Introduction
Lung cancer is one of the most common cancers
worldwide, with non-small-cell lung cancer (NSCLC) accounting for
∼80% of the cases (1, 2). The risk of lung cancer clearly
increases with advancing age and, with the prolongation of life
expectancy, the number of elderly patients with lung cancer is
expected to rapidly increase (3–5).
According to the USA National Surveillance, Epidemiology and End
Results database, approximately half of the patients with lung
cancer were aged ≥ 70 years. In addition, 14% of the patients with
lung cancer were aged at least 80 years (6, 7). As
a significant proportion of the NSCLC patients who are aged ≥ 80
years present with advanced disease, a poor performance status (PS)
at diagnosis and several comorbidities, there is a need for the
development of suitable chemotherapy for such patients.
Recently, several reports described the safety and
efficacy of gefitinib in patients with epidermal growth factor
receptor (EGFR) mutation-positive NSCLC aged >70 years
and/or with a poor PS (2–4, 7–9). The
safety and efficacy of erlotinib in elderly patients with NSCLC
were also reported (10, 11). Gefitinib and erlotinib are orally
active EGFR-tyrosine kinase inhibitors (EGFR-TKIs), which have
displayed notable efficacy in patients with advanced NSCLC
(2, 12–14).
Since the hematological toxicity of EGFR-TKIs is lower compared to
that of cytotoxic chemotherapy, they may be of value as treatment
for elderly patients and/or patients with poor PS.
However, the efficacy and toxicity of EGFR-TKIs in
NSCLC patients aged ≥ 80 years have not been fully evaluated.
Whether standard anticancer therapy in extremely elderly patients
with lung cancer is always safe in clinical practice remains
unclear. In this study, we retrospectively assessed the value and
safety of EGFR-TKIs in such patients.
Patients and methods
Patients
A total of 23 patients aged ≥ 80 years with NSCLC
were treated with EGFR-TKIs between January, 2009 and December,
2013 at Kainan Hospital (Yatomi, Aichi, Japan). Two patients were
excluded from this analysis, as they were administered EGFR-TKIs
for only a few days and data on treatment efficacy and toxicity
were not available. Accordingly, a total of 21 patients were
included in this analysis. The gefitinib group included patients
who were treated with gefitinib alone or with gefitinib followed by
erlotinib. The erlotinib group included patients treated with
erlotinib alone or with erlotinib followed by gefitinib. In the
gefitinib group, the patients were treated with oral gefitinib 250
mg daily, 250 mg every other day, or 250 mg every 3 days. In the
erlotinib group, the patients were treated with oral erlotinib 150,
100, or 50 mg daily. The administration of EGFR-TKIs was continued
until worsening of the general status, the development of
unacceptable toxicity regardless of dose reduction, or the
patients' refusal to continue treatment.
This study was approved by the Ethics committee of
Kainan Hospital, Aichi Prefectural Welfare Federation of
Agricultural Cooperatives. Written informed consent was obtained
from all the patients prior to the initiation of EGFR-TKI
treatment.
Patient characteristics and evaluation
of response to treatment and toxicity
The patient characteristics, including the type of
EGFR mutation, were retrospectively obtained from medical
records. EGFR mutations were analyzed using the peptide
nucleic acid-locked nucleic acid polymerase chain reaction clamp
method (15) up to July, 2012.
From August, 2012 onwards, EGFR exon 19 deletion was determined by
fragment analysis and L858R point mutation was detected using the
cycleave polymerase chain reaction technique (16). The patients were staged according
to the 7th edition of the Union for International Cncer Control TNM
classification prior to treatment initiation (17). The patients' adverse events,
tolerance and response to treatment were retrospectively analyzed.
Tumor response were classified as complete response (CR), partial
response (PR), stable disease (SD) or progressive disease (PD), in
accordance with the Response Evaluation Criteria for Solid tumors,
version 1.1 (18). Toxicities were
assessed according to the Common Terminology Criteria for Adverse
Events, version 4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40)).
Statistical analysis
Progression-free survival (PFS) and overall survival
(OS) rates were analyzed by the Kaplan-Meier method. PFS was
measured from the date of initiation of gefitinib or erlotinib
therapy to the date of progressive disease, death, or last
follow-up. OS was defined as the time between the initiation of
treatment and death or last follow-up. All the statistical analyses
were performed with EZR statistical software (Saitama Medical
Center, Jichi Medical University, Saitama, Japan), which is a
graphical user interface for R (The R Foundation for Statistical
Computing, Vienna, Austria). More precisely, EZR is a modified
version of R commander designed to add statistical functions
frequently used in biostatistics (19).
