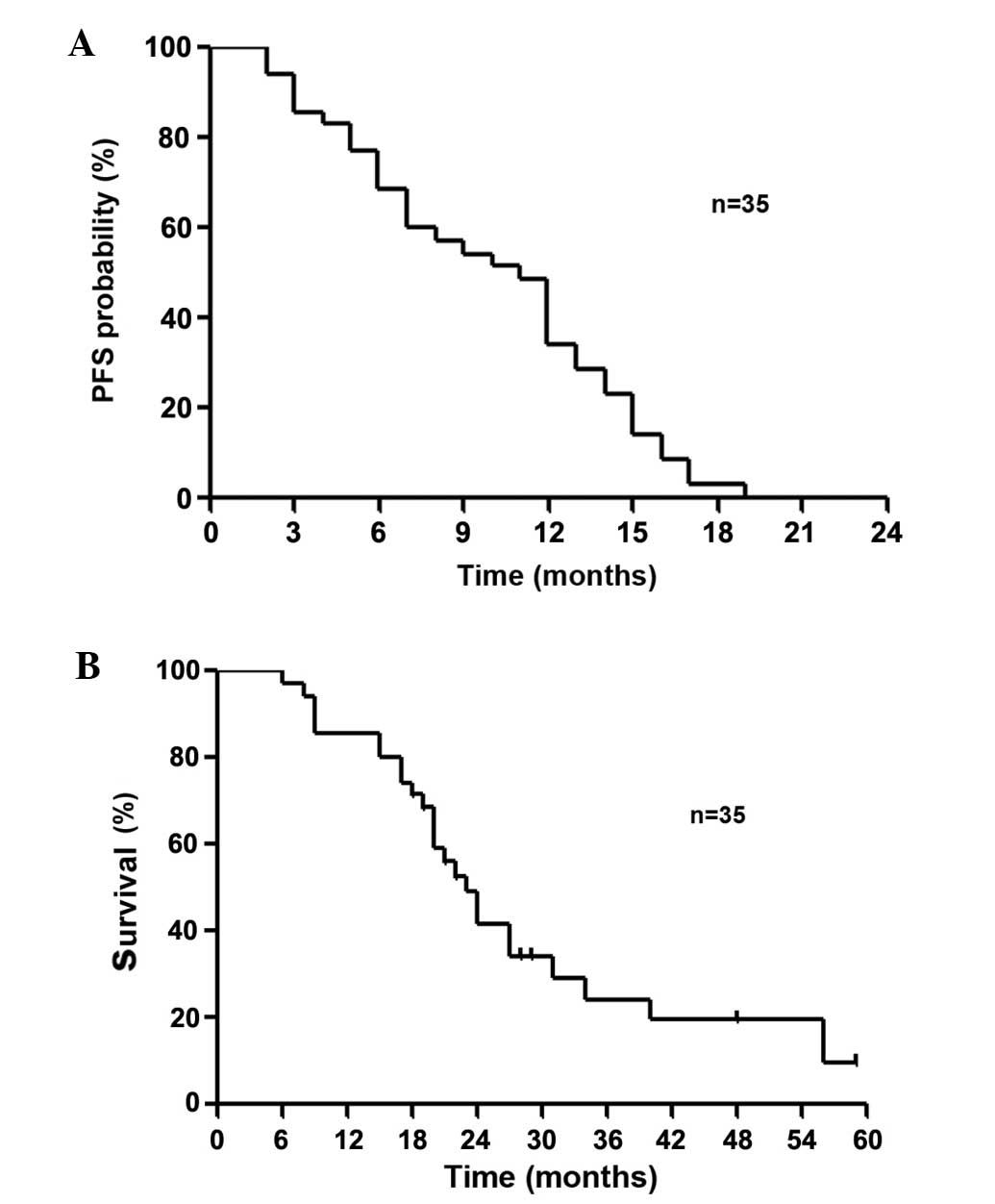
chemotherapy. The median PFS and OS were 11.0 and 23.0 months,
respectively (Fig. 2).
