Introduction
Colorectal cancer is the third most common type of
cancer and the second or third most common cause of cancer-related
mortality worldwide. Approximately one-third of the tumors arise in
the rectum, whereas in the remaining cases the tumor arises in the
colon and the majority of the cases are carcinomas (1). The preoperative assessment of the
depth of cancer invasion of the rectal wall and regional lymph node
metastasis is crucial in determining the surgical approach for the
treatment of rectal cancer.
Multiple modalities are available for the staging of
rectal cancer, including digital examination, endorectal
ultrasonography (ERUS), computerized tomography (CT), magnetic
resonance imaging (MRI) and, more recently, positron emission
tomography (PET)-CT. Clinical staging by digital examination is
standard, but does not allow assessment of the nodal status.
Although CT scanning is useful for assessing extramural tumor
spread, it is less accurate in assessing the depth of invasion
within the wall or in predicting nodal disease. The accuracy of MRI
and PET-CT were found to be higher, but are more costly. ERUS,
being a non-invasive modality, has been proven to be fast, safe,
cost-effective, efficient and is currently a widely used diagnostic
tool in the assessment of the depth of cancer invasion and lymph
node involvement (2,3).
There has been extensive research regarding the
diagnostic performance of ERUS in the staging of rectal cancer
(4–6); however, its accuracy, reliability and
validity remain controversial. The majority of the studies include
<100 patients and represent only the initial institutional
experience with this technique. There is currently no consensus
regarding the performance of ERUS at each stage of rectal cancer.
Furthermore, ERUS is highly dependent on examiner and institutional
experience. Although most of these reports mention that there is a
learning curve in performing and interpreting these examinations,
the available information regarding the length and steepness of
that curve, or the early training required when using this
modality, is currently limited. Thus, the purpose of our study, was
to further assess the accuracy and learning curve of ERUS in the
assessment of preoperative staging of rectal cancer in the Shanxi
Province Tumor Hospital.
Patients and methods
Patient selection
The patients were selected based on four inclusion
criteria as follows: i) all the patients required a positive biopsy
report (rectal cancer); ii) all the patients underwent ERUS prior
to treatment; iii) all the tumors were surgically excised and
assessed histopathologically; and iv) ERUS diagnosis was directly
compared with the histopathological findings. Patients with
stenotic tumors and those undergoing incomplete examination were
excluded.
All the rectal cancer patients presenting to the
Shanxi Province Tumor Hospital between January, 2007 and March,
2010, were preoperatively assessed by ERUS. Any tumor within 12 cm
of the anal verge on rigid sigmoidoscopy was defined as a rectal
tumor, but only patients with histologically proven adenocarcinoma
were included in the study. A total of 319 rectal cancer patients
(175 men and 144 women), with a mean age of 59 years (range, 22–82
years) were included in this study. Of the 319 patients, 311
underwent radical surgery (abdominoperineal or low anterior
resection) and 8 underwent transanal local excision.
The research protocols were approved by the Ethics
Comittee of the Shan Xi province Tumor Hospital and all the
patients provided written informed consent prior to enrolment.
Patient and physician classification
For the purposes of our analysis, the patients were
divided into three groups, namely A, B and C, depending on whether
the examination was performed between January and December, 2007
(n=38), between January and December, 2008 (n=35), or between
January, 2009 and March, 2010 (n=146). Five physicians with no
prior experience in ERUS performed the examinations. Two of the
physicians performed only 15% of the examinations (47/319) and were
excluded from our analysis of accuracy by physician. The remaining
three physicians, namely D, E and F, performed 162, 64 and 46
examinations, respectively.
Procedure
After undergoing an enema 1 h prior to the ERUS
preparation of the lower bowel, a rigid proctoscopy was performed
on each patient to determine the distance of the tumor from the
anal verge, the tumor size and the wall of the rectum on which the
tumor was located. The patients were examined in the left lateral
position. The ERUS was performed with a 7.5-MHz scanner with a 360°
rotating ultrasonographic probe (Water Balloon Endo-P-Probe;
Siemens AG, Munich, Germany). The probe was fitted with a rubber
sheath and filled with degassed water to minimize acoustic
artifacts and ensure acoustic coupling. The probe was lubricated
using water-soluble gel and introduced under ultrasound control
without the use of a rigid sigmoidoscope.