Results
Patient characteristics
The patients' characteristics are summarized in
Table I. There were 8 male (38.1%)
and 13 female (61.9%) patients, with a median age of 85 years
(range, 80–96 years). A total of 8 patients (38.1%) had an Eastern
Cooperative Oncology Group PS of 2–3, whereas the remaining 13
patients had a PS of 0–1. A total of 15 patients (71.4%) were never
smokers and 6 (28.6%) were former smokers. Adenocarcinoma comprised
the majority of the cancers (85.7%). EGFR status was
analyzed in 20 patients and EGFR mutations were identified
in 17 patients (81%). Of the 21 patients, 4 (19%) had clinical
stage IIIA disease at diagnosis, whereas the remaining 17 (81%) had
stage IV disease. A total of 5 patients received prior anticancer
therapy; namely, 2 patients received cytotoxic chemotherapy
(non-platinum-based chemotherapy), 2 patients received radiation
therapy and 1 patient underwent surgery.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Patient no.
(n=21) |
|---|
| Age, years | 85 (80–96) |
| Median (range) | |
| Sex | |
| Male | 8 |
|
Female | 13 |
| ECOG performance
status | |
| 0 | 4 |
| 1 | 9 |
| 2 | 5 |
| 3 | 3 |
| 4 | 0 |
| Smoking status | |
|
Never | 15 |
|
Former | 6 |
|
Current | 0 |
| Histology | |
|
Adenocarcinoma | 18 |
| Squamous
cell carcinoma | 2 |
|
Unknown | 1 |
| Clinical stage at
diagnosis | |
| IIIA | 4 |
| IIIB | 0 |
| IV | 17 |
| EGFR
mutation | |
| Exon 19
deletion | 5 |
|
L858R | 12 |
|
Negative | 3 |
|
Unknown | 1 |
| Prior therapy | |
| None | 16 |
|
Radiation | 2 |
| Cytotoxic
chemotherapy | 2 |
|
Surgery | 1 |
Patient EGFR status, treatment and
outcomes
The characteristics and outcomes of the enrolled
patients are listed in Table II.
Of the 21 patients, 18 were administered gefitinib and 3 were