 | Table IIChemotherapeutic regimens administered
after disease progression following first-line chemotherapy. |
Table II
Chemotherapeutic regimens administered
after disease progression following first-line chemotherapy.
| Regimens | Second-line | ≥Third-line | Total |
|---|
| Platinum
combination | 19 | 7 | 26 |
| Docetaxel | 0 | 9 | 9 |
| Pemetrexed | 2 | 2 | 4 |
| Erlotinib | 3 | 5 | 8 |
| Gefitinib
rechallenge | 0 | 6 | 6 |
| S1 | 0 | 7 | 7 |
| Gemcitabine | 0 | 4 | 4 |
| Amrubicin | 0 | 2 | 2 |
| Others | 0 | 5 | 5 |
| Investigational
agent | 0 | 0 | 0 |
Association between OS and PFS, PPS and
tumor shrinkage
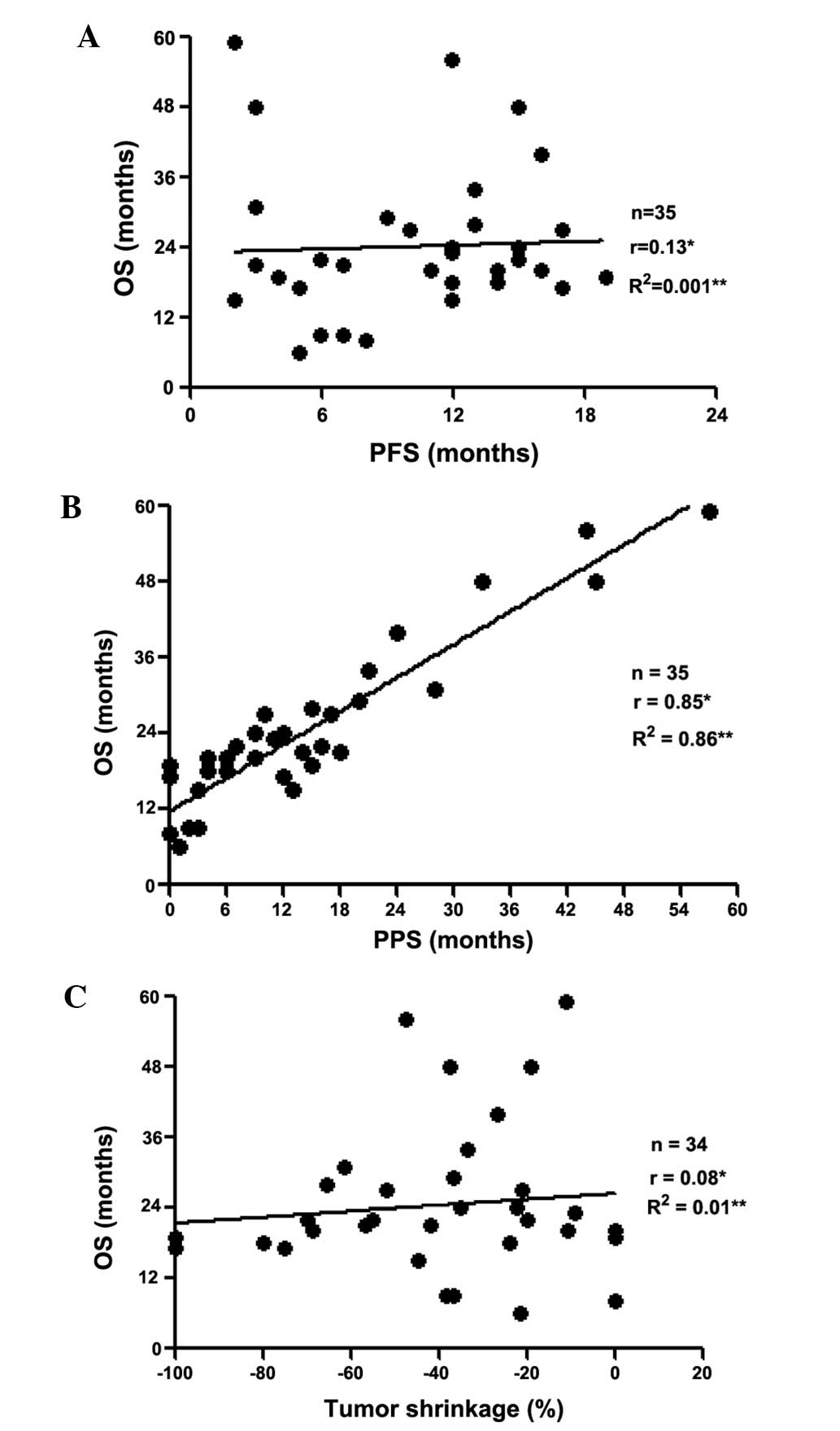
The associations between OS and PFS, PPS and tumor
shrinkage are shown in Fig. 3A, B and
C, respectively. Based on Spearman’s rank correlation
coefficients and linear regression, PPS was found to be strongly
associated with OS (r=0.85, P<0.05, R2=0.86), unlike
PFS (r=0.13, P=0.45, R2=0.001) and tumor shrinkage
(r=0.08, P=0.61, R2=0.01).
Factors affecting PPS
The univariate analysis demonstrated that the
factors associated with PPS (P<0.05) were age at the beginning
