Introduction
Hepatocellular carcinoma (HCC) is the most common
neoplasm worldwide (1). HCC
principally develops on a background of chronic liver disease,
particularly cirrhosis caused by hepatitis C or hepatitis B virus
infection (1). In Japan, the
median age of patients with HCC has been increasing gradually since
1986 (2). Elderly cancer patients
often present with multiple comorbidities and age-related changes
in the pharmacokinetics and pharmacodynamics of anticancer drugs
that may affect chemotherapeutic regimens (3). The clinical benefits of treatment of
elderly patients with advanced HCC remain unclear. A previously
published study demonstrated that investigations in elderly
patients were less intense, that such patients were more likely to
receive conservative therapy and that the median survival was worse
compared to that among younger patients (4). However, the treatments for HCC have
progressed significantly over the last few years and Mirici-Cappa
et al (5) demonstrated that
the overall applicability of radical or effective HCC treatment may
not be affected by age. Moreover, Suda et al (2) suggested that the therapeutic approach
to HCC should not be restricted by patient age.
Sorafenib is an oral tyrosine kinase inhibitor that
targets multiple molecular pathways. In a pivotal study, sorafenib
provided an overall survival (OS) advantage in patients with
advanced HCC, with the median survival increasing by ~3 months in
sorafenib-treated patients, compared to those receiving placebo
therapy (6). Sorafenib is the only
globally approved drug for the treatment of HCC; however, it is not
curative and is only indicated for Child-Pugh class A patients who
have preserved hepatic function. Hepatic arterial infusion
chemotherapy (HAIC) is an alternative option for advanced HCC and,
based on the Japanese HCC management guidelines, it is recommended
for patients with the same indications for sorafenib (7). Although HAIC is widely used in Japan,
as it tends to be associated with a favorable response rate (RR) in
patients with HCC, randomized controlled trials have not been
conducted and there is currently no evidence of a survival benefit
for HAIC. HAIC may reduce HCC stage (8) and is indicated for patients
exhibiting a moderate reduction in hepatic reserve function
(9). In patients who achieve a
complete response (CR) with HAIC, a long-term survival benefit was
reported (10,11). The efficacy of sorafenib treatment
in elderly patients with advanced HCC has been investigated in
several studies (12–16); however, to the best of our
knowledge, there are no available reports regarding the efficacy of
HAIC in such patients and there are currently no satisfactory
strategies for the management of advanced HCC as a function of age.
The aim of this study was to compare the feasibility and safety of
HAIC to those of sorafenib in elderly patients with advanced
HCC.
Patients and methods
Patients
We retrospectively analyzed data from elderly
patients with advanced unresectable HCC, who were treated at our
hospital between March, 2002 and June, 2013. Eligible patients
included those aged ≥70 years with histologically or clinically
confirmed advanced HCC. HCC was considered as unresectable in
patients who presented with severe vascular invasion or multiple
intrahepatic lesions (i.e., ≥5 nodules), or in those with
progressive disease (PD) following surgical or locoregional therapy
intervention. A total of 20 eligible patients were identified.
The study protocol was approved by our Institutional
Review Board and informed consent was obtained from all the
patients prior to treatment.
Treatment
In the HAIC group (n=8), an implantable drug
delivery system was used for arterial infusion of the
chemotherapeutic agents. Between February, 2003 and March, 2009,
HAIC consisted of 5-fluorouracil (5-FU) at a dose of 300
mg/m2/day for 5 days during the 1st and 2nd weeks,
combined with intramuscular or subcutaneous administration of
interferon-α 3 times per week for 4 weeks. Interferon-α dosing
consisted of either natural interferon-α, 5 million units;
recombinant interferon-α, 12 million units; or interferon-α 2b, 3
million units. From April, 2009 onwards, HAIC was performed with
5-FU plus cisplatin (CDDP) at a dose of 20 mg/m2/day on
days 1 and 8, combined with intramuscular interferon-α
administration, as described above (17). The treatment cycle was repeated
until disease progression or unacceptable drug toxicity.
In the sorafenib group (n=12), a limited number of
patients received sorafenib 200–600 mg/day as an initial dose. In
the absence of adverse events (AEs), the dose of sorafenib was
increased to 400 mg twice daily. Treatment was discontinued on the
same basis as in the HAIC group. However, if the performance status
and liver function of patients with PD was preserved, sorafenib was
continued until the occurrence of severe AEs in order to prevent
rapid tumor growth associated with treatment cessation.
