Introduction
The reliable determination of predictive factors of
breast cancer is a prerequisite for selection of optimal
therapeutic strategy. Estrogen receptor (ER) and progesterone
receptor are currently the established tumor markers guiding
routine use of endocrine therapy (ET). Neoadjuvant chemotherapy
(NAC) is the standard treatment in the early years for patients
with locally advanced breast cancer, and thus far, it is an
increasingly used option for operable breast cancer (1,2).
Certain studies have reported that the ER and progesterone receptor
status may alter during the course of NAC (3–6).
However, limited studies have been performed regarding whether
patients with a hormone receptor change from positive to negative
following NAC could gain any benefit from adjuvant ET. The present
study aimed to determine whether ET could provide any benefit in
this subpopulation of patients with breast cancer.
Materials and methods
Patients
A total of 97 out of 687 (14.1%) primary breast
cancer patients who were treated with NAC at the Comprehensive
Breast Health Center (Ruijin Hospital, Shanghai, China) between
December 2000 and November 2010 were retrospectively collected. The
eligibility criteria included female unilateral breast cancer
patients treated with ≥3 and ≤6 cycles of NAC. The following data
were also required: Age, menopausal status, clinical tumor size and
lymph node status, pathological axillary lymph node involvement and
follow-up information. Only the patients with a
positive-to-negative switch in the hormone receptor status
following NAC were included in the study. All the patients were
classified into two groups on the basis of ET administration: 57
ET-administered and 40 ET-naïve patients.
Treatment modalities
For the majority of patients, NAC treatment was
supplemented with one of the following three protocols: NE (25
mg/m2 vinorelbine on day 1 and day 8 plus 60
mg/m2 epirubicin on day 1, every 21 days), CEF (500
mg/m2 cyclophosphamide on day 1, 75 mg/m2
epirubicin on day 1 and 500 mg/m2 fluorouracil on day 1,
every 21 days) and ED (75 mg/m2 epirubicin on day 1 plus
75 mg/m2 docetaxel on day 1, every 21 days). The other
NAC regimens included PCb (paclitaxel and carboplatin) and DCb
(docetaxel and carboplatin).
All the patients underwent radical surgery. Adjuvant
chemotherapy was administered following surgery, and a total of 6–8
courses of chemotherapy were completed according to the preference
of the physician treating the patient on the basis of the clinical
and pathological findings following the surgery. Radiotherapy was
applied subsequent to the completion of the adjuvant chemotherapy.
The prescription of ET and the choice of the specific drugs were
also determined according to the physician and/or the patient’s
preferences. Only one patient with human epidermal growth factor
receptor 2 (HER2)-positive disease received trastuzumab.
Assessment of response
The clinical assessment of the response was based on
the clinical measurements in the longest diameter of the tumor and
node according to the Response Evaluation Criteria in Solid Tumors
(RECIST) 1.1 (7) and was
classified as follows: Complete response (CR), the disappearance of
the disease; partial response (PR), ≥30% decrease; progressive
disease (PD), ≥20% increase in the sum of the longest diameter of
the target lesions or the appearance of new lesions; and stable
disease (SD), neither sufficient shrinkage to qualify for PR or a
sufficient increase to qualify for PD. The pathological complete
response (pCR) was defined as the absence of invasive tumor in the
breast and axillary lymph node. The patients with pCR following NAC
were excluded from the analysis, as the hormone receptor status
subsequent to surgery could not be accurately evaluated.
Hormone receptor and HER2 status
determination
All the patients underwent a 14-gauge core needle
biopsy (CNB) prior to NAC. The ER, progesterone receptor and HER2
status of CNB and the surgical specimens were determined by
immunohistochemistry (IHC). Positive staining for ER/progesterone
receptor was defined as nuclear staining in ≥10% of the tumor
cells. The hormone receptor was defined as positive if the ER
and/or progesterone receptor were positive and as negative if the
ER and progesterone receptor were negative. HER2 positivity was
considered as HER2 3+ by IHC or positive on fluorescence in
situ hybridization (FISH), whereas cases with 0–1+ or 2+
without FISH detecting were regarded as negative. The following
antibodies were used for the IHC test: Anti-ER, clone 1D5 (rabbit
monoclonal; Gene Corp., Capinteria, CA, USA); progesterone
receptor, clone PR636 (mouse monoclonal; Dako, Carpinteria, CA,
USA); and HER2, c-erbB-2 (2000–2008, rabbit polyclonal; Dako) or
4B5 (2009–2010, rabbit monoclonal; Roche, Basel, Switzerland).
Statistical analysis
The χ2 test was applied to evaluate the
association between the administration of ET and the other
parameters studied. Fisher’s exact test was performed when
necessary. The disease-free survival (DFS) interval was defined as
the time from the date of the first administration of NAC to the
earliest occurrence of all the local, regional or distant
recurrences, all the second cancers and contralateral breast
cancers, and all the fatalities. The overall survival (OS) was
defined as the time from the date of the first administration of
NAC to all the mortalities, whether they were breast cancer-related
or not. DFS and OS were estimated using the Kaplan-Meier analysis
and the survival curves were compared using the log-rank test.