of first-line treatment; performance status (PS) at the beginning
and at the end of first-line treatment and at the beginning of
second-line treatment; the best response to second-line treatment;
and the number of regimens employed after disease progression
following first-line chemotherapy (Table III). Subsequently, the
multivariate analysis revealed that the clinical factors affecting
PPS were the PS at the end of the first-line treatment, best
response to second-line treatment (non-PD vs. PD) and number of
regimens employed after disease progression following first-line
chemotherapy (P<0.05, Table
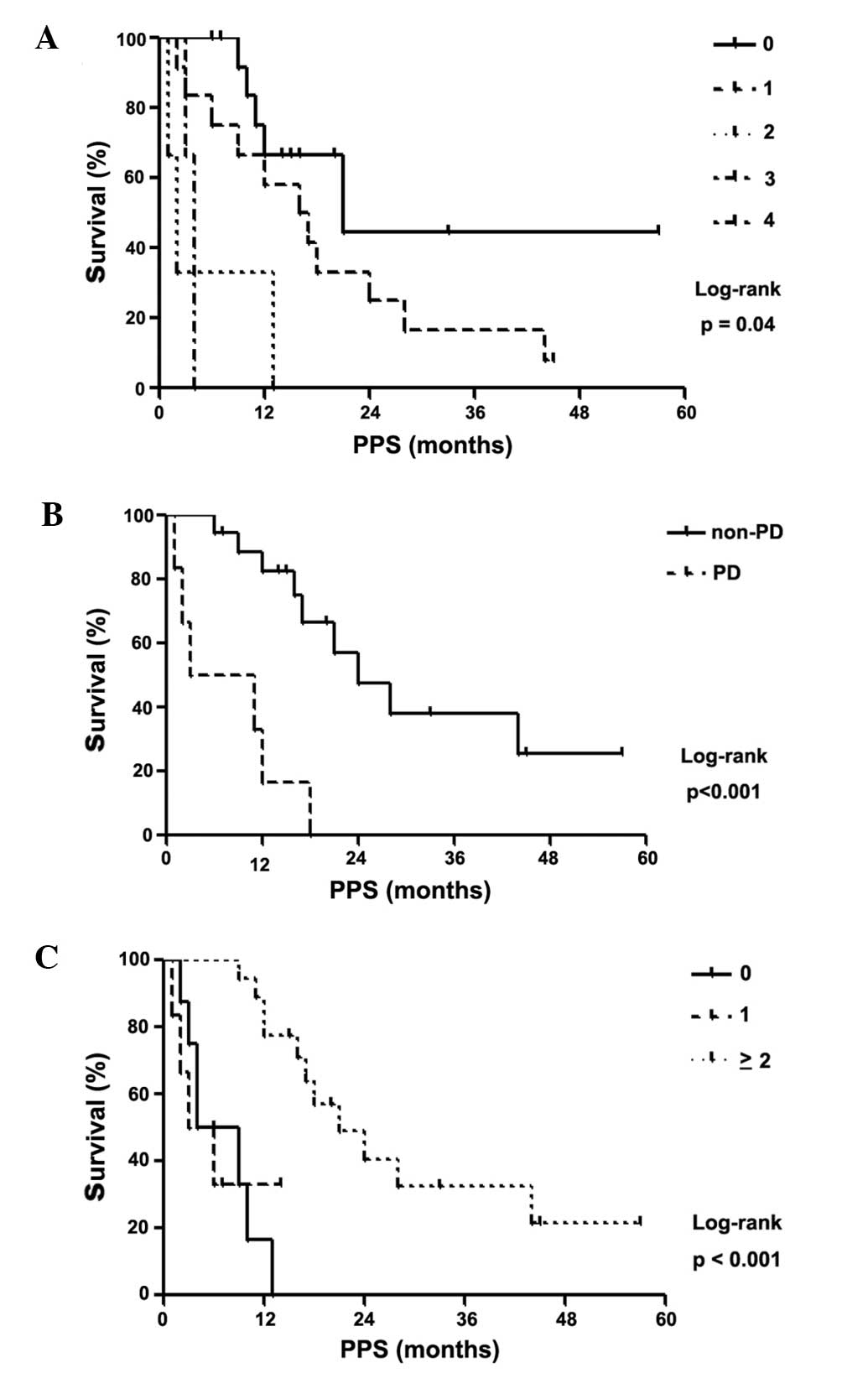
IV). Log-rank tests were used to confirm that these 3 factors
were significantly associated with PPS (log-rank test, P<0.05;
Fig. 4). Patients with PS 0 at the
end of first-line treatment had a PPS of 21.0 months, patients with
PS 1 had a PPS of 16.5 months, patients with PS 2 had a PPS of 2.0
months, patients with PS 3 had a PPS of 4.0 months and patients
with PS 4 had an undefined PPS (log-rank test, P=0.04; Fig. 4A). Furthermore, patients with
non-PD had a median PPS of 24.0 months, whereas their PD
counterparts had a median PPS of 7.0 months (log-rank test,
P<0.001; Fig. 4B). The PPS for
patients who did not receive additional treatment regimens after
disease progression following first-line chemotherapy was 6.5
months; with 1 additional regimen, the PPS was 4.5 months; and with
≥2 regimens, the PPS was 21.0 months (log-rank test, P<0.001;
Fig. 4C). These results remained
consistent following adjustment in the Cox proportional hazards
model (Table IV).
 | Table IIIUnivariate Cox regression analysis of
the association between baseline patient characteristics and
post-progression survival. |
Table III
Univariate Cox regression analysis of
the association between baseline patient characteristics and
post-progression survival.
| Post-progression
survival |
|---|
|
|
|---|
|
Characteristics | Hazard ratio | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 1.53 | 0.57–3.73 | 0.37 |
| Age at the
beginning of first-line treatment | 1.07 | 1.02–1.12 | <0.01 |
| PS at the beginning
of first-line treatment | 1.73 | 1.05–2.69 | 0.03 |
| Stage (IIIB vs.