Response assessment
Tumor response was determined using dynamic computed
tomography or magnetic resonance imaging, according to Response
Evaluation Criteria in Solid Tumors, version 1.1. RR was defined as
the combined percentages of patients experiencing a CR and those
with a partial response (PR). Tumor control rate (TCR) was defined
as the combined percentages of patients experiencing CR, PR and
stable disease (SD). HAIC was evaluated every 6 or 8 weeks and
sorafenib treatment was evaluated every 4 or 12 weeks. OS was
calculated from the date of treatment initiation to the date of the
last follow-up or death. Progression-free survival (PFS) was
calculated from the date of treatment initiation to the date of the
last follow-up or PD. Drug-related AEs were evaluated according to
the Common Toxicity Criteria for Adverse Events, version 4.0 (Japan
Clinical Oncology Group/Japan Society of Clinical Oncology
edition).
Additional therapy
Of the 20 patients, 8 received additional treatment,
including surgery, radiofrequency ablation (RFA), transcatheter
arterial chemoembolization (TACE), HAIC using 5-FU and low-dose
CDDP without interferon-α administration (low-dose FP), arterial
CDDP infusion and irradiation therapy.
Statistical analyses
The results are expressed as means ± standard
deviation. The differences between the two groups were examined for
statistical significance using the Mann-Whitney U test, the
Fisher’s exact test and the Chi-square test. The survival curves
for OS and PFS were analyzed using the Kaplan-Meier method and the
differences were evaluated using a log-rank test. The 95%
confidence intervals (CIs) of median OS and median PFS were
calculated. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The baseline patient clinical characteristics are
summarized in Table I. The mean
age of the sorafenib group was significantly higher compared to
that of the HAIC group (P=0.039). There were no significant
differences by blood cell counts, blood coagulation tests,
biochemical tests, or Child-Pugh classifiction. In addition, a
comparison of tumor-related background factors between the two
groups did not reveal any significant differences in TNM stage,
main tumor diameter, or serum α-fetoprotein levels.
 | Table IClinical characteristics of patients
treated with sorafenib and hepatic arterial infusion chemotherapy
(HAIC). |
Table I
Clinical characteristics of patients
treated with sorafenib and hepatic arterial infusion chemotherapy
(HAIC).
| Variables | Sorafenib (n=12) | HAIC (n=8) | P-value |
|---|
| Age (years) | 80.2±5.4 | 74.9±3.4 | 0.039a |
| Gender (M/F) | 6/6 | 6/2 | NSb |
| White cell count
(×102/μl) | 48.0±13.2 | 59.4±27.4 | NSa |
| Lymphocyte count
(×102/μl) | 14.6±8.5 | 14.9±6.2 | NSa |
| Platelet count
(×104/μl) | 16.7±6.1 | 14.1±6.7 | NSa |
| PT-INR | 1.10±34.6 | 1.16±0.19 | NSa |
| ALT (IU/l) | 35.5±0.12 | 41.9±0.19 | NSa |
| Total bilirubin
(mg/dl) | 0.67±0.40 | 0.76±0.27 | NSa |
| Albumin (g/dl) | 3.5±0.4 | 3.3±0.8 | NSa |
| Cirrhosis (Child-Pugh
A/B/C) | 10/2/0 | 4/4/0 | NSb |
| TNM stage
(I/II/III/IV-A/IV-B) | 0/2/3/2/5 | 0/2/3/2/1 | NSc |
| Largest tumor
(mm) | 42.3±21.2 | 49.7±28.2 | NSa |
| AFP | 2,027±5,219 | 279±418 | NSa |
Clinical response
The mean daily dose and duration of sorafenib
treatment were 544 mg and 5.3 months, respectively. The mean number
of treatment cycles in the HAIC group was 1.8 (~2.2 months). The
treatment responses are summarized in Table II. The RR was significantly
different between the two groups, as patients in the sorafenib
group failed to respond to treatment (P=0.049). However, there was
no significant difference in TCR between the two groups. Two
patients in the HAIC group achieved a sustained CR after receiving
additional RFA: one initially achieved a CR in response to HAIC and
the other initially demonstrated a PR in response to HAIC.
 | Table IIComparison of best response between
sorafenib and hepatic arterial infusion chemotherapy (HAIC). |
Table II
Comparison of best response between
sorafenib and hepatic arterial infusion chemotherapy (HAIC).