ERUS staging
Tumor invasion of the rectal wall was staged by the
ultrasound tumor (T staging) and node classification (N staging)
proposed by Hildebrandt and Feifel (7). According to this system and the 7th
TNM classification (8), a uT1
lesion is confined to the mucosa and submucosa; a uT2 tumor
penetrates the muscularis but is confined within the rectal wall,
so that the outer hyperechoic layer remains intact; a uT3 tumor
disrupts the outer hyperechoic layer, indicating invasion of the
perirectal fat; a uT4a tumor penetrates to the surface of the
visceral peritoneum; and a uT4b tumor directly involves surrounding
organs or is adherent to other organs or structures. Normal,
non-enlarged lymph nodes are similar in echogenicity to the
hyperechoic perirectal tissues and, therefore, are not usually
identified. Pathological lymph nodes, which were described as uN1,
were defined as circular structures >5 mm in diameter, with a
similar echogenicity to the tumor according to the description by
Beynon et al (9). Nodes
<5 mm in diameter, which were defined as uN0, were considered to
be normal or inflammatory.
Comparison of ERUS results with
histopathological findings and statistical analysis
The ERUS results were compared with the
postoperative histopathological findings for each resected
specimen. The accuracy of T and N staging after each additional 20
patients was calculated for three physicians, namely D, E and F and
the series of cumulative accuracies were displayed. The learning
curve was indicated by a plateau of the cumulative accuracies. The
comparison of the accuracies within the T and N staging results and
between groups was performed using the χ2 test by SPSS
statistical software, version 19.0 (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
T staging
The overall accuracy for T staging was 67%, with 16%
of the tumors being overstaged and 17% understaged. Of the 319
primary rectal tumors, 6 were uT1, 58 uT2, 170 uT3, 81 uT4a and 4
uT4b. The accuracy of T1 staging could not be meaningfully
calculated due to the limited number of cases (Table I).
 | Table IAssessment of rectal wall invasion
with ERUS (uT) vs. pathological examination (pT). |
Table I
Assessment of rectal wall invasion
with ERUS (uT) vs. pathological examination (pT).
| Pathological
examination | | | |
|---|
|
| | | |
|---|
| ERUS | pT1 (n) | pT2 (n) | pT3 (n) | pT4a (n) | pT4b (n) | Accuracy (%) | Overstaged (%) | Understaged (%) |
|---|
| uT1 | 2 | 1 | 2 | 1 | 0 | NA | NA | NA |
| uT2 | 1 | 25 | 29 | 3 | 0 | 43 | 2 | 55 |
| uT3 | 0 | 16 | 137 | 17 | 0 | 81 | 9 | 10 |
| uT4a | 0 | 7 | 26 | 48 | 0 | 59 | 41 | |
| uT4b | 0 | 0 | 1 | 0 | 3 | 75 | 25 | |
N staging
The overall accuracy for N staging was 66%; when
incorrect, understaging tended to occur more frequently compared to
overstaging (23 vs. 11%, respectively) (Table II). A total of 311 nodes (nodal
diameter, 2.0–33.4 cm) were examined with ERUS in the 311 patients
who underwent radical surgery and 120 nodes were found to be
positive for metastasis.
 | Table IIAssessment of lymph node metastasis
with ERUS (uN) vs. pathological examination (pN). |
Table II
Assessment of lymph node metastasis
with ERUS (uN) vs. pathological examination (pN).
| Pathological
examination | | | |
|---|
|
| | | |
|---|
| ERUS | pN1 (n) | pN0 (n) | Total (n) | Accuracy (%) | Overstaged (%) | Understaged (%) |
|---|
| uN1 | 85 | 35 | 120 | 71 | 29 | |
| uN0 | 70 | 121 | 191 | 63 | | 37 |
| Total | 155 | 156 | 311 | 66 | 11 | 23 |
Diagnostic accuracy by physician during
three different time periods
Physicians D, E and F completed the examination of
272 of the 319 cases. The total T and N staging accuracy of
physicians D, E and F were 75 and 72%; 59 and 59%; and 50 and 52%,
respectively. The diagnostic accuracy of the physicians during time
periods A, B and C, is shown in Table III.