IV) | 2.03 | 0.47–6.06 | 0.29 |
| EGFR mutation
status (exon 19 del vs. exon 21 L858R) | 0.48 | 0.21–1.08 | 0.07 |
| Sum of longest
diameter of target lesions | 1.01 | 0.98–1.03 | 0.24 |
| Best response at
first-line treatment |
| PR vs. non-PR | 0.69 | 0.30–1.54 | 0.36 |
| Non-PD vs. PD | 0.22 | 0.04–4.22 | 0.24 |
| PS at the end of
first-line treatment | 2.01 | 1.38–2.96 | <0.01 |
| Age at the
beginning of second-line treatment | 1.06 | 0.99–1.15 | 0.07 |
| PS at the beginning
of second-line treatment | 3.6 | 1.20–13.6 | 0.02 |
| Best response to
second-line treatment |
| PR vs. non-PR | 0.33 | 0.07–1.09 | 0.07 |
| Non-PD vs. PD | 0.13 | 0.03–0.46 | <0.01 |
| Number of regimens
after progression beyond first-line chemotherapy | 0.57 | 0.41–0.76 | <0.01 |
 | Table IVMultivariate Cox regression analysis
of the association between post-progression survival and age at the
beginning of first-line treatment, PS at the end of first-line
treatment, best response to second-line treatment and number of
regimens employed after progression beyond first-line
chemotherapy. |
Table IV
Multivariate Cox regression analysis
of the association between post-progression survival and age at the
beginning of first-line treatment, PS at the end of first-line
treatment, best response to second-line treatment and number of
regimens employed after progression beyond first-line
chemotherapy.
| Post-progression
survival |
|---|
|
|
|---|
| Variables | Hazard ratio | 95% CI | P-value |
|---|
| Age at the
beginning of first-line treatment | 1.03 | 0.93–1.14 | 0.53 |
| PS at the end of
first-line treatment | 3.67 | 1.00–17.9 | 0.04 |
| Best response to
second-line treatment (non-PD vs. PD) | 0.11 | 0.01–0.54 | <0.01 |
| Number of regimens
after progression beyond first-line chemotherapy | 0.47 | 0.24–0.75 | <0.01 |
Discussion
We investigated the associations between OS and PFS,
PPS and tumor shrinkage at the individual patient level. PPS was
found to be strongly associated with OS, unlike PFS and tumor
shrinkage. Additionally, PPS was affected by the PS at the end of
first-line treatment, best response to second-line treatment
(non-PD vs. PD) and number of regimens employed after disease
progression following first-line chemotherapy.
The validity of surrogate endpoints has been
determined by previous meta-analyses (18,19).
Biostatisticians have also proposed measures for validating
surrogate endpoints (20,21). Tumor response and PFS are potential
surrogate endpoints for OS in extensive-stage small-cell lung
cancer (22), although their
validity in advanced NSCLC is controversial (23). Broglio and Berry (2) recently focused on PPS, which they
defined as survival post-progression (OS minus PFS), in a
hypothetical clinical trial situation; their study hypothesized
that treatment affected PFS, but not PPS. Recently, PPS was found
to be strongly associated with OS after first-line chemotherapy for
advanced NSCLC (8,9).
Our results do not correspond to those of certain
previous studies indicating that tumor response and PFS may be
surrogate endpoints for OS in advanced NSCLC (24,25).
In our patients with advanced NSCLC harboring EGFR mutations
who received first-line gefitinib, PFS and tumor response did not
reflect OS. PPS was more closely associated with OS rather than
PFS; the association between PPS and OS was linear (Fig. 3A and B). PPS accounted for a large
part of OS, suggesting that chemotherapy was too weak for PFS to
prolong OS. Therefore, in clinical trials with patients expected to
have a short PFS after first-line chemotherapy, such as those in
our study, we must control for factors that affect the PPS.