| Response | Sorafenib (n=12) | HAIC (n=8) | P-value |
|---|
| CR | 0 (0.0) | 1 (12.5) | NS |
| PR | 0 (0.0) | 2 (25.0) | NS |
| SD | 6 (50.0) | 4 (50.0) | NS |
| PD | 6 (50.0) | 1 (12.5) | NS |
| RR (CR+PR) | 0 (0.0) | 3 (37.5) | 0.049a |
| TCR (CR+PR+SD) | 6 (50.0) | 7 (87.5) | NS |
Clinical course and additional
therapy
Fig. 1 shows the
clinical course of patients who were treated with sorafenib
(Fig. 1A) or HAIC (Fig. 1B). In the sorafenib group,
treatment was discontinued in 11 patients; for 7 patients (patients
2, 3, 4, 5, 7, 9 and 11), this was due to drug-related AEs, whereas
the remaining patients (patients 6, 8, 10 and 11) developed PD. In
the HAIC group, none of the patients discontinued 5-FU and CDDP
infusion, but interferon-α administration was discontinued in 1
patient (patient 1). Four patients in each group (patients 7, 9, 10
and 11, Fig. 1A; and patients 3,
6, 7 and 8, Fig. 1B) received
various additional therapies, including arterial CDDP infusion
(patients 7 and 11, Fig. 1A; and
patients 3 and 8, Fig. 1B);
operation (patient 9, Fig. 1A; and
patient 6, Fig. 1B); TACE
(patients 7 and 11, Fig. 1A; and
patient 8, Fig. 1B); low-dose FP
(patient 6, Fig. 1B); RFA
(patients 7 and 8, Fig. 1B); and
radiation therapy (patient 8, Fig.
1B). Patients 9 and 10 (Fig.
1A) underwent HAIC immediately after sorafenib failure, whereas
patient 3 (Fig. 1B) received
sorafenib immediately after HAIC failure. Overall, no patients in
the sorafenib group demonstrated a curative response following
these treatments, whereas for 3 patients in the HAIC group, the