Multivariate Cox regression analysis with stepwise selection was
used to estimate the hazard ratio (HR), 95% confidence interval
(CI) and the effects of the clinical and pathological variables.
All the statistical tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference. The
software package SPSS 16.0 for Windows XP (SPSS, Inc., Chicago, IL,
USA) was used for analysis.
Results
Patient characteristics and
treatment
A total of 97 eligible patients without pCR
following NAC were identified to have a positive-to-negative change
in the hormone receptor status. The median age was 51 years (range,
31–74 years) and 55.7% of these patients were premenopausal. A
total of 30 patients (30.9%) had stage IIb disease, 37 (38.1%)
exhibited stage IIIa disease and the remaining patients (30.9%) had
stage IIIb-IIIc.
The majority of the patients received one of the
following three NAC regimens: NE (38.1%), CEF (36.1%) and ED
(14.4%). The remaining patients received either the PCb (7.2%) or
DCb (4.1%) regimen. As for the clinical tumor response according to
RECIST 1.1, CR was documented in 20 patients (20.6%), 53 (54.6%)
obtained PR, SD was observed in 21 (21.6%) and three (3.1%) had
PD.
A total of 57 patients (58.8%) received ET (47 with
tamoxifen only, 39 with aromatase inhibitors only and 11 with
tamoxifen and aromatase inhibitors). The correlation between the
characteristics of the patients and ET administration are
summarized in Table I. The
patients treated with or without ET did not differ significantly in
their clinical or pathological characteristics.
 | Table IPatient characteristics, stratified by
the use of adjuvant endocrine therapy. |
Table I
Patient characteristics, stratified by
the use of adjuvant endocrine therapy.
| Adjuvant endocrine
therapy | |
|---|
|
| |
|---|
| Characteristics | No
n=40 (%) | Yes
n=57 (%) | P-value |
|---|
| Age, years | | | |
| <50 | 18 (45.0) | 27 (47.4) | 0.839 |
| >50 | 22 (55.0) | 30 (52.6) | |
| Menopausal
status | | | |
| Premenopausal | 23 (57.5) | 31 (54.4) | 0.837 |
| Postmenopausal | 17 (42.5) | 26 (45.6) | |
| Pre-NAC ER | | | |
| Positive | 38 (95.0) | 53 (93.0) | 1.000 |
| Negative | 2 (5.0) | 4 (7.0) | |
| Pre-NAC progesterone
receptor | | | |
| Positive | 35 (87.5) | 48 (84.2) | 0.773 |
| Negative | 5 (12.5) | 9 (15.8) | |
| Pre-NAC HER2 | | | |
| Positive | 6 (15.0) | 4 (7.0) | 0.310 |
| Negative | 34 (85.0) | 53 (93.0) | |
| NAC | | | |
| NE | 16 (40.0) | 21 (36.8) | 0.663 |
| CEF | 12 (30.0) | 23 (40.4) | |
| ED | 6 (15.0) | 8 (14.0) | |
| Others | 6 (15.0) | 5 (8.8) | |
| NAC cycles | | | |
| 3–4 | 31 (77.5) | 48 (84.2) | 0.436 |
| 5–6 | 9 (22.5) | 9 (15.8) | |
| Initial clinical
stage | | | |
| IIb | 10 (25.0) | 20 (35.1) | 0.575 |
| IIIa | 16 (40.0) | 21 (36.8) | |
| IIIb–IIIc | 14 (35.0) | 16 (28.1) | |
| Clinical tumor
response | | | |
| CR | 5 (12.5) | 15 (26.3) | 0.248 |
| PR | 25 (62.5) | 28 (49.1) | |
| SD+PD | 10 (25.0) | 14 (24.6) | |
| pALN | | | |
| Positive | 26 (65.0) | 42 (73.7) | 0.377 |
| Negative | 14 (35.0) | 15 (26.3) | |
| Adjuvant
chemotherapy | | | |
| Yes | 37 (92.5) | 50 (87.7) | 0.517 |
| No | 3 (7.5) | 7 (12.3) | |
| Adjuvant radiation
therapy | | | |
| Yes | 32 (80.0) | 45 (78.9) | 1.000 |
| No | 8 (20.0) | 12 (21.1) | |
Hormone receptor status and HER2
status
A total of 77 patients (79.4%) had
ER-positive/progesterone receptor-positive tumors, 14 (14.4%) had
ER-positive/progesterone receptor-negative tumors and six (6.2%)
exhibited ER-negative/progesterone receptor-negative tumors. A
positive pre- and post-NAC HER2 status was observed in 10 (10.3%)
and 11 patients (11.3%), respectively. Two patients had a
positive-switch of HER2 status following NAC and a negative-switch
of HER2 status was observed in one patient. The pre-NAC
ER/progesterone receptor status and pre/post-NAC HER2 status are
shown in Table II.