 | Table IIIDiagnostic accuracy of ERUS by
physician during three different time periods. |
Table III
Diagnostic accuracy of ERUS by
physician during three different time periods.
| | Period A
(January–December, 2007) | Period B
(January–December, 2008) | Period C (January,
2009–March, 2010) |
|---|
| |
|
|
|
|---|
| Physicians | Patient no. | T stage (%) | N stage (%) | T stage (%) | N stage (%) | T stage (%) | N stage (%) |
|---|
| D | 162 | 55 (12/22)a | 41 (9/22)b | 72 (46/64) | 73 (45/62) | 84 (64/76)a | 81 (58/72)b |
| E | 64 | | | 43 (10/23) | 52 (12/23) | 66 (27/41) | 63 (26/41) |
| F | 46 | 44 (4/9) | 33 (3/9) | 50 (14/28) | 57 (16/28) | 56 (5/9) | 56 (5/9) |
Learning curve
Two of the physicians performed only 15% of the
examinations (47/319) and were excluded from our analysis of
accuracy by physician. For the remaining three physicians, namely
D, E and F, the accuracy of T and N staging after each additional
set of 20 patients was calculated. The series of cumulative
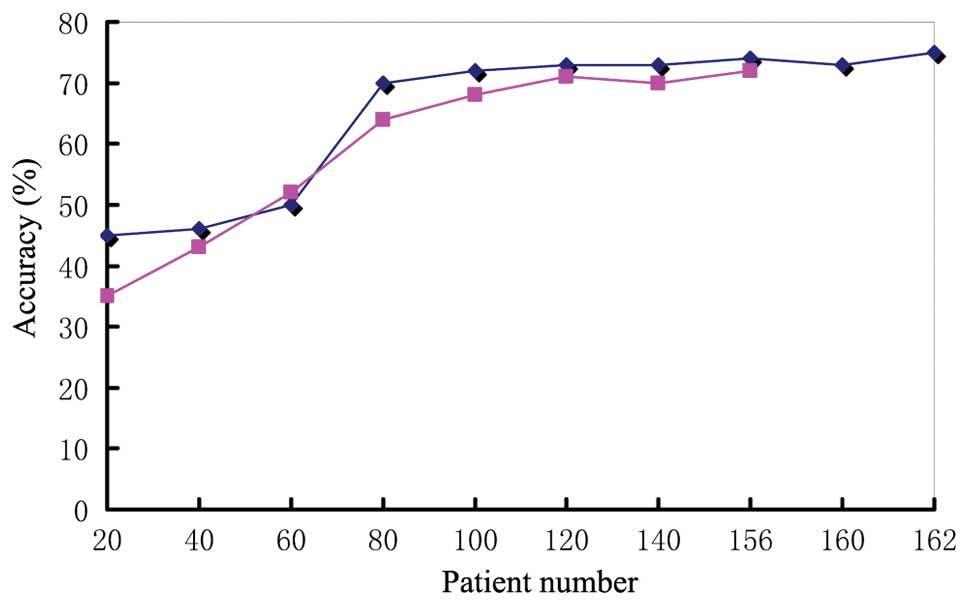
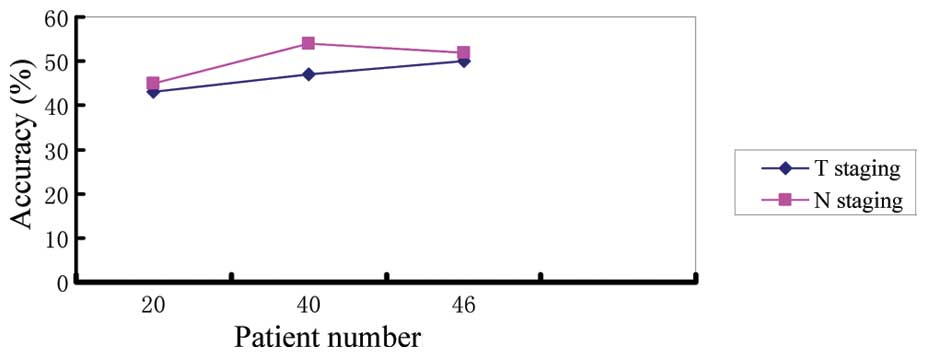
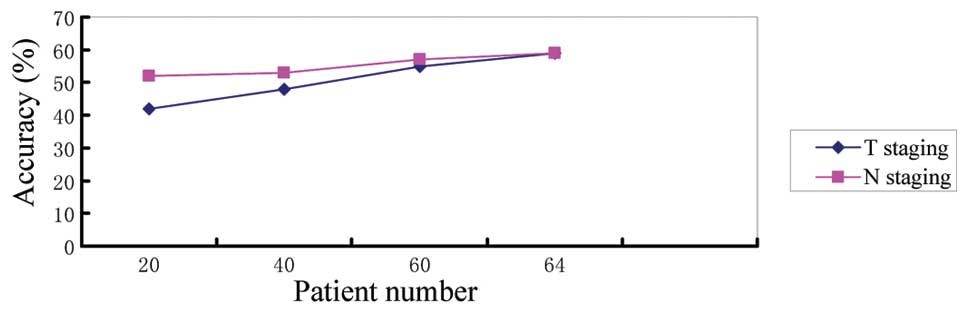
accuracies are displayed in Figs.