additional treatment was significantly curative (P=0.049).
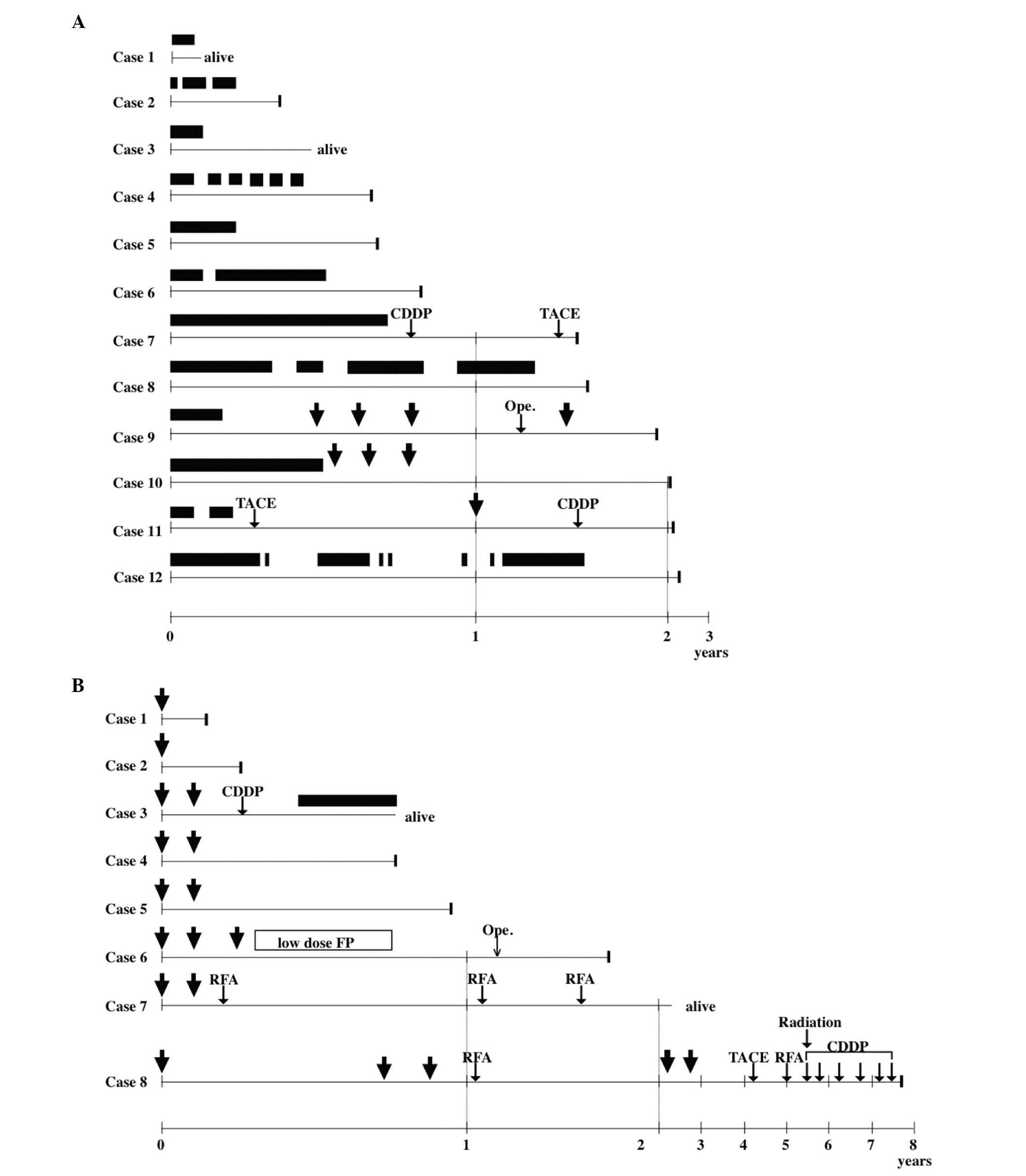 | Figure 1Clinical course of (A) the sorafenib
and (B) hepatic arterial infusion chemotherapy (HAIC) groups. The
best clinical responses were complete response in 1 patient (case
8) in the HAIC group, partial response in 2 patients (cases 6 and
7) in the HAIC group, stable disease in 10 patients (cases 1, 6, 9,
10, 11 and 12 in the sorafenib group and cases 2, 3, 4 and 5 in the
HAIC group) and progressive disease in 7 patients (cases 2, 3, 4,
5, 7 and 8 in the sorafenib group and case 1 in the HAIC group).
Although patients 1 and 3 in the sorafenib group and patients 3 and
7 in the HAIC group remained alive, other patients succumbed to the
disease at the indicated time points. Closed bars, sorafenib
administration. Arrows, HAIC. CDDP, cisplatin infusion; TACE,
transcatheter arterial chemoembolization; Ope., operation; low-dose
FP, continuous 5-fluorouracil and low-dose cisplatin infusion; RFA,
radiofrequency ablation. |
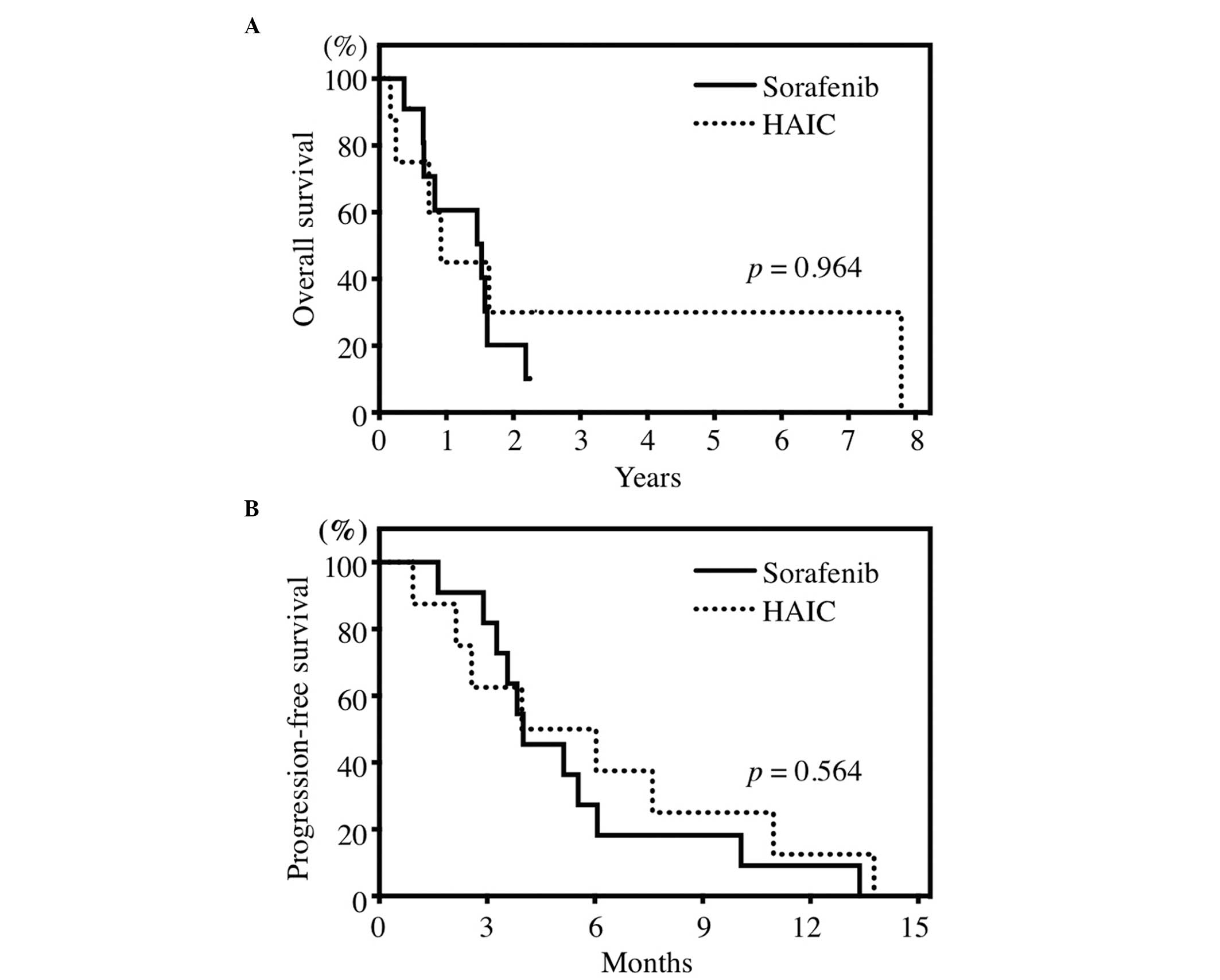
Survival
The median OS of the total patient population was
17.8 months (0.93–94.7 months). The median OS was 18.6 months (95%
CI: 13.8–23.4) and 11.7 months (95% CI: 0–31.5) in the sorafenib
and HAIC groups, respectively (Fig.
2A). The median PFS was 4.0 months (95% CI: 2.1–5.9) and 5.0
months (95% CI: 2.6–7.4) in the sorafenib and HAIC groups,
respectively (Fig. 2B). The median
OS and PFS were not significantly different between the two groups
(P=0.964 and 0.562, respectively).
Safety