 | Table IINumber of patients classified by ER,
progesterone receptor and HER2 statuses prior and subsequent to
neoadjuvant chemotherapy (n=97). |
Table II
Number of patients classified by ER,
progesterone receptor and HER2 statuses prior and subsequent to
neoadjuvant chemotherapy (n=97).
| Pre-NAC
ER/progesterone receptor | Pre/post-NAC
HER2 |
|---|
|
|---|
| +/+ | +/− | −/+ | −/− |
|---|
| +/+ | 7 | 0 | 2 | 68 |
| +/− | 2 | 0 | 0 | 12 |
| −/+ | 0 | 1 | 0 | 5 |
DFS and OS
After a median follow-up of 68 months (range, 14–103
months), the overall DFS and OS rates were 61.9 and 70.1%,
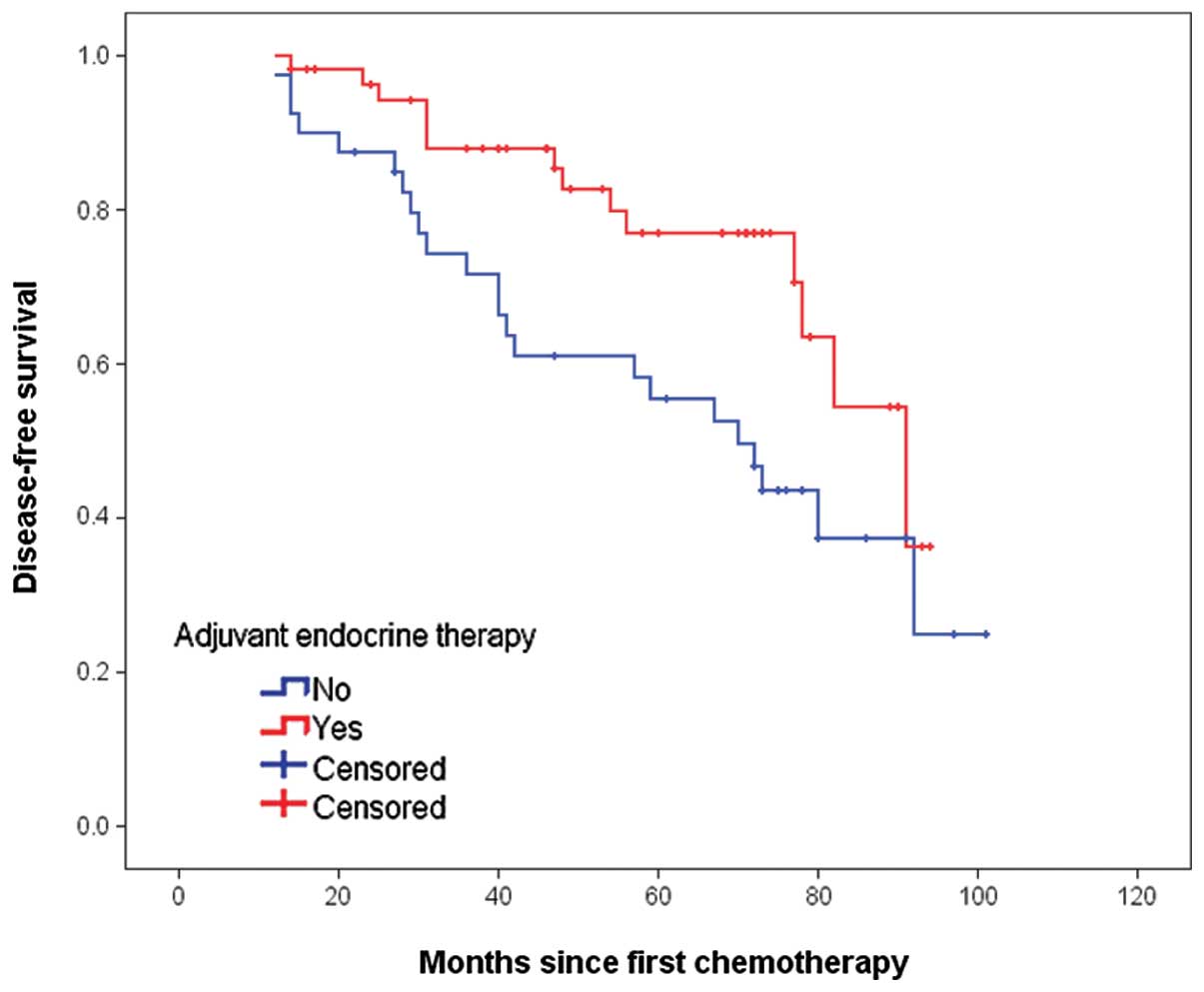
respectively. The Kaplan-Meier curves for DFS in the two groups are
shown in Fig. 1. The differences
between the two curves were statistically significant, as
determined by the log-rank test (P=0.018). The 5-year DFS rates in
ET-administered and ET-naïve patients were 77.0 and 55.5%,
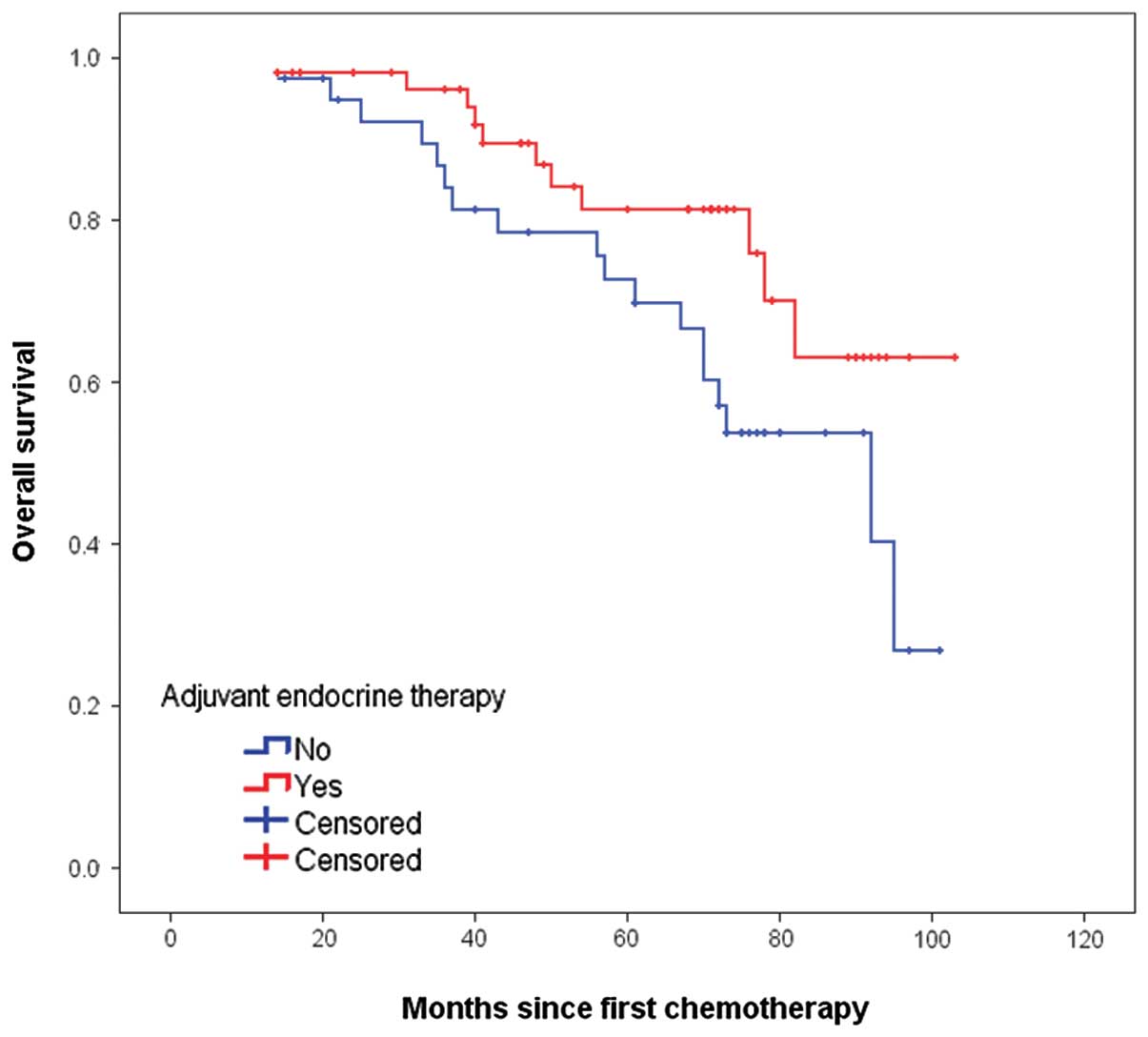