1–3.
Discussion
The modern treatment of rectal cancer is currently
stage-oriented (10). Accurate
preoperative clinical staging is crucial for planning treatment and
determining the prognosis of patients on an individual basis. Over
the last few years, transanal local excision has become an accepted
surgical alternative for selected patients with rectal tumors
confined within the rectal wall. Preoperative chemoradiation is the
standard therapy in patients with advanced rectal cancer. The aim
of preoperative treatment is to achieve downstaging and/or
downsizing, with the intention to increase the resectability rate,
enable sphincter-sparing surgery, reduce local recurrence and
possibly improve long-term survival (11,12).
However, the therapeutic strategies that attempt treatment by stage
require a precise knowledge of the depth of tumor invasion of the
rectal wall and of the presence of affected regional lymph nodes
prior to the operation.
Over the last decade, although ERUS has become the
most common diagnostic modality for local staging of rectal cancer,
the accuracy of ERUS staging has been controversial. A review of
the literature revealed that the accuracy of T staging with ERUS
ranges between 81 and 95% (13–15).
These differences are mainly affected by factors such as the number
of cases included in the study, experience with ERUS, patient
selection (with or without radiation) and type of endorectal probe
(2D and 3D). The number of patients in the majority of the studies
is <100, although it may be higher for stage T3–4 cases.
Garcia-Aguilar et al (13)
reviewed the charts of 545 patients with rectal carcinomas staged
by ERUS and compared the ERUS staging with the pathological
findings based on the surgical specimens. The overall accuracy of T
staging was 69%, with 18% of the tumors being overstaged and 13%
understaged.
In our study, the overall accuracy of T staging was
67%, which is lower compared to that previously reported, but
similar to that reported by Garcia-Aguilar, with 16% of the tumors
overstaged and 17% understaged, which is similar to the percentages
(2.0–23.9% and 2.5–17.0%, respectively) reported in the literature
(16). ERUS accurately identified
2 of 3 pT1 tumors and 188 (71%) of 264 pT3 or pT4 tumors that were
candidates for preoperative chemoradiation therapy. However, the
number of pT1 patients in those studies was inadequate to reach
meaningful conclusions. Approximately 9% of T2 lesions may be
overstaged due to a desmoplastic response, whereas 41% of T3
lesions may be understaged due to the presence of microscopic
metastases. The poor endosonographic diagnosis of T2 and T4a tumors
(43 and 59%, respectively) is worse compared to that previously
reported, except for the study of Sailer et al (17), who reported an accuracy of 41% in
T2 tumors. The low accuracy of ERUS in the characterization of T2
tumors emphasizes the need to plan the final treatment after local
excision based on the pathological rather than on the
ultrasonographic stage. Yamashita et al (18) demonstrated that the overestimation
of the extent of cancer invasion was increased in proportion to the
degree of peritumoral inflammation, which makes hyperechoic layers
in the rectal wall appear hypoechoic and may lead to disappearance
of the outer hyperechoic layer, resulting in overstaging of T2
lesions. In our study, the tumor-induced inflammation was falsely
diagnosed on preoperative ERUS as bladder invasion (uT4b). During
the operation, we observed adhesion formation between the rectum
and the posterior wall of bladder and resected that part of the
bladder wall. After surgery, the pathological examination confirmed
chronic inflammation of the bladder wall, whereas the tumor only
invaded perirectal fat tissue (pT3). When initially applying this
technique, due to the operator’s limited experience, the
water-filled balloon was quite sizeable, applying excessive
pressure on the intestine, making it difficult to distinguish the
rectal wall layers. As seen in Table
III, with increasing experience, the accuracy of physician D
improved, with a statistically significant difference in T staging
accuracy between time periods A and C (χ2=6.65,
P<0.01).