The major AEs are listed in Table III. A total of 7 patients (58.3%)
in the sorafenib group discontinued treatment due to grade 3 AEs [4
patients, anorexia; and 1 patient each with hand-foot (HF)
syndrome, ascites and hepatic encephalopathy], whereas no patients
demonstrated intolerance to HAIC. The discontinuation rate in the
sorafenib group was significantly higher compared to that in the
HAIC group (P=0.015). Among sorafenib-treated patients, the most
frequent AEs were mild in severity (grade 1/2) and included HF
syndrome, anorexia, hypoalbuminemia and diarrhea. Grade 3 AEs
included HF syndrome, anorexia and hypertension. One Child-Pugh
class A patient developed hepatic failure (hepatic encephalopathy)
and sorafenib was discontinued. There were no grade 4 AEs. Among
HAIC group patients, the most frequent AEs were mostly mild in
severity (grade 1/2) and included decreased platelet count, anemia,
fever, malaise, anorexia, hypoalbuminemia, decreased white blood
cell count and decreased neutrophil count. In total, 6
hematological AEs of grade 3/4 were recorded in 4 patients. In the
HAIC group, 1 patient (12.5%) experienced catheter occlusion as a
catheter-related complication. In addition, 5 patients in the
sorafenib group changed Child-Pugh class from A to B, whereas none
of the patients in the HAIC group changed Child-Pugh class. These
changes were mostly caused by the development of hypoalbuminemia in
sorafenib-treated patients; there was no significant change in the
prothrombin time-international normalized ratio (PT-INR).
 | Table IIIAdverse events. |
Table III
Adverse events.