respectively. The Kaplan-Meier curves for OS are shown in Fig. 2. The 5-year OS rate for
ET-administered was higher than that of the ET-naïve patients (81.3
vs. 72.7%, P=0.053), but the difference between the two groups did
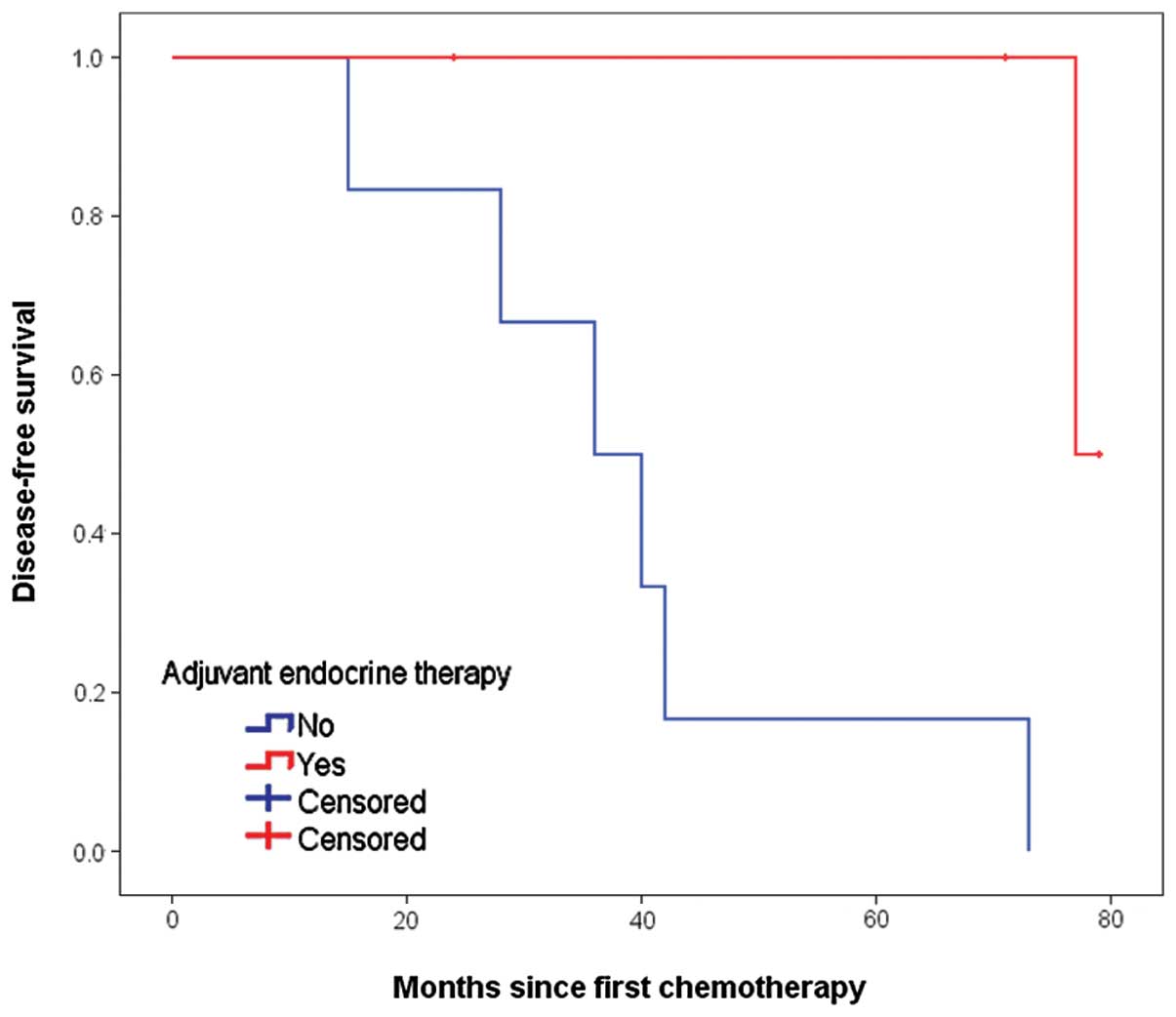
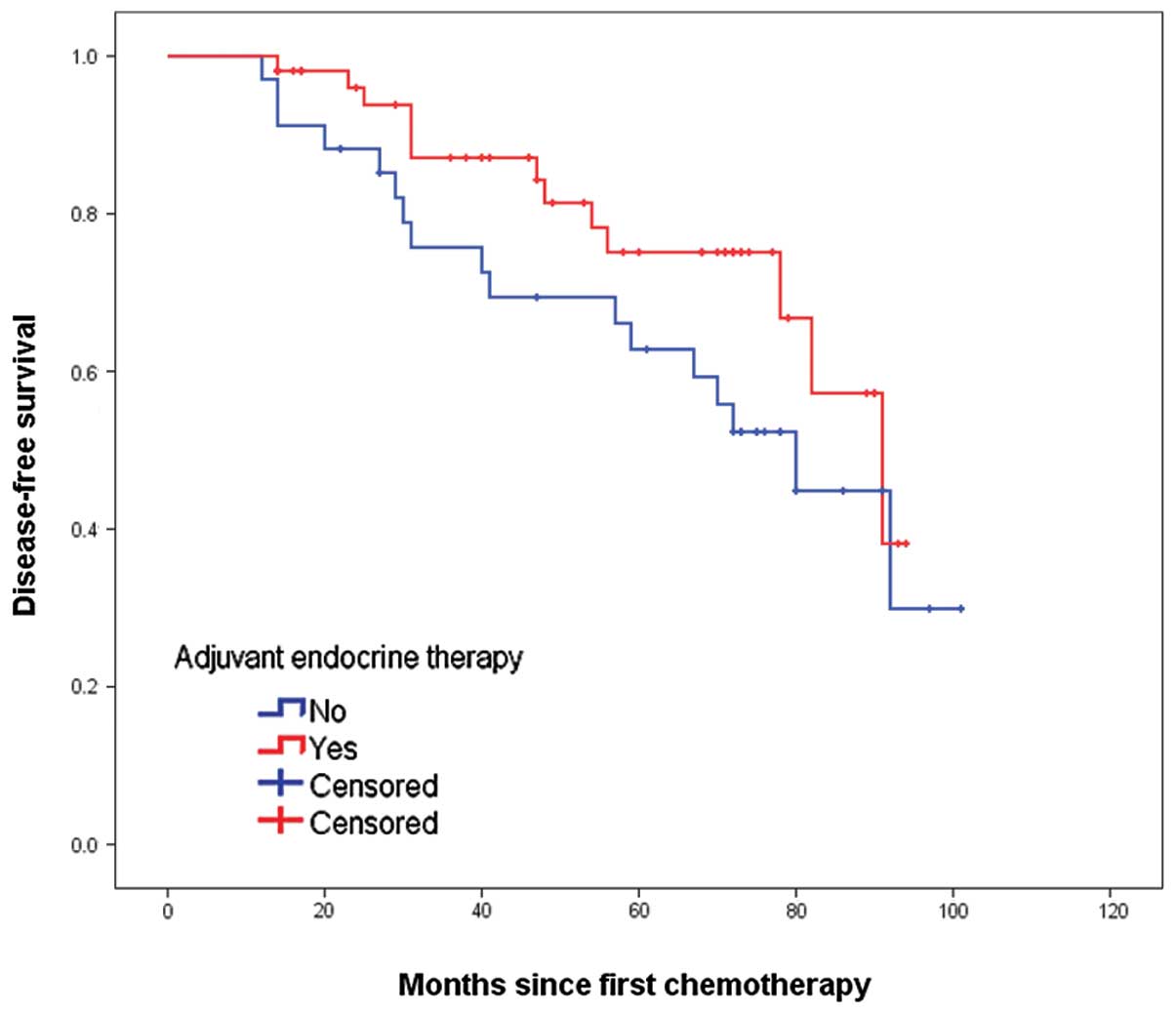
not reach statistical significance. In the exploratory subgroup
analysis according to HER2 status, administration of ET was also
associated with an improved DFS, with P-values of 0.013 and 0.122
in the cases of HER2-positive and -negative disease, respectively
(Figs. 3 and 4). The other results of univariate
analysis are summarized in Table
III. The factors associated with an inferior DFS were pre-NAC
HER2 positivity, higher clinical stage, involvement of lymph nodes
and omission of ET (P<0.05). Similarly, higher clinical stage
and involvement of lymph node were associated with an inferior OS
(P<0.05).
 | Table IIIUnivariate and multivariate prognostic
analysis of DFS and OS. |
Table III