A previous clinical trial for advanced NSCLC
demonstrated that a long PPS was associated with a good PS, the use
of first-line monotherapy and a molecularly targeted agent
(8). However, no studies have yet
investigated individual patient data to determine factors that
affect PPS in advanced NSCLC with EGFR mutations. We aimed
to determine whether baseline factors were prognostic for PPS and
found that the PS at the end of first-line treatment, best response
to second-line treatment and number of regimens employed after
disease progression were strongly associated with PPS; this was
confirmed by using log-rank tests. To the best of our knowledge,
this study is the first to report individual-level factors that
affect PPS in patients with advanced NSCLC with targeted
therapy-sensitive EGFR mutations. Our findings suggest that
patients with a good PS at the end of first-line treatment are able
to achieve SD after disease progression. These patients are also
likely to be able to continue chemotherapy and achieve a prolonged
PPS, which is associated with a prolonged OS. The large number of
treatment regimens used after disease progression is likely the
result of the increasing number of active compounds, such as
docetaxel, pemetrexed, S1, gemcitabine, amrubicin and erlotinib,
which are currently available for second- and third-line
chemotherapy for advanced NSCLC (Table II). Furthermore, re-administration
of gefitinib is reported to be effective and is therefore a
treatment option for patients with NSCLC who are initially
responsive to gefitinib, but acquire resistance following
subsequent chemotherapy (26).
This study had several limitations. First, the
sample size was small. Only a small number of advanced NSCLC
patients harboring therapy-sensitive EGFR mutations who were
treated with first-line gefitinib were seen at our single
institution, leading to this limitation. Our sample size was also
limited by our attempt to analyze patients with similar
backgrounds. However, our institution treats a relatively large
number of such cases and our practices and policies are largely
unified, since this is a single institution. Understanding the
nature of the sources of bias in this study ensures that the
results are meaningful. A future study that includes a larger
patient cohort is required. Second, we were unable to thoroughly
evaluate the treatments administered after disease progression
following second-line chemotherapy. However, our results are
meaningful, as only a limited number of patients received
third-line or subsequent chemotherapy. Third, individual physicians
decided on the date on which responses were recorded, possibly
introducing variance into the measurements of PFS and tumor
response. Fourth, the patients in this study received gefitinib,
and not erlotinib, as first-line chemotherapy. However, this
reflects the clinical setting, in which the majority of patients
are treated with gefitinib.
In conclusion, using individual-level data, we
observed that PPS, but not PFS or tumor response, was a surrogate
for OS in patients with advanced NSCLC with targeted
therapy-sensitive EGFR mutations. Additionally, a PFS
advantage was not associated with increased OS, whereas PPS exerted
a more significant influence on OS. Furthermore, the PS at the end
of first-line treatment, best response to second-line treatment
(non-PD/PD) and number of regimens employed after disease
progression following first-line chemotherapy were identified as
prognostic factors for PPS. We suggest that the treatment course
after disease progression following first-line chemotherapy
significantly affects OS. We recommend that these results are
validated to determine whether they can be generalized to larger
populations.
Acknowledgements
We would like to thank Drs Tomohito Kuwako, Yosuke
Miura, Yasuki Iwasaki, Shinichi Ishihara, Satoshi Tsuchiya and
Satoru Watanabe for their assistance with this manuscript.
References
|
1
|
Siegel R, DeSantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broglio KR and Berry DA: Detecting an
overall survival benefit that is derived from progression-free
survival. J Natl Cancer Inst. 101:1642–1649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soria JC, Massard C and Le Chevalier T:
Should progression-free survival be the primary measure of efficacy
for advanced NSCLC therapy? Ann Oncol. 21:2324–2332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saad ED, Katz A and Buyse M: Overall
survival and post-progression survival in advanced breast cancer: a
review of recent randomized clinical trials. J Clin Oncol.