| Sorafenib
(n=12)
Grade (CTCAE v4.0) | HAIC
(n=8)
Grade (CTCAE v4.0) |
|---|
|
|
|
|---|
| Adverse events | 1 | 2 | 3 | 4 | Any | 3–4 | 1 | 2 | 3 | 4 | Any | 3–4 |
|---|
| Anemia | 4 | 1 | | | 5 (41.7) | 0 | 2 | 5 | | | 7 (87.5) | 0 |
| Decreased WBC | | 1 | | | 1 (8.3)a | 0 | 2 | 3 | 1 | | 6 (75.0)a | 1 (12.5) |
| Decreased
neutrophil count | | 1 | | | 1 (8.3)a | 0 | 3 | 2 | 1 | | 6 (75.0)a | 1 (12.5) |
| Decreasedlatelet
count | 4 | 3 | 1 | | 8 (66.7) | 1 (8.3) | 3 | | 3 | 1 | 7 (87.5) | 4 (50.0) |
| Malaise | 5 | 2 | | | 7 (58.3) | 0 | 4 | 3 | | | 7 (87.5) | 0 |
| Fever | | | | | 0b | 0 | 6 | 1 | | | 7 (87.5)b | 0 |
| Anorexia | 2 | 4 | 3 | | 9 (75.0) | 3 (25.0) | 3 | 4 | | | 7 (87.5) | 0 |
| Nausea | 1 | | | | 1 (8.3) | 0 | 2 | | | | 2 (25.0) | 0 |
| Vomiting | | | | | 0 | 0 | | | | | 0 | 0 |
| Diarrhea | 2 | 7 | | | 9 (75.0) | 0 | 2 | | | | 2 (25.0) | 0 |
| Mucositis | 1 | 2 | | | 3 (25.0) | 0 | | 1 | | | 1 (12.5) | 0 |
| Hand-foot
syndrome | 4 | 1 | 4 | | 9 (75.0)a | 4 (33.3) | | | | | 0a | 0 |
| Hepatic
encephalopathy | | | 1 | | 1 (8.3) | 1 (8.3) | | | | | 0 | 0 |
| Ascites | | 3 | | | 3 (25.0) | 0 | | | | | 0 | 0 |
| Bleeding | | | | | 0 | 0 | | | | | 0 | 0 |
| Cardiological | | | | | 0 | 0 | | | | | 0 | 0 |
| Hypertension | 2 | 3 | 3 | | 8 (66.7)a | 3 (25.0) | | | | | 0a | 0 |
| Pancreatitis | | | | | 0 | 0 | | | | | 0 | 0 |
| Infection | | 1 | | | 1 (8.3) | 0 | | 2 | | | 2 (25.0) | 0 |
|
Hyperbilirubinemia | 2 | 1 | | | 3 (25.0) | 0 | 2 | | | | 2 (25.0) | 0 |
|
Hypoalbuminemia | 2 | 8 | | | 10 (83.3) | 0 | 1 | 5 | | | 6 (75.0) | 0 |
| Increased AST | 6 | | | | 6 (50.0) | 0 | 2 | | | | 2 (25.0) | 0 |
| Increased ALT | 3 | | | | 3 (25.0) | 0 | 1 | | | | 1 (12.5) | 0 |
| Increased
creatinine | 1 | | | | 1 (8.3) | 0 | 1 | 2 | | | 3 (37.5) | 0 |
| Increased serum
amylase | 3 | 2 | | | 5 (41.7) | 0 | | | | | 0 | 0 |
Discussion
In the present study, we demonstrated the
feasibility and safety of HAIC in elderly patients with advanced
HCC. Several previous studies demonstrated the efficacy and safety
of sorafenib in elderly patients (12,14–16);
however, to the best of our knowledge, there are no studies
performing a comparison of efficacy and safety between sorafenib
and HAIC in elderly patients with HCC. It should be noted that the
definition of ‘elderly’ may be controversial. We selected the
cut-off age of 70 years, as the majority of age-related changes
occur after this age (3). There
are some studies available comparing sorafenib and HAIC for the
treatment of HCC, but they were not performed in elderly patients
(18,19).
In the present study, the RR of the HAIC group was
significantly higher compared to that of the sorafenib group, but
the TCR was similar between the two groups. Our findings were
concurrent with those of previous studies of
interferon-α-containing HAIC that demonstrated a RR of 24.6–73.0%
(11,17,20–24),
indicating that interferon-α-containing HAIC is a feasible
treatment for elderly patients with advanced HCC.
An important finding of the present study is that,
in the HAIC group, over a third of the patients achieved a CR or PR
and, among these patients, 3 achieved long-term survival with
additional curative therapy. This observation has important
implications in understanding the indications for HAIC in elderly
patients. There were no significant differences in median OS and
PFS between the two groups. The median PFS with sorafenib was
similar to that reported by previous investigations in elderly
patients, but the median OS was longer (12,14–16).