Univariate and multivariate prognostic
analysis of DFS and OS.
| DFS | OS |
|---|
|
|
|
|---|
| Characteristics | Univariate
P-value |
Multivariate
P-value | HR (95% CI) | Univariate
P-value |
Multivariate
P-value | HR (95% CI) |
|---|
| Age, years |
| <50 | 0.658 | NS | | 0.498 | NS | |
| >50 | | | | | | |
| Menopausal
status |
| Premenopausal | 0.896 | NS | | 0.798 | NS | |
|
Postmenopausal | | | | | | |
| Pre-NAC HER2 |
| Positive | 0.016a | 0.008a | 3.412
(1.370–8.499)a | 0.242 | 0.028a | 3.533
(1.149–10.869)a |
| Negative | | | 1a | | | 1a |
| NAC |
| NE | 0.406 | NS | | 0.299 | NS | |
| CEF | | | | | | |
| ED | | | | | | |
| Others | | | | | | |
| NAC cycle |
| 3–4 | 0.274 | NS | | 0.292 | NS | |
| 5–6 | | | | | | |
| Initial clinical
stage |
| IIb | 0.004a | 0.027a | 1a | 0.005a | 0.021a | 1a |
| IIIa | | | 3.930
(1.095–14.096)a | | | 15.438
(1.680–141.856)a |
| IIIb–IIIc | | | 5.843
(1.600–21.340)a | | | 23.271
(2.445–221.488)a |
| Clinical tumor
response |