Lymph node metastasis was found to be an independent
prognostic factor for patient survival and local disease
recurrence. According to the literature, the accuracy for detection
of malignant lymph nodes ranges between 64 and 84% (13–15).
In a meta-analysis, Bipat et al (19) reported that the sensitivity of
ERUS, CT and MRI for lymph node metastasis was 67, 55 and 66%,
respectively, whereas their specificity was 78, 74 and 76%,
respectively, indicating that there were no significant differences
among ERUS, CT and MRI in nodal staging. In our study, the overall
accuracy of N staging was 66%, which is lower compared to that
previously reported, with 11% of the tumors being overstaged and
23% understaged. Lymph node staging is difficult and challenging.
Several studies adopted 5 mm as a cut-off size for discriminating
between malignant and benign nodes; however, nodal size is not a
reliable discriminator. Imaging is mainly based on the size of the
lymph nodes, but cannot identify micrometastases. In a study of 424
surgical rectal cancer specimens including a total of 12,759 nodes,
the mean nodal diameter was 3.34 mm and the mean diameter of
metastasis was 3.84 mm (20). In
another pathological study of 698 lymph nodes, 70 of 132 (53%)
nodes containing metastases were <5 mm in diameter (21). In our study, the minimum diameter
of the lymph nodes identified with ultrasound was 2.0 mm and the
maximum 33.4 mm. A total of 71% (85/120) lymph nodes >5 mm were
found to be metastatic, as were 53% (10/19) of those sized <4
mm. Thus, diameter alone cannot determine the presence of
metastasis in a lymph node. In addition, benign nodal hyperplasia
was a common finding. The overstaging of lymph nodes is primarily
caused by the presence of reactive enlarged lymph nodes that may be
misdiagnosed as malignant. In addition, small blood vessels and the
seminal vesicles are occasionally mistaken for metastatic lymph
nodes (22). It was previously
demonstrated that endoscopic ultrasound-guided fine-needle
aspiration and 3D transrectal ultrasound may improve T and N
staging accuracy (23,24).
Although ERUS is an operator-dependent procedure and
its results are closely associated with operator experience, only a
limited number of studies in the literature provide information on
the learning curve. One such study by Orrom et al (25) reported on 77 rectal cancer patients
assessed ultrasonically at the University of Minnesota. In that
study, the patients were divided into three consecutive groups as
follows: group A, 27 patients in the first 15 months; group B, 30
patients in the next 10 months; and group C, 20 patients in the
last 6 months. The T staging accuracy improved from 58% in the
first group to 77% in the second group and, finally, to 95% in the
third group. The difference between the accuracies of the first and
second group was statistically significant, as was the difference
in the accuracies between the first and third group. No conclusions
were drawn with regards to a learning curve for N staging.
A more recent study from the Colorectal Surgical
Society of Australia and New Zealand was published by Morris et
al (26). A prospective study
of ERUS for staging rectal cancer by a single surgeon from
commencement of consultant practice was performed. The results were
compared over three time periods: the first within a single year,
followed by 2- and 3-year periods. A total of 233 cases were
assessable for T staging and 142 for N staging. The overall
accuracy was 82% for T staging and 73% for N staging. The accuracy
for T and N staging did not change significantly over the three
time periods (P>0.05). The authors suggested that accuracy does
not improve with further experience; however, an ERUS accreditation
scheme should be established for future trainees. As shown in
Fig. 1, the T staging accuracy of
physician D increased from 45% (20 cases) to 70% (80 cases) after
the curve had stabilized and the difference was significant
(P<0.05). The N staging accuracy increased from 35 to 64% and
the difference was also statistically significant (P<0.05).