The reasons underlying the prolongation of OS in the sorafenib
group in the present study are unknown, but one possibility is that
the sorafenib group included 2 patients who received HAIC
immediately after disease progression, which may skew the data. Two
patients in the HAIC group achieved a CR after additional RFA.
Other studies have demonstrated that a CR may improve long-term
survival, although this was demonstrated in elderly patients
(10,11).
The rate of treatment discontinuation due to severe
AEs was significantly higher in the sorafenib group compared to
that in the HAIC group. Multiple AEs have been associated with
5-FU, CDDP and interferon-α therapy; however, life-threatening AEs
rarely occur, even in patients with liver cirrhosis (11,17,20,23).
In this study, AEs in HAIC-treated patients were more severe than
previously reported (11,17,20,23),
particularly thrombocytopenia, although none resulted in treatment
discontinuation or required any additional management. The
evaluation of AEs in this patient population may be challenging, as
the majority of the patients already presented with pancytopenia
due to underlying liver cirrhosis. However, a high AE-induced
discontinuation rate was apparent among sorafenib-treated patients,
mostly as a result of anorexia or hypoalbuminemia, which may lead
to ascites. In the present study, patients with a mean age of 80.2
years comprised 75% of all the grades of anorexia. This is
concordant with the observations of Morimoto et al (13), who indicated that the incidence of
anorexia was significantly higher among patients aged ≥75 years.
Our results and those of Morimoto et al (13) differ from the results of the SHARP
and Asia-Pacific trials (6,25);
however, in those studies, the age and incidence of all-grade
anorexia was 64.9 years (mean) and 51 years (median) and 14 and
12.8%, respectively (6,25). The results of those studies and our
present results suggest that elderly patients are more prone to
sorafenib-induced anorexia. In addition, Montella et al
(15) suggested that the changes
reported in Child-Pugh scores, as a result of changes in
hypoalbuminemia and PT-INR, appeared to be associated with liver
function and worsening of cirrhosis, rather than to the drugs
administered. However, in the elderly patients in this study, the
PT-INR did not change, suggesting preserved hepatic protein
synthesis, indicating that hypoalbuminemia may be associated with
the anorexia, rather than liver dysfunction. Accordingly, the
results of the present study suggest that hypoalbuminemia is an
important AE in elderly patients. In summary, HAIC may be a safer
option compared to sorafenib for the treatment of elderly patients
with HCC.
There were several limitations in the interpretation
of the data presented in this study. First, the retrospective
design and limited number of patients enrolled may give rise to
selection bias. The mean age of the sorafenib group was higher
compared to that of the HAIC group, which may explain why the
incidence of AEs was higher in the sorafenib group. Moreover,
according to the initial response to treatment, additional
therapies were performed without limitation, which may affect OS.
All the patients in the sorafenib group who received additional
therapies developed PD or severe AEs, while some of the patients in
the HAIC group who received additional therapies achieved a CR or
PR. However, in part, the present study provided significant
information regarding the management of HCC in elderly
patients.
In conclusion, HAIC appears to be a feasible and
safe treatment option for elderly patients with advanced HCC.
However, further study of HAIC in a larger population of elderly
patients is required to assess its potential as an alternative
option for HCC management.
Acknowledgements
This study was supported, in part, by a grant from
the Clinical Trial and Advanced Medical Center of University of
Fukui.
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suda T, Nagashima A, Takahashi S, et al:
Active treatments are a rational approach for hepatocellular
carcinoma in elderly patients. World J Gastroenterol. 19:3831–3840.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balducci L: Geriatric oncology: challenges
for the new century. Eur J Cancer. 36:1741–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collier JD, Curless R, Bassendine MF and
James OF: Clinical features and prognosis of hepatocellular
carcinoma in Britain in relation to age. Age Ageing. 23:22–27.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirici-Cappa F, Gramenzi A, Santi V, et
al: Treatments for hepatocellular carcinoma in elderly patients are
as effective as in younger patients: a 20-year multicentre
experience. Gut. 59:387–396. 2010.PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudo M, Izumi N, Kokudo N, et al; HCC
Expert Panel of Japan Society of Hepatology. Management of
hepatocellular carcinoma in Japan: Consensus-Based Clinical
Practice Guidelines proposed by the Japan Society of Hepatology
(JSH) 2010 updated version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar
|
|
8
|
Meric F, Patt YZ, Curley SA, et al:
Surgery after downstaging of unresectable hepatic tumors with
intra-arterial chemotherapy. Ann Surg Oncol. 7:490–495. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyaki D, Aikata H, Honda Y, et al:
Hepatic arterial infusion chemotherapy for advanced hepatocellular
carcinoma according to Child-Pugh classification. J Gastroenterol
Hepatol. 27:1850–1857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ando E, Tanaka M, Yamashita F, et al:
Hepatic arterial infusion chemotherapy for advanced hepatocellular
carcinoma with portal vein tumor thrombosis: analysis of 48 cases.