| CR | 0.329 | NS | | 0.590 | NS | |
| PR | | | | | | |
| SD+PD | | | | | | |
| pALN |
| Positive | 0.003a | 0.035a | 2.819
(1.074–7.404)a | 0.002a | 0.031a | 5.068
(1.161–22.125)a |
| Negative | | | 1a | | | 1a |
| Adjuvant endocrine
therapy |
| Yes | 0.018a | 0.039a | 0.479
(0.238–0.964)a | 0.053 | 0.092 | 0.497
(0.221–1.121) |
| No | | | 1 | | | 1 |
| Adjuvant
chemotherapy |
| Yes | 0.961 | NS | | 0.844 | NS | |
| No | | | | | | |
| Adjuvant radiation
therapy |
| Yes | 0.439 | NS | | 0.055 | NS | |
| No | | | | | | |
Multivariate analysis
The results of the multivariate stepwise Cox
regression analysis of DFS and OS are shown in Table III. The following variables were
included as probable prognostic factors in the Cox proportional
hazard model: Age (<50 vs. >50 years), menopausal status
(premenopausal vs. postmenopausal), pre-NAC HER2 status (positive
vs. negative), NAC regimen (NE vs. CEF vs. ED vs. others), NAC
cycle (3–4 vs. 5–6), initial clinical stage (IIb vs. IIIa vs.