After 80 cases, the staging accuracy of physician D reached a
plateau. In Figs. 2 and 3, the difference between the accuracy of
physician B and C and was not statistically significant
(P>0.05); however, with the increase in the number of patients
examined, their accuracies improved. Our experience suggests that
there is a learning curve about ERUS.
This study had certain limitations. First, this was
a retrospective, observational study conducted by a single center.
Second, all the examinations were performed by five physicians. The
strength of this study lies with the fact that we performed more
examinations (319 patients), which were divided into three groups
depending on the visiting time. Although in the abovementioned
studies the overall accuracy appears to be better compared to ours,
there are a few methodical differences between the studies. First,
five board-certified physicians performed all the assessments in
our study, whereas in the study by Morris et al (26), a single consultant radiologist
conducted all the examinations. Second, the five physicians in our
study had no prior experience with ERUS. As shown in Table III, the T staging accuracy of
physician D increased from 55% (period A) to 84% (period C) and the
difference was statistically significant (P<0.01). The N staging
accuracy increased from 41 to 81% and the difference was also
statistically significant (P<0.01). However, the number of
patients examined by physicians E and F was limited; therefore,
there was not statistically significant difference in their staging
accuracy between period B and period C (P>0.05). Third, the
five-layer rectal wall model was the standard interpretation
throughout our study. The three-layer model was used for the first
two groups in the Minnesota study, with the five-layer model used
for the last group of patients. Therefore, in the study by Orrom
et al (25), none of the
groups were interpreted with consistent criteria, making
conclusions on the learning curve for ERUS difficult. We sought to
overcome these problems by using consistent parameters. Of note,
although constant definitions are preferable, there is a debate
over the number of definable layers (7,9). The
muscularis propria of the rectal wall has been suggested as being a
two- or three-layer structure (7,27).
Therefore, uT2 lesions may be subdivided. However, as this would
not significantly affect clinical management, we did not consider
it was important to make such a distinction.
ERUS is a valuable diagnostic tool. Provided that
the examiner completes a certain number of examinations (~80
cases), accumulates experience and masters the technique, ERUS may
be used for the preoperative staging of rectal cancer. In
conclusion, there is a learning curve in the preoperative staging
of rectal carcinoma by ERUS. However, the results of ERUS during
the learning process must be interpreted with caution for clinical
decision making. The combination of ERUS with other diagnostic
methods may lead to a more accurate prediction of the local stage
in rectal cancer patients.
References
|
1
|
Nedrebø BS, Søreide K, Eriksen MT, Kvaløy
JT, Søreide JA and Kørner H: Excess mortality after curative
surgery for colorectal cancer changes over time and differs for
patients with colon versus rectal cancer. Acta Oncol. 52:933–940.
2013.PubMed/NCBI
|
|
2
|
Samee A and Selvasekar CR: Current trends
in staging rectal cancer. World J Gastroenterol. 17:828–834. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernández-Esparrach G, Ayuso-Colella JR,
Sendino O, et al: EUS and magnetic resonance imaging in the staging
of rectal cancer: a prospective and comparative study. Gastrointest
Endosc. 74:347–354. 2011.PubMed/NCBI
|
|
4
|
Cârţână ET, Pârvu D and Săftoiu A:
Endoscopic ultrasound: current role and future perspectives in
managing rectal cancer patients. J Gastrointestin Liver Dis.