administered erlotinib. As regards EGFR mutations, 12
patients carried the L858R mutation in exon 21 and 5 carried
deletions in exon 19. The median treatment duration was 197 days
(range, 12–965 days). No patients achieved CR, whereas 14 patients
(66.7%) achieved PR. The disease control rate, defined as the
percentage of patients who achieved CR, PR, or SD, was 90.5%. Dose
reduction of EGFR-TKIs due to adverse events was required in 15
patients (71.4%). A total of 7 patients (33.3%) continued with the
EGFR-TKI therapy beyond PD. In the gefitinib group, 4 patients
received subsequent erlotinib therapy following termination of
gefitinib therapy. The average follow-up duration was 413 days
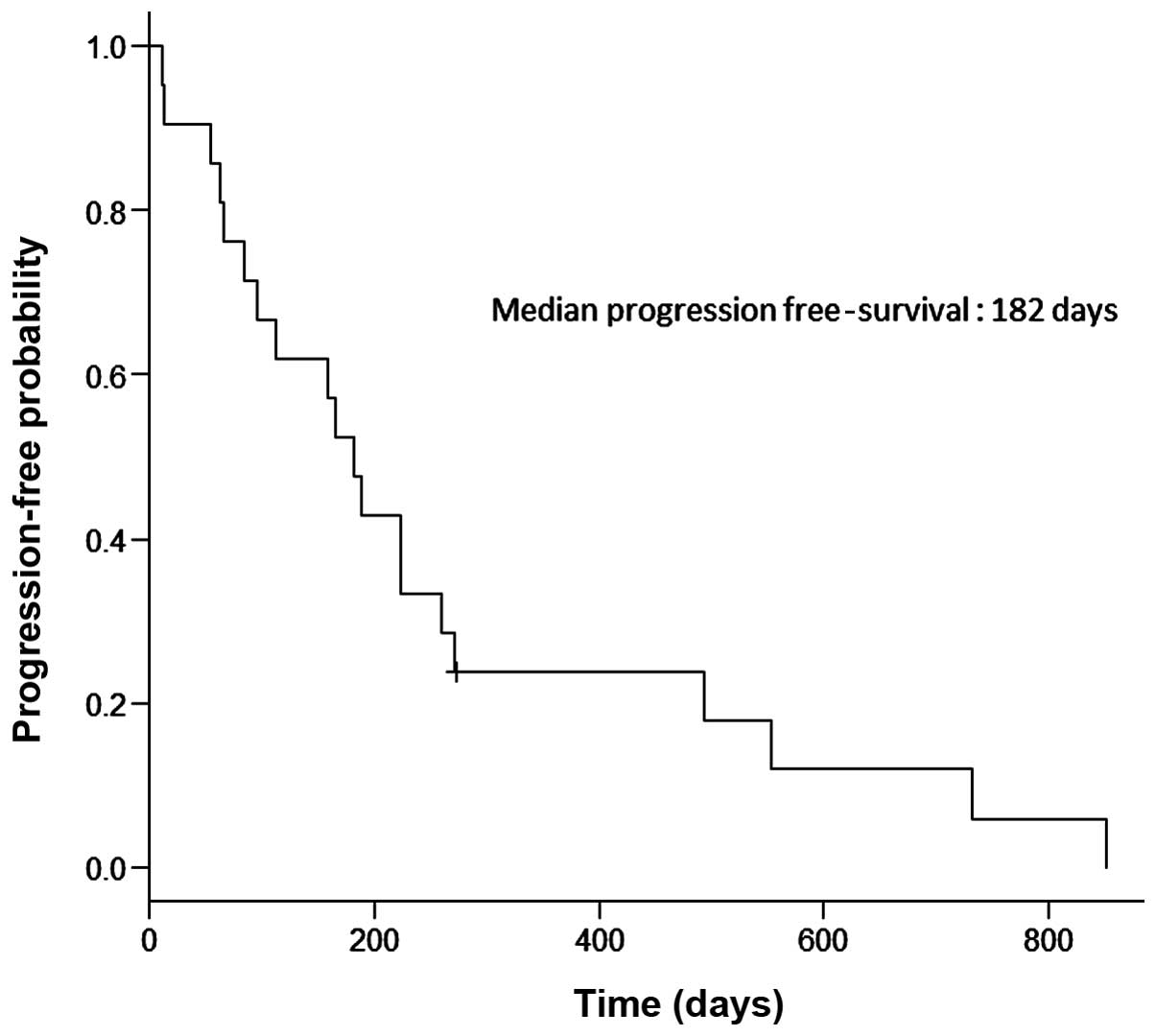
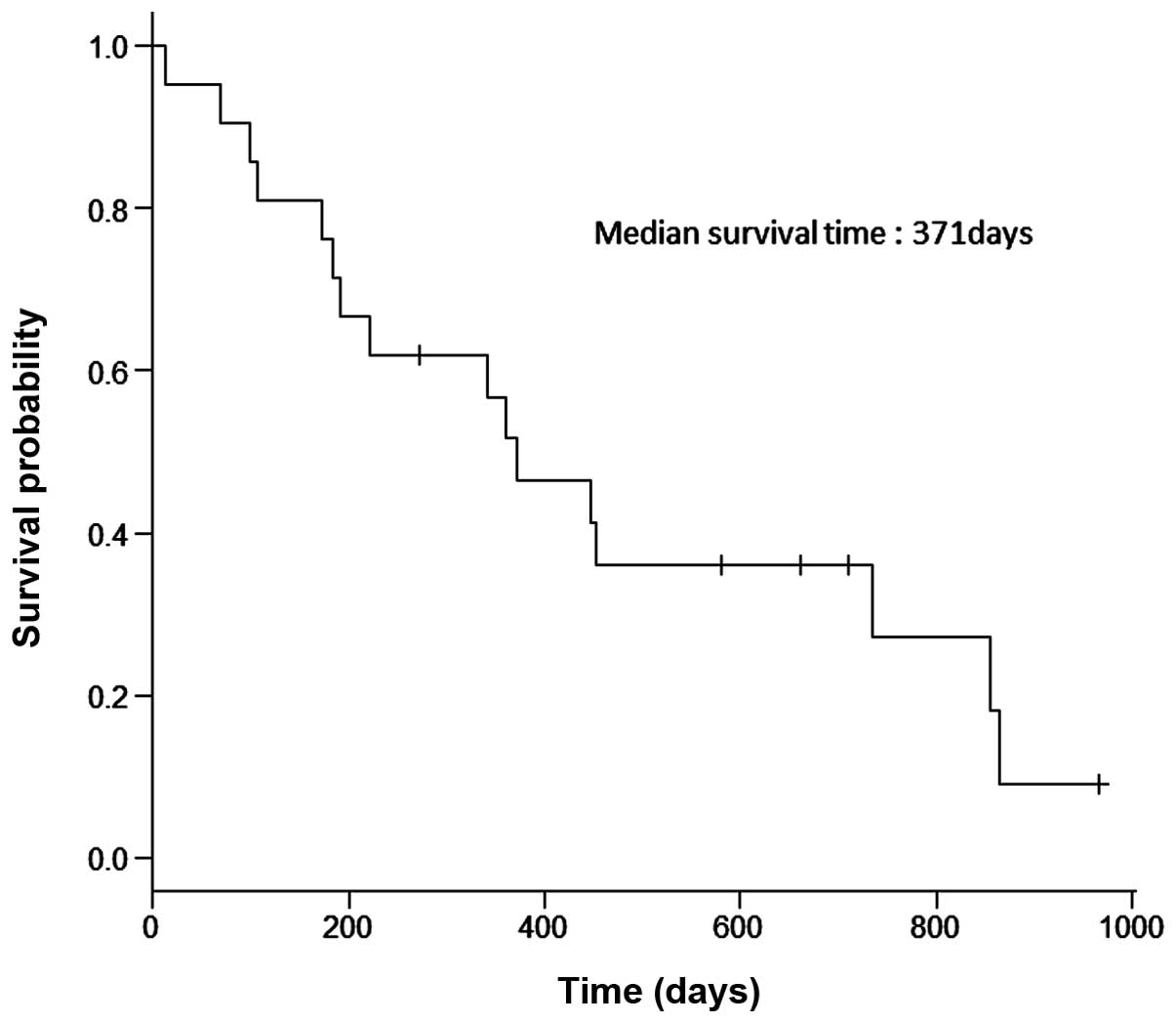
(range, 12–965 days). The median PFS was 182 days (Fig. 1) and the median OS was 371 days
(Fig. 2). Of the 17 patients
harboring EGFR mutations, 13 (76.5%) achieved PR and the
median PFS and OS were 223 and 452 days, respectively.
 | Table II.Patient characteristics and response
to gefitinib or erlotinib therapy. |
Table II.
Patient characteristics and response
to gefitinib or erlotinib therapy.
| Case no. | Age (yrs) | Gender | EGFR
mutationa | Type of
EGFR-TKI | Dose
reductionb | Response | Time to progression
(days) | Survival
(days) | Subsequent
therapy | Beyond
PDc |
|---|
| 1 | 85 | F | L858R | Gefitinib | Yes | PR | 260 | 452 | None | Yes |
| 2 | 85 | F | Wild-type | Gefitinib | No | PD | 13 | 70 | None | No |
| 3 | 82 | F | L858R | Gefitinib | Yes | PR | 224 | 446 | None | Yes |
| 4 | 81 | F | L858R | Gefitinib | Yes | PR | 493 | 865 | Pemetrexed | No |
| 5 | 86 | F | Unkown | Gefitinib | Yes | PR | 159 | 192 | None | No |
| 6 | 81 | F | Exon 19 del | Gefitinib | Yes | PR | 223 | 371 | None | Yes |