IIIb-IIIc), clinical tumor response (CR vs. PR vs. SD+PD),
pathological axillary lymph node status (positive vs. negative) and
adjuvant therapies (ET, chemotherapy and radiation therapy; yes vs.
no). Four of these variables (pre-NAC HER2 status, initial clinical
stage, pathological axillary lymph node status and the use of
adjuvant ET) were identified as the independent predictors for DFS
by the stepwise Cox regression model. Similarly, three of these
variables (pre-NAC HER2 status, initial clinical stage and
pathological axillary lymph node status) were identified as the
variables affecting the OS.
Discussion
The discordance in hormone receptor and HER2 status
between CNB and excision specimens has been reported in numerous
retrospective studies with inconsistent results. A meta-analysis by
Chen et al (8) confirmed
the high diagnostic accuracy of CNB in evaluating hormone receptor
and HER2 status compared to open-excision biopsy in breast cancer
patients without NAC. The meta-analysis by Zhang et al
(9) concluded that in the patients
receiving NAC, the hormone receptor status was significantly
altered by the chemotherapy and re-evaluation of the hormone
receptor status following NAC should be considered. Regarding the
high accuracy of CNB in evaluating hormone receptor status, this
type of discordance is primarily caused by NAC.
A small number of retrospective studies have
reported the discordances in the hormone receptor status to range
from 8–33% (3–6), and the reported changes are equally
distributed between a positive and negative switch in the hormone
receptor status. Therefore, ET may be significant in patients with
a negative-to-positive switch of hormone receptor status and more
attention was administered to the effect of ET in patients with
hormone receptor status changed from positive to negative in the
present study. To the best of our knowledge, the present study is
the first to focus on the effect of ET in patients who exhibited a
positive-to-negative switch in the hormone receptor status
following NAC. In the study, the long-term benefit of ET in this
subpopulation of patients was confirmed. The 5-year DFS rates in
ET-administered were significantly higher than that of the ET-naïve
patients (77.0 vs. 55.5%), whereas the 5-year OS rates had a
non-statistically significant increase subsequent to the use of ET
(81.3 vs. 72.7%). Similar results have been reported in a previous
study of 59 patients who showed hormone receptor status conversion
following NAC; the DFS of 47 ET-administered was significantly
longer compared to the 12 ET-naïve patients (HR, 0.19, 95% CI:
0.06–0.60, P<0.004) (4).
However, the study only included 30 patients with a
positive-to-negative change of the hormone receptor status and all
those ET-naïve patients exhibited a negative-to-positive
change.
The mechanisms for the change in the hormone
receptor status in breast cancer caused by chemotherapy are
complicated. Chemotherapy has been indicated to be able to target
chemosensitive tumor cells (such as hormone receptor-negative
cells) and leave the insensitive tumor cells with a different
biology (such as hormone receptor-positive cells) behind in the
residual disease, leading to a negative-to-positive change of the
hormone receptor. Another possible explanation for a
negative-to-positive change of the hormone receptor may be the
heterogeneity of the breast carcinoma, as well as the insufficiency
of CNB material. The mechanism of the positive-to-negative switch
of the hormone receptor status during NAC is more complex. The
critical review by van de Ven et al (10) hypothesized that this could be the
result of lowered circulating levels of estrogens caused by ovarian
insufficiency during chemotherapy, which may cause downregulation
of the ER and/or progesterone receptor of the tumor leading to
estrogen-independent growth. This is, at least in part, a plausible
explanation for the clear downregulation of the hormone receptor
following NAC. The present study revealed that this type of breast
cancer responded to ET, regardless of the underlying mechanism.