20:407–413. 2011.PubMed/NCBI
|
|
5
|
Kav T and Bayraktar Y: How useful is
rectal endosonography in the staging of rectal cancer? World J
Gastroenterol. 16:691–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puli SR, Bechtold ML, Reddy JB, et al: Can
endoscopic ultrasound predict early rectal cancers that can be
resected endoscopically? A meta-analysis and systematic review. Dig
Dis Sci. 55:1221–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildebrandt U and Feifel G: Preoperative
staging of rectal cancer by intrarectal ultrasound. Dis Colon
Rectum. 28:42–46. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: 2010
|
|
9
|
Beynon J, Foy DM, Temple LN, Channer JL,
Virjee J and Mortensen NJ: The endoscopic appearances of normal
colon and rectum. Dis Colon Rectum. 29:810–813. 1986. View Article : Google Scholar
|
|
10
|
Ayuso Colella JR, Pagés Llinás M and Ayuso
Colella C: Staging rectal cancer. Radiologia. 52:18–29. 2010.(In
Spanish).
|
|
11
|
Popek S and Tsikitis VL: Neoadjuvant vs.
adjuvant pelvic radiotherapy for locally advanced rectal cancer:
which is superior? World J Gastroenterol. 17:848–854. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sermeus A, Leonard W, Engels B and De
Ridder M: Advances in radiotherapy and targeted therapies for
rectal cancer. World J Gastroenterol. 20:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia-Aguilar J, Pollack J, Lee SH,
Hernandez de Anda E, Mellgren A, Wong WD, Finne CO, Rothenberger DA
and Madoff RD: Accuracy of endorectal ultrasonography in
preoperative staging of rectal tumors. Dis Colon Rectum. 45:10–15.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nesbakken A, Lovig T, Lunde OC and Nygaard
K: Staging of rectal carcinoma with transrectal ultrasonography.
Scand J Surg. 92:125–129. 2003.PubMed/NCBI
|
|
15
|
Mackay SG, Pager CK, Joseph D, et al:
Assessment of the accuracy of transrectal ultrasonography in
anorectal neoplasia. Br J Surg. 90:346–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin S, Luo G, Gao X, et al: Application of
endoscopic sonography in preoperative staging of rectal cancer:
six-year experience. J Ultrasound Med. 30:1051–1057.
2011.PubMed/NCBI
|
|
17
|
Sailer M, Leppert R, Bussen D, Fuchs KH
and Thiede A: Influence of tumor position accuracy of endorectal
ultrasound staging. Dis Colon Rectum. 40:1180–1186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashita Y, Machi J, Shirouzu K, Morotomi
T, Isomoto H and Karegawa T: Evaluation of endorectal ultrasound
for the assessment of wall invasion of rectal cancer: report of a
case. Dis Colon Rectum. 31:617–623. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bipat S, Glas AS, Slors FJ, Zwinderman AH,
Bossuyt PM and Stoker J: Rectal cancer: local staging and
assessment of lymph node involvement with endoluminal US, CT, and
MRI - a meta-analysis. Radiology. 232:773–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dworak O: Morphology of lymphnodes in the
resected rectum of patients with rectal carcinoma. Pathol Res
Pract. 187:1020–1024. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monig SP, Baldus SE, Zirbes TK, et al:
Lymph node size and metastatic infiltration in colon cancer. Ann
Surg Oncol. 6:579–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kruskal JB, Kane RA, Sentovitch SM and
Longmaid HE: Pitfalls and sources of error in staging rectal cancer
with endorectal US. Radiographics. 17:606–626. 1997.PubMed/NCBI
|
|
23
|
Knight CS, Eloubeidi MA, Crowe R, et al:
Utility of endoscopic ultrasound-guided fine-needle aspiration in
the diagnosis and staging of colorectal carcinoma. Diagn
Cytopathol. 41:1031–1037. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan KK and Tsang CB: Staging of rectal
cancer - technique and interpretation of evaluating rectal
adenocarcinoma, uT1–4, N disease: 2D and 3D evaluation. Semin Colon
Rectal Surg. 21:197–204. 2010.
|
|
25
|
Orrom WJ, Wong WD, Rothenberger DA, Jensen
LL and Goldberg SM: Endorectal ultrasound in the preoperative
staging of rectal tumors: a learning experience. Dis Colon Rectum.
33:654–659. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris OJ, Draganic B and Smith S: Does a
learning curve exist in endorectal two-dimensional ultrasound
accuracy? Tech Coloproctol. 15:301–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katsura Y, Yamada K, Ishizawa T, Yoshinaka
H and Shimazu H: Endorectal ultrasonography for the assessment of
wall invasion and lymph node metastasis in rectal cancer. Dis Colon
Rectum. 35:362–368. 1992. View Article : Google Scholar : PubMed/NCBI
|