28:1958–1962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundar S, Wu J, Hillaby K, Yap J and
Lilford R: A systematic review evaluating the relationship between
progression free survival and post progression survival in advanced
ovarian cancer. Gynecol Oncol. 125:493–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrelli F and Barni S: Correlation of
progression-free and post-progression survival with overall
survival in advanced colorectal cancer. Ann Oncol. 24:186–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotta K, Kiura K, Fujiwara Y, et al: Role
of survival post-progression in phase III trials of systemic
chemotherapy in advanced non-small-cell lung cancer: a systematic
review. PLoS One. 6:e266462011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi H, Okamoto I, Morita S, Taguri M
and Nakagawa K: Postprogression survival for first-line
chemotherapy of patients with advanced non-small-cell lung cancer.
Ann Oncol. 23:1537–1541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imai H, Takahashi T, Mori K, et al:
Individual-level data on the relationships of progression-free
survival, post-progression survival, and tumor response with
overall survival in patients with advanced non-squamous non-small
cell lung cancer. Neoplasma. 61:233–240. 2014. View Article : Google Scholar
|
|
11
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitsudomi T, Morita S, Yatabe Y, et al;
West Japan Oncology Group. Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): an
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar
|
|
13
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inoue A, Kobayashi K, Maemondo M, et al:
Updated overall survival results from a randomized phase III trial
comparing gefitinib with carboplatin-paclitaxel for chemo-naive
non-small cell lung cancer with sensitive EGFR gene mutations
(NEJ002). Ann Oncol. 24:54–59. 2013. View Article : Google Scholar
|
|
15
|
Nagai Y, Miyazawa H, Huqun, et al: Genetic
heterogeneity of the epidermal growth factor receptor in non-small
cell lung cancer cell lines revealed by a rapid and sensitive
detection system, the peptide nucleic acid-locked nucleic acid PCR
clamp. Cancer Res. 65:7276–7282. 2005. View Article : Google Scholar
|
|
16
|
Yatabe Y, Hida T, Horio Y, Kosaka T,
Takahashi T and Mitsudomi T: A rapid, sensitive assay to detect
EGFR mutation in small biopsy specimens from lung cancer. J Mol
Diagn. 8:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
18
|
Johnson KR, Ringland C, Stokes BJ, et al:
Response rate or time to progression as predictors of survival in
trials of metastatic colorectal cancer or non-small-cell lung
cancer: a meta-analysis. Lancet Oncol. 7:741–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hotta K, Fujiwara Y, Matsuo K, et al: Time
to progression as a surrogate marker for overall survival in
patients with advanced non-small cell lung cancer. J Thorac Oncol.
4:311–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weir CJ and Walley RJ: Statistical
evaluation of biomarkers as surrogate endpoints: a literature
review. Stat Med. 25:183–203. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fleischer F, Gaschler-Markefski B and
Bluhmki E: A statistical model for the dependence between
progression-free survival and overall survival. Stat Med.
28:2669–2686. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foster NR, Qi Y, Shi Q, et al: Tumor
response and progression-free survival as potential surrogate
endpoints for overall survival in extensive stage small-cell lung
cancer: findings on the basis of North Central Cancer Treatment
Group trials. Cancer. 117:1262–1271. 2011. View Article : Google Scholar
|
|
23
|
Berghmans T, Pasleau F, Paesmans M, et al:
ELCWP: Surrogate markers predicting overall survival for lung
cancer: ELCWP recommendations. Eur Respir J. 39:9–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujino K, Kawaguchi T, Kubo A, et al:
Response rate is associated with prolonged survival in patients
with advanced non-small cell lung cancer treated with gefitinib or
erlotinib. J Thorac Oncol. 4:994–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Liu S, Gu H and Wang D: Surrogate
end points for survival in the target treatment of advanced
non-small-cell lung cancer with gefitinib or erlotinib. J Cancer
Res Clin Oncol. 138:1963–1969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomizawa Y, Fujita Y, Tamura A, et al:
Effect of gefitinib re-challenge to initial gefitinib responder
with non-small cell lung cancer followed by chemotherapy. Lung
Cancer. 68:269–272. 2010. View Article : Google Scholar : PubMed/NCBI
|