With regards to a recent International Expert
Consensus, breast cancer can be divided into four intrinsic
subtypes on the basis of ER, progesterone receptor, HER2 and Ki67
status (11). Patients with
different subtypes of breast cancer exhibit distinct responsiveness
to systemic treatment, as well as diverse outcomes (12–14).
All the patients in the present study had luminal breast cancer
prior to NAC and basal-like or HER2-positive breast cancer
following surgery. The results indicated that although the breast
cancer was apparently endocrine non-responsive (including
basal-like or HER2-positive subtypes) subsequent to surgery, breast
cancers with a luminal subtype that was confirmed prior to NAC act
like hormonal responsive breast cancer and had a favorable
prognosis with the use of ET. Furthermore, luminal breast cancers
have been reported to exhibit varied sensitivity to ET, which may
be largely, but not exclusively, affected by HER2 status. The
multivariate analysis indicated that the benefit of ET was
independent of the HER2 status. HER2-positive breast cancers
appeared to be more responsive than HER2-negative ones. Due to the
limited sample size of the subgroup analysis, this result required
further study in a large cohort of patients.
In conclusion, the present study demonstrated that
the hormone receptor status should be evaluated not only in the CNB
specimens obtained prior to NAC, but also in specimens obtained
during post-NAC surgery. The mechanism of the positive-to-negative
switch of the hormone receptor status during NAC remains unclear,
however, ET is warranted in patients with a pre-NAC-positive
hormone receptor status, even though it becomes negative
postoperatively.
Acknowledgements
The authors would like to thank the patients and
family members for their willingness to cooperate in the present
study. The study was supported in part by grants from the Leading
Academic Discipline Project of Shanghai Municipal Education
Commission (project no. J50208).
References
|
1
|
Berruti A, Generali D, Kaufmann M, Puztai
L, Curigliano G, Aglietta M, Gianni L, Miller WR, Untch M, Sotiriou
C, et al: International expert consensus on primary systemic
therapy in the management of early breast cancer: highlights of the
Fourth Symposium on Primary Systemic Therapy in the Management of
Operable Breast Cancer; Cremona, Italy. 2010; J Natl Cancer Inst
Monogr. 2011. pp. 147–151. 2011, View Article : Google Scholar
|
|
2
|
Kaufmann M, von Minckwitz G, Mamounas EP,
Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant
M, Harris JR, et al: Recommendations from an international
consensus conference on the current status and future of
neoadjuvant systemic therapy in primary breast cancer. Ann Surg
Oncol. 19:1508–1516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burcombe RJ, Makris A, Richman PI, Daley
FM, Noble S, Pittam M, Wright D, Allen SA, Dove J and Wilson GD:
Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to
neoadjuvant anthracycline chemotherapy for operable breast cancer.
Br J Cancer. 92:147–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirata T, Shimizu C, Yonemori K, Hirakawa
A, Kouno T, Tamura K, Ando M, Katsumata N and Fujiwara Y: Change in
the hormone receptor status following administration of neoadjuvant
chemotherapy and its impact on the long-term outcome in patients
with primary breast cancer. Br J Cancer. 101:1529–1536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain V, Landry M and Levine EA: The
stability of estrogen and progesterone receptors in patients
receiving preoperative chemotherapy for locally advanced breast
carcinoma. Am Surg. 62:162–165. 1996.PubMed/NCBI
|
|
6
|
Tacca O, Penault-Llorca F, Abrial C,
Mouret-Reynier MA, Raoelfils I, Durando X, Achard JL, Gimbergues P,
Curé H and Chollet P: Changes in and prognostic value of hormone
receptor status in a series of operable breast cancer patients
treated with neoadjuvant chemotherapy. Oncologist. 12:636–643.
2007. View Article : Google Scholar
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
8
|
Chen X, Yuan Y, Gu Z and Shen K: Accuracy
of estrogen receptor, progesterone receptor, and HER2 status
between core needle and open excision biopsy in breast cancer: a
meta-analysis. Breast Cancer Res Treat. 134:957–967. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang N, Moran MS, Huo Q, Haffty BG and
Yang Q: The hormonal receptor status in breast cancer can be
altered by neoadjuvant chemotherapy: a meta-analysis. Cancer
Invest. 29:594–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van de Ven S, Smit VT, Dekker TJ, Nortier
JW and Kroep JR: Discordances in ER, PR and HER2 receptors after
neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev.
37:422–430. 2011.PubMed/NCBI
|
|
11
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members. Strategies for
subtypes - dealing with the diversity of breast cancer: highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar
|
|
12
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001.PubMed/NCBI
|
|
14
|
Chen XS, Wu JY, Huang O, Chen CM, Wu J, Lu
JS, Shao ZM, Shen ZZ and Shen KW: Molecular subtype can predict the
response and outcome of Chinese locally advanced breast cancer
patients treated with preoperative therapy. Oncol Rep.
23:1213–1220. 2010.PubMed/NCBI
|