Introduction
Colorectal cancer (CRC) is a major health concern
worldwide and is one of the most frequent causes of cancer-related
mortality (1). Despite the
advances in diagnosis and treatment, ~25% of CRC patients
eventually succumb to their disease (2). The most common causes of treatment
failure and/or death in patients with CRC are tumor invasion and
metastasis (3). The mechanism
underlying CRC development has not been clearly determined,
although a series of genetic and epigenetic events are considered
to be involved in colorectal carcinogenesis, including oncogene
activation and anti-oncogene inactivation (4). The second step in the physiologically
critical β-oxidation pathway of fatty acid metabolism in the
mitochondria is catalyzed by enoyl coenzyme A hydratase, short
chain, 1 (ECHS1), which is assigned to chromosome 10q26.2-q26.3 and
promotes proliferation of several tumor cells, such as
hepatocellular carcinoma cells (5). Yeh et al (6) reported that ECHS1 may play an
important role in human colorectal carcinogenesis via microarray
bioinformatics analysis. However, definite conclusions regarding
the extent of ECHS1 expression in human CRCs and its significance
have not yet been reached. In this study, we investigated the
expression of ECHS1 by immunohistochemical analysis in human CRC
samples and proximal non-cancerous colorectal tissues and its
association with clinicopathological characteristics and prognosis.
The expression of the ECHS1 protein in fresh CRC specimens and
normal colorectal tissues was detected by western blot
analysis.
Materials and methods
Specimens and clinicopathological
data
We retrospectively collected samples from 148
patients who had undergone surgical treatment for pathologically
confirmed primary CRC at the Chenggong Hospital Affiliated to
Xiamen University, between March, 2010 and January, 2013 (Table I). None of the patients had
received chemotherapy or radiotherapy prior to surgery. This study
was conducted in accordance with the ethical principles of the
Declaration of Helsinki and approved by the Review Committee for
the Use of Human or Animal Subjects of Xiamen University. Informed
consent was obtained from all the participants. A total of 78 men
and 70 women, with a median age of 51.5 years (range, 33–71 years),
were included in this study. The post-surgery pathological reports
confirmed that all the CRCs were adenocarcinomas. The median
follow-up was 19 months (range, 10–26 months). According to the
International Union Against Cancer-TNM stage, the patients were
divided into 30 stage I, 44 stage II, 48 stage III and 26 stage IV
cases. A total of 38 proximal non-cancerous colorectal tissues were
included as controls. In addition, 46 fresh tissue samples of CRC
and 22 normal colorectal tissues were collected for western blot
analysis.
 | Table IAssociation between ECHS1 expression
and clinicopathological characteristics in CRC and proximal
non-cancerous colorectal tissues. |
Table I
Association between ECHS1 expression
and clinicopathological characteristics in CRC and proximal
non-cancerous colorectal tissues.
| | ECHS1 | |
|---|
| |
| |
|---|
| Variables | n | Negative | Positive | P-value |
|---|
| Tissue type |
| CRC | 148 | 64 | 84 | <0.001 |
| Paraneoplastic | 38 | 36 | 2 | |
| Age, years |
| <55 | 76 | 40 | 36 | 0.419 |
| ≥55 | 72 | 42 | 30 | |
| Gender |
| Male | 78 | 38 | 40 | 0.623 |
| Female | 70 | 36 | 34 | |
| Differentiation |
| High/Moderate | 86 | 34 | 52 | 0.028 |
| Low | 62 | 20 | 42 | |
| Stage | | | | 0.015 |
| I | 30 | 6 | 24 | |
| II | 44 | 10 | 34 | |
| III | 48 | 12 | 36 | |
| IV | 26 | 4 | 22 | |
| LN metastasis |
| N0 | 38 | 20 | 18 | 0.011 |
| N1 | 54 | 24 | 30 | |
| N2 | 56 | 14 | 42 | |
Immunohistochemistry and evaluation
criteria
The specimens were fixed in 10% formalin and
embedded in paraffin, according to standard procedures. Tissue
sections (4 μm) were prepared and endogenous peroxidase activity
was blocked by incubation in 0.3% H2O2 for 20
min. The sections were incubated with rabbit anti-ECHS1 monoclonal
antibody (1:400 dilution; Abcam, Cambridge, UK) overnight at 4°C.
The secondary antibody used was goat anti-rabbit IgG (1:5,000
dilution). The specimens were examined using the Envision kit (Dako
Canada Inc., Burlington, ON, Canada) using diaminobenzidine as a
chromogen.