| 7 | 81 | M | L858R | Gefitinib | Yes | PR | 96 | 183 | None | Yes |
| 8 | 85 | F | Exon 19 del | Gefitinib | Yes | SD | 733 | 854 | None | Yes |
| 9 | 88 | F | L858R | Gefitinib | Yes | SD | 851 | 965 | None | No |
| 10 | 83 | F | Wild-type | Erlotinib | No | SD | 63 | 99 | None | No |
| 11 | 81 | F | Exon 19 del | Gefitinib | Yes | PR | 188 | 734 | Erlotinib | No |
| 12 | 96 | M | L858R | Erlotinib | Yes | PR | 84 | 107 | None | No |
| 13 | 86 | F | L858R | Gefitinib | Yes | PR | 272 | 710 | None | Yes |
| 14 | 81 | M | L858R | Gefitinib | No | SD | 554 | 661 | None | No |
| 15 | 83 | F | L858R | Gefitinib | Yes | PR | 112 | 580 | Erlotinib | No |
| 16 | 80 | M | Wild-type | Erlotinib | Yes | SD | 55 | 361 | Radiation | No |
| 17 | 85 | M | Exon 19 del | Gefitinib | No | PD | 12 | 12 | None | No |
| 18 | 85 | M | L858R | Gefitinib | No | PR | 66 | 172 | Erlotinib | No |
| 19 | 90 | M | Exon 19 del | Gefitinib | Yes | PR | 166 | 341 | Erlotinib | Yes |
| 20 | 80 | M | L858R | Gefitinib | Yes | PR | 182 | 221 | None | No |
| 21 | 89 | F | L858R | Gefitinib | No | PR | 273 | 273 | None | No |
Adverse events
The treatment-related adverse events are summarized
in Table III. The most common
adverse event associated with EGFR-TKIs was skin toxicities
(57.1%). Skin toxicities ≥ grade 2 were as follows: skin rash, 9
patients (42.9%); dry skin, 6 patients; (28.6%); pruritus, 5
patients (23.8%); and paronychia, 3 patients (14.3%). Liver
function test abnormalities ≥ grade 2 were observed in 7 patients
(33.3%). Other adverse events ≥ grade 2 were as follows: anorexia,
3 patients (14.3%); diarrhea, 2 patients (9.5%); general fatigue, 2
patients (9.5%); and anemia, leukopenia, elevated amylase and
nausea, 1 patient each (4.8%). Adverse events ≥ grade 4 or
interstitial lung disease (ILD) were not observed in this
study.
 | Table III.Adverse events. |
Table III.
Adverse events.
| Grade, no. | | |
|---|
|
| | |
|---|
| Toxicity | 1 | 2 | 3 | 4 | ≥Grade 1, no.
(%) | ≥Grade 2, no.
(%) |
|---|
| Skin rash | 5 | 4 | 5 | 0 | 14 (66.7) | 9 (42.9) |
| Dry skin | 3 | 4 | 2 | 0 | 9 (42.9) | 6 (28.6) |
| Pruritus | 1 | 3 | 2 | 0 | 6 (28.6) | 5 (23.8) |
| Paronychia | 1 | 3 | 0 | 0 | 4 (19.0) | 3 (14.3) |
| Diarrhea | 7 | 2 | 0 | 0 | 9 (42.9) | 2 (9.5) |
| Nausea | 2 | 1 | 0 | 0 | 3 (14.3) | 1 (4.8) |
| Vomiting | 2 | 0 | 0 | 0 | 2 (9.5) | 0 (0.0) |
| Anorexia | 3 | 2 | 1 | 0 | 6 (28.6) | 3 (14.3) |
| Stomatitis | 3 | 0 | 0 | 0 | 3 (14.3) | 0 (0.0) |
| Dysgeusia | 2 | 0 | 0 | 0 | 2 (9.5) | 0 (0.0) |
| Dry mouth | 1 | 0 | 0 | 0 | 1 (4.8) | 0 (0.0) |
| Elevated
AST/ALT/ALP | 4 | 4 | 3 | 0 | 11 (52.4) | 7 (33.3) |
| Elevated
amylase | 0 | 0 | 1 | 0 | 1 (4.8) | 1 (4.8) |
| Interstitial lung
disease | 0 | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) |
| Leukopenia | 1 | 1 | 0 | 0 | 2 (9.5) | 1 (4.8) |
| Anemia | 0 | 1 | 0 | 0 | 1 (4.8) | 1 (4.8) |
|
Thrombocytopenia | 0 | 0 | 0 | 0 | 0 (0.0) | 0 (0.0) |
| Epistaxis | 2 | 0 | 0 | 0 | 2 (9.5) | 0 (0.0) |
| Increased
creatinine | 1 | 0 | 0 | 0 | 1 (4.8) | 0 (0.0) |
| General
fatigue | 1 | 2 | 0 | 0 | 3 (14.3) | 2 (9.5) |
| Leg edema | 1 | 0 | 0 | 0 | 1 (4.8) | 0 (0.0) |
Discussion
Gefitinib and erlotinib have displayed acceptable
efficacy in NSCLC patients aged ≥ 80 years. However, a dose
reduction of EGFR-TKIs due to adverse events were required in >
70% of our study subjects. To the best of our knowledge, this is
the first study targeting extremely elderly patients with NSCLC who
were treated with EGFR-TKIs.
Several recent studies described the efficacy of
gefitinib and erlotinib for elderly patients with NSCLC (3, 4,
7–11, 20,
21). In studies of elderly
patients not selected by EGFR mutation status, the overall
response rates (RRs) were 10–25%, the median PFS was 2.7-4 months
and the median OS was 7.6-11.9 months (4, 7,
10, 11, 20). By contrast, in studies of
EGFR mutation-positive elderly patients, the overall RRs
were 59–74%, the median PFS was 12.3-13.8 months and the median OS
was 17.4-29.1 months (3, 8, 9,
21). In our study, the overall RR
was 66.7%, the median PFS was 6.1 months and the median OS was 12.4
months. A total of 17 patients (81%) had sensitive EGFR
mutations in this study and, among these patients, PR was observed
in 76.5% of patients and the median PFS and OS were 7.4 and 15.1
months, respectively. The high prevalence of EGFR mutation
is considered to reflect that mutation-positive patients are more
likely to receive gefitinib or erlotinib therapy compared to
EGFR mutation-negative patients. As regards the efficacy of
EGFR-TKI therapy, considering the age of our study population, the
results of our study may be considered acceptable in comparison to
those of previous studies.
Elderly patients with lung cancer prefer to receive
less toxic anticancer chemotherapy (7). Such patients generally present with
more comorbidities and more compromised organ functions compared to
younger patients; even elderly patients with a good PS may be at
higher risk of severe toxicity compared to younger patients
(3, 22). A subgroup analysis of the BR.21
study revealed that elderly patients with NSCLC who were treated
with erlotinib displayed similar efficacy as younger patients, but
experienced more significant toxicity (3, 23).
In addition, advanced age has been reported to be a risk factor for
ILD in Japanese subjects during gefitinib treatment (24). Therefore, extremely elderly
patients who were treated with EGFR-TKIs should be carefully
monitored for adverse events. In previous studies, dose reduction
or discontinuation of EGFR-TKIs due to adverse events was observed
in 19–51.5% of the patients (3,
7, 10, 11,
25, 26), whereas in our study, 76.1% of the
patients required dose reduction or discontinuation (dose reduction
in 15 patients and treatment discontinuation in 1 patient, due to
liver function test abnormalities). There were no fatal toxicities,
including ILD, whereas almost all types of toxicity were manageable
by dose modification in both EGFR-TKI groups. Satoh et al
(25) reported that low-dose
gefitinib was clinically comparable to standard-dose gefitinib for
NSCLC in patients with sensitive EGFR mutations. Therefore,
we may be able to suggest that reduced-dose gefitinib and erlotinib
therapy may be suitable for extremely elderly patients.
The limitations of our study were the small sample
size, retrospective nature, heterogeneity of the treatment regimens
and being a single-arm study. As our study was based on clinical
data from a small sample of patients in a single facility, larger
prospective trials on patients aged ≥ 80 years treated with
EGFR-TKIs should be conducted to reveal the true efficacy and
toxicity of this treatment. In addition, as Togashi et al
reported that 150 mg erlotinib daily was associated with more
toxicity and less tolerability compared to 250 mg gefitinib daily
(12), an independent research
program regarding gefitinib and erlotinib therapy should be
conducted.
In conclusion, reduced-dose gefitinib or erlotinib
therapy may be an effective and tolerable regimen for NSCLC
patients aged ≥ 80 years, particularly those with EGFR
mutations. The information presented in our study may provide some
directions for clinical research on the treatment of such patients.
However, further large studies are required to validate our
findings.
References
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoue A, Kobayashi K, Usui K, et al:
First-line gefitinib for patients with advanced non-small-cell lung
cancer harboring epidermal growth factor receptor mutations without
indication for chemotherapy. J Clin Oncol. 27:1394–1400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Minegishi Y, Inoue A, et al:
First-line gefitinib in patients aged 75 or older with advanced
non-small cell lung cancer harboring epidermal growth factor
receptor mutations: NEJ 003 study. J Thorac Oncol. 7:1417–1422.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hotta K, Ueoka H, Kiura K, et al: Safety
and efficacy of gefitinib treatment in elderly patients with
non-small-cell lung cancer: Okayama Lung Cancer Study Group
experience. Acta Oncol. 44:717–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bunn PA Jr and Lilenbaum R: Chemotherapy
for elderly patients with advanced non-small-cell lung cancer. J
Natl Cancer Inst. 95:341–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Owonikoko TK, Ragin CC, Belani CP, et al:
Lung cancer in elderly patients: an analysis of the surveillance,
epidemiology, and end results database. J Clin Oncol. 25:5570–5577.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi M, Matsui K, Katakami N, et al
West Japan Oncology Group: Phase II study of gefitinib as a
first-line therapy in elderly patients with pulmonary
adenocarcinoma: West Japan thoracic oncology group study 0402. Jpn
J Clin Oncol. 41:948–952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uruga H, Kishi K, Fujii T, et al: Efficacy
of gefitinib for elderly patients with advanced non-small cell lung
cancer harboring epidermal growth factor receptor gene mutations: a
retrospective analysis. Intern Med. 49:103–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asami K, Koizumi T, Hirai K, et al:
Gefitinib as first-line treatment in elderly epidermal growth
factor receptor-mutated patients with advanced lung adenocarcinoma:
results of a Nagano Lung Cancer Research Group study. Clin Lung
Cancer. 12:387–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rossi D, Dennetta D, Ugolini M, et al:
Activity and safety of erlotinib as second- and third-line
treatment in elderly patients with advanced non-small cell lung
cancer: a phase II trial. Target Oncol. 5:231–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jackman DM, Yeap BY, Lindeman NI, et al:
Phase II clinical trial of chemotherapy-naive patients > or = 70
years of age treated with erlotinib for advanced non-small-cell
lung cancer. J Clin Oncol. 25:760–766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Togashi Y, Masago K, Fujita S, et al:
Differences in adverse events between 250 mg daily gefitinib and
150 mg daily erlotinib in Japanese patients with non-small cell
lung cancer. Lung Cancer. 74:98–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al: Erlotinib in previously treated non-small-cell lung
cancer. N Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 21:2237–2246.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagai Y, Miyazawa H, Huqun, et al: Genetic
heterogeneity of the epidermal growth factor receptor in non-small
cell lung cancer cell lines revealed by a rapid and sensitive
detection system, the peptide nucleic acid-locked nucleic acid PCR
clamp. Cancer Res. 65:7276–7282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida K, Yatabe Y, Park JY, et al:
Prospective validation for prediction of gefitinib sensitivity by
epidermal growth factor receptor gene mutation in patients with
non-small cell lung cancer. J Thorac Oncol. 2:22–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstraw P, Crowley J, Chansky K, et al
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA1, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebi N, Semba H, Tokunaga SJ, et al: A
phase II trial of gefitinib monotherapy in chemotherapy-naive
patients of 75 years or older with advanced non-small cell lung
cancer. J Thorac Oncol. 3:1166–1171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tateishi K, Ichiyama T, Hirai K, et al:
Clinical outcomes in elderly patients administered gefitinib as
first-line treatment in epidermal growth factor receptor-mutated
non-small-cell lung cancer: retrospective analysis in a Nagano Lung
Cancer Research Group study. Med Oncol. 30:4502013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chrischilles EA, Pendergast JF, Kahn KL,
et al: Adverse events among the elderly receiving chemotherapy for
advanced non-small-cell lung cancer. J Clin Oncol. 28:620–627.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wheatley-Price P, Ding K, Seymour L, Clark
GM and Shepherd FA: Erlotinib for advanced non-small-cell lung
cancer in the elderly: an analysis of the National Cancer Institute
of Canada Clinical Trials Group study BR.21. J Clin Oncol.
26:2350–2357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudoh S, Kato H, Nishiwaki Y, et al:
Interstitial lung disease in Japanese patients with lung cancer: a
cohort and nested case-control study. Am J Respir Crit Care Med.
177:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Satoh H, Inoue A, Kobayashi K, et al:
Low-dose gefitinib treatment for patients with advanced non-small
cell lung cancer harboring sensitive epidermal growth factor
receptor mutations. J Thorac Oncol. 6:1413–1417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goto K, Nishio M, Yamamoto N, et al: A
prospective, phase II, open-label study (JO22903) of first-line
erlotinib in