Cancer. 95:588–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obi S, Yoshida H, Toune R, et al:
Combination therapy of intraarterial 5-fluorouracil and systemic
interferon-alpha for advanced hepatocellular carcinoma with portal
venous invasion. Cancer. 106:1990–1997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong H, Tang YF, Yao TJ, et al: The
outcomes and safety of single-agent sorafenib in the treatment of
elderly patients with advanced hepatocellular carcinoma (HCC).
Oncologist. 16:1721–1728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morimoto M, Numata K, Kondo M, et al:
Higher discontinuation and lower survival rates are likely in
elderly Japanese patients with advanced hepatocellular carcinoma
receiving sorafenib. Hepatol Res. 41:296–302. 2011. View Article : Google Scholar
|
|
14
|
Di Costanzo GG, Tortora R, De Luca M, et
al: Impact of age on toxicity and efficacy of sorafenib-targeted
therapy in cirrhotic patients with hepatocellular carcinoma. Med
Oncol. 30:4462013.PubMed/NCBI
|
|
15
|
Montella L, Addeo R, Cennamo G, et al:
Sorafenib in elderly patients with advanced hepatocellular
carcinoma: a case series. Oncology. 84:265–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo M, Yasui K, Kirishima T, et al:
Efficacy and safety of sorafenib in very elderly patients aged 80
years and older with advanced hepatocellular carcinoma. Hepatol
Res. Feb 14–2014.(Epub ahead of print).
|
|
17
|
Yamashita T, Arai K, Sunagozaka H, et al:
Randomized, phase II study comparing interferon combined with
hepatic arterial infusion of fluorouracil plus cisplatin and
fluorouracil alone in patients with advanced hepatocellular
carcinoma. Oncology. 81:281–290. 2011. View Article : Google Scholar
|
|
18
|
Hiramine Y, Uto H, Imamura Y, et al:
Sorafenib and hepatic arterial infusion chemotherapy for
unresectable advanced hepatocellular carcinoma: A comparative
study. Exp Ther Med. 2:433–441. 2011.PubMed/NCBI
|
|
19
|
Jeong SW, Jang JY, Lee JE, et al: The
efficacy of hepatic arterial infusion chemotherapy as an
alternative to sorafenib in advanced hepatocellular carcinoma. Asia
Pac J Clin Oncol. 8:164–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasai K, Ushio A, Kasai Y, et al:
Therapeutic efficacy of combination therapy with intra-arterial
5-fluorouracil and systemic pegylated interferon alpha-2b for
advanced hepatocellular carcinoma with portal venous invasion.
Cancer. 118:3302–3310. 2012. View Article : Google Scholar
|
|
21
|
Enjoji M, Morizono S, Kotoh K, et al:
Re-evaluation of antitumor effects of combination chemotherapy with
interferon-alpha and 5-fluorouracil for advanced hepatocellular
carcinoma. World J Gastroenterol. 11:5685–5687. 2005.PubMed/NCBI
|
|
22
|
Ota H, Nagano H, Sakon M, et al: Treatment
of hepatocellular carcinoma with major portal vein thrombosis by
combined therapy with subcutaneous interferon-alpha and
intra-arterial 5-fluorouracil; role of type 1 interferon receptor
expression. Br J Cancer. 93:557–564. 2005. View Article : Google Scholar
|
|
23
|
Nagano H, Wada H, Kobayashi S, et al:
Long-term outcome of combined interferon-alpha and 5-fluorouracil
treatment for advanced hepatocellular carcinoma with major portal
vein thrombosis. Oncology. 80:63–69. 2011. View Article : Google Scholar
|
|
24
|
Uka K, Aikata H, Takaki S, et al:
Pretreatment predictor of response, time to progression, and
survival to intraarterial 5-fluorouracil/interferon combination
therapy in patients with advanced hepatocellular carcinoma. J
Gastroenterol. 42:845–853. 2007. View Article : Google Scholar
|
|
25
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|