The evaluation of the grade of staining was
independently performed by an experienced pathologist. The criteria
used for assessment were previously reported as follows: −,
negative; +, 1–25% positive cells; ++, 25–75% positive cells; and
+++, 75–100% positive cells (7).
Western blot analysis
The proteins (20 μg) were subjected to 10% SDS
polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes (Millipore Corp., Billerica,
MA, USA) using the Mini-Protean system (Bio-Rad, Hercules, CA,
USA). The membranes were incubated for 1 h at room temperature in
blocking buffer and then incubated with the appropriate antibodies
(anti-ECHS1 antibody at 1:400 dilution, Abcam; and anti-tubulin
antibody at 1:2,000 dilution, Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) overnight at 4°C. After washing with Tris-buffered
saline, Tween-20 and Triton X-100, the membranes were incubated
with horseradish peroxidase-conjugated anti-rabbit antibody
(1:8,000 dilution; ZSGB-BIO, Beijing, China) for 2 h at 37°C. The
blots were briefly incubated with enhanced chemiluminescence
solution (Applygen Technologies, Inc., Beijing, China) and exposed
to X-ray films.
Statistical analysis
Statistical analyses were performed using SPSS.17.0
software (SPSS Inc., Chicago, IL, USA). The Pearson’s Chi-square
test was used for recepteur d’origine nantais expression positive
rate comparison. The Kaplan-Meier method was used for evaluating
survival curves and the long-rank test was used for testing
survival rates. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of ECHS1 protein in CRC and
proximal non-cancerous colorectal tissues
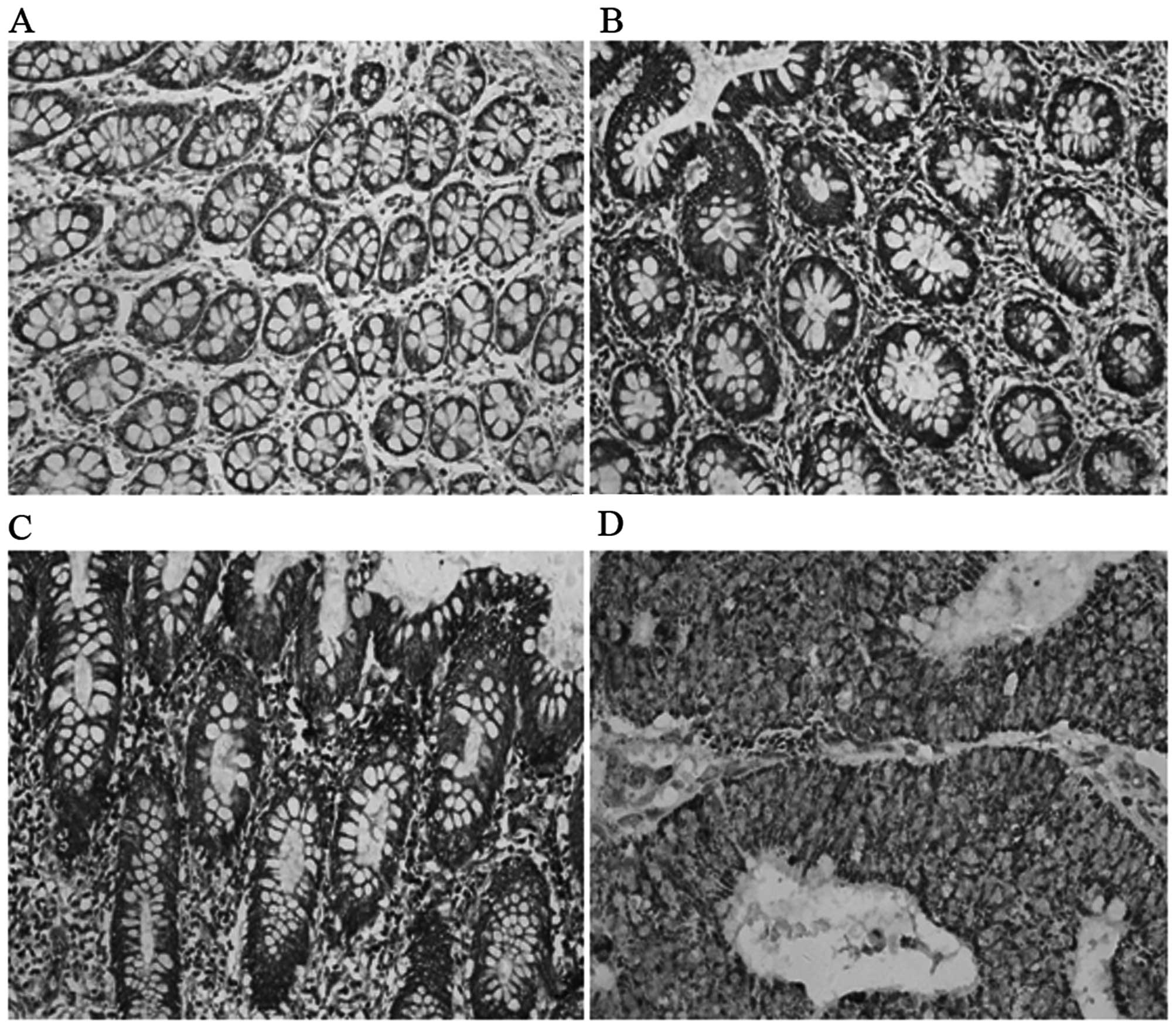
ECHS1 protein expression was detected in the
cytoplasm of CRC cells (Fig. 1).
The percentage of positively stained carcinoma cells and the
staining intensity were recorded. The positive expression rate of
the ECHS1 was 56.76% (84/148) in CRC cases and only 5.26% (2/38) in
proximal non-cancerous colorectal tissues. The difference between
the two groups was statistically significant (χ2=13.06;
P<0.001) (Table I).
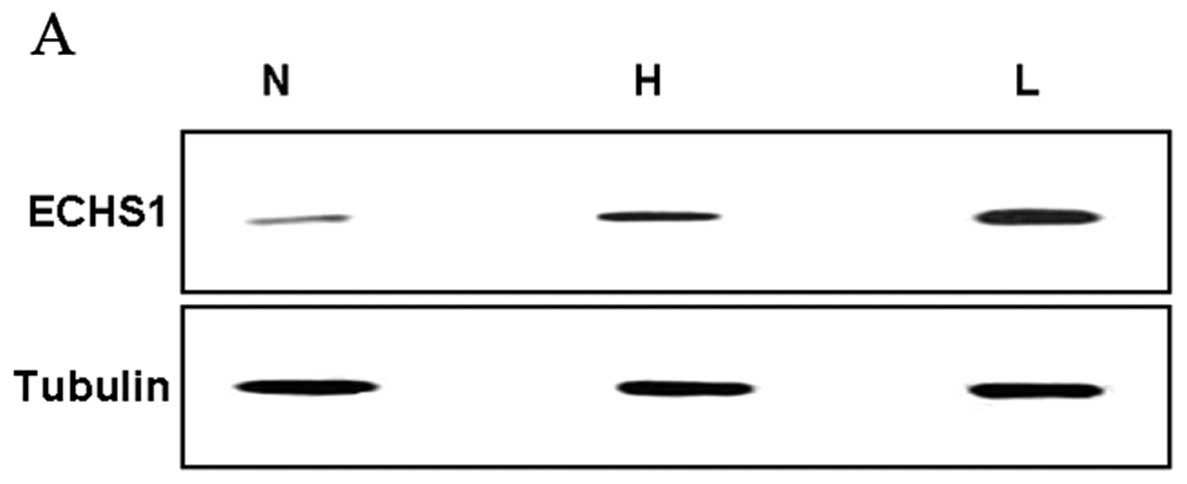
ECHS1 protein expression in fresh
tissues
The expression of the ECHS1 protein in 46 fresh CRC
and 22 normal colorectal tissue samples was detected by western
blot analysis. We observed that the expression level of the ECHS1
protein in fresh CRC tissues was significantly higher compared to
that in normal colorectal tissues. Furthermore, the expression
level of the ECHS1 protein in fresh samples of poorly
differentiated CRC was significantly higher compared to that in CRC
samples of high or moderate differentiation (Fig. 2A and B).
Association between ECHS1 expression and
clinicopathological characteristics
The ECHS1 protein was strongly expressed in CRC
tissues classified as the high TNM stage group, positive lymph node
metastasis group and poorly differentiated group (Fig. 1 and Table I). The expression of the ECHS1
protein was positively correlated with clinical TNM stage
(χ2=10.46; P=0.015), lymph node metastasis
(χ2=7.93; P=0.011) and histological differentiation
(χ2=5.73; P=0.024), with statistically significance
differences, which was not the case for gender or age (P>0.05)
(Table I).
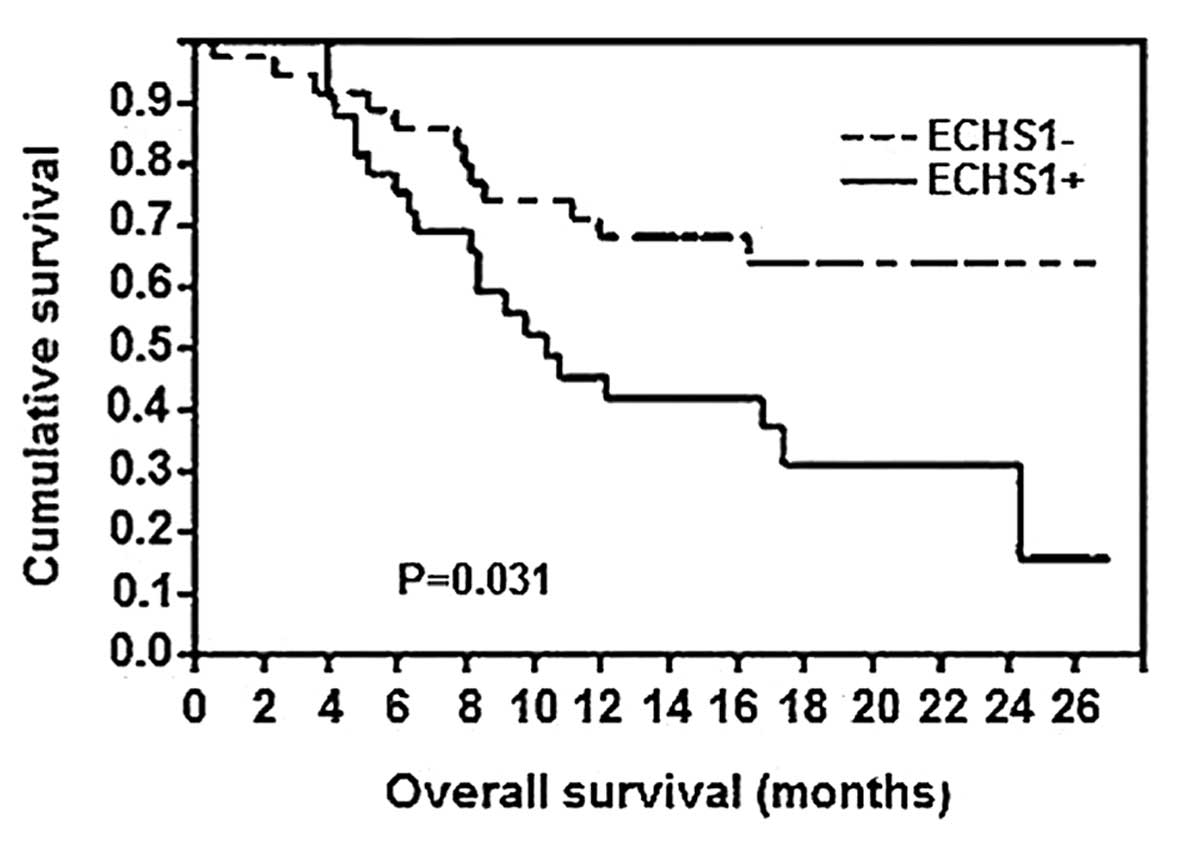
ECHS1 expression and survival
Of the 148 CRC patients, 110 patients remained alive
at the time of this study, whereas 34 patients had succumbed to
distant metastases and 4 patients had succumbed to locoregional
disease recurrence. As regards the Kaplan-Meier survival analysis
(Fig. 3), a significant difference
in the overall survival rate was observed between patients with
positive expression of the ECHS1 protein and those with negative
ECHS1 expression (P=0.031). Therefore, the expression of the ECHS1
protein was associated with a poor prognosis in patients with
CRC.
Discussion
Under physiological conditions, ECHS1 is essential
for embryonic development and is critical in the regulation of
certain physiological processes (8,9).
Inappropriate activation or altered expression of ECHS1 is involved
in several regulatory processes associated with the occurrence and
progression of tumors. Elevated expression of the ECHS1 protein has
been detected in several malignant tumors, including liver,
colorectal, breast, bladder and prostate cancers (10). In this study, we observed a
significant increase in the expression of the ECHS1 protein in CRC
tissues compared to that in proximal non-cancerous colorectal
tissues. The western blot analysis confirmed that the expression of
the ECHS1 protein was significantly increased in CRC vs. normal
tissues, indicating that ECHS1 may be involved in the development
of CRC and inferring that ECHS1 may be a novel tumor marker for
CRC.
Through the analysis of the association between the
expression of ECHS1 and clinicopathological characteristics, we
demonstrated that the positive expression rate of the ECHS1 protein
in CRC was correlated with the TNM stage (11–13).
Lymphogenous metastases are usually an early step during cancer
progression, with lymph nodes commonly being the first metastatic
site (13,14). The presence of lymph node
metastases is known to be associated with poor prognosis in a
number of tumors (15,16). Lymph node metastasis and
histological differentiation were also found to be correlated with
ECHS1 protein expression. These data suggest that ECHS1 may be
involved in the progression, invasion and metastasis of CRC.
Previous studies on ECHS1 expression and prognostic significance
demonstrated that elevated ECHS1 expression was associated with a
significantly poorer prognosis in several types of tumors,
including breast, liver and colorectal cancers (5,6,9).
Of note, we observed that the expression of the
ECHS1 protein was positively correlated with histological
differentiation. The ECHS1 protein expression was significantly
higher in poorly differentiated CRC tissues compared to that in CRC
tissues of high or moderate differentiation. In our study, there
was also a significant association between the expression of the
ECHS1 protein and the overall survival rate. These findings suggest
that positive ECHS1 expression may be useful as a novel prognostic
marker in CRC patients and a potential target for therapeutic
intervention.
Acknowledgements
This study was supported by grants from the
Scientific Research Foundation of Health Bureau of Xiamen City (no.
3502Z20134025) and the Medical Technology Innovation Project of
Nanjing Military (no. MS090), P.R. China.
References
|
1
|
Fehlker M, Huska MR, Jöns T, et al:
Concerted down-regulation of immune-system related genes predicts
metastasis in colorectal carcinoma. BMC Cancer. 14:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Debunne H and Ceelen W: Mucinous
differentiation in colorectal cancer: molecular, histological and
clinical aspects. Acta Chir Belg. 113:385–390. 2013.PubMed/NCBI
|
|
3
|
Chen YS, Xu SX, Ding YB, et al:
Helicobacter pylori infection and the risk of colorectal
adenoma and adenocarcinoma: an updated meta-analysis of different
testing methods. Asian Pac J Cancer Prev. 14:7613–7619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ewing I, Hurley JJ, Josephides E, et al:
The molecular genetics of colorectal cancer. Frontline
Gastroenterol. 5:26–30. 2014. View Article : Google Scholar
|
|
5
|
Zhu XS, Dai YC, Chen ZX, et al: Knockdown
of ECHS1 protein expression inhibits hepatocellular carcinoma cell
proliferation via suppression of Akt activity. Crit Rev Eukaryot
Gene Expr. 23:275–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeh CS, Wang JY, Cheng TL, et al: Fatty
acid metabolism pathway play an important role in carcinogenesis of
human colorectal cancers by microarray-bioinformatics analysis.
Cancer Lett. 233:297–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CW, Li AF, Chi CW, et al: Human gastric
cancer kinase profile and prognostic significance of MKK4 kinase.
Am J Pathol. 156:2007–2015. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Preter V, Arijs I, Windey K, et al:
Impaired butyrate oxidation in ulcerative colitis is due to
decreased butyrate uptake and a defect in the oxidation pathway.
Inflamm Bowel Dis. 18:1127–1136. 2012.PubMed/NCBI
|
|
9
|
Liu X, Feng R and Du L: The role of
enoyl-CoA hydratase short chain 1 and peroxiredoxin 3 in
PP2-induced apoptosis in human breast cancer MCF-7 cells. FEBS
Lett. 584:3185–3892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Resch A and Langner C: Lymph node staging
in colorectal cancer: old controversies and recent advances. World
J Gastroenterol. 19:8515–8526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lou X, Qi X, Zhang Y, Long H and Yang J:
Decreased expression of microRNA-625 is associated with tumor
metastasis and poor prognosis in patients with colorectal cancer. J
Surg Oncol. 108:230–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miglio U, Mezzapelle R, Paganotti A, et
al: Mutation analysis of KRAS in primary colorectal cancer and
matched metastases by means of highly sensitivity molecular assay.
Pathol Res Pract. 209:233–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawamata F, Homma S, Kamachi H, et al:
C-ERC/mesothelin provokes lymphatic invasion of colorectal
adenocarcinoma. J Gastroenterol. 49:81–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang B, Mason J, Jewett A, et al: Cell
budding from normal appearing epithelia: a predictor of colorectal
cancer metastasis? Int J Biol Sci. 9:119–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Jingyan G, Baorong S, et al:
Expression of EGFR, Her2 predict lymph node metastasis
(LNM)-associated metastasis in colorectal cancer. Cancer Biomark.
11:219–226. 2012.PubMed/NCBI
|
|
16
|
Toiyama Y, Yasuda H, Saigusa S, et al:
Increased expression of Slug and vimentin as novel predictive
biomarkers for lymph node metastasis and poor prognosis in
colorectal cancer. Carcinogenesis. 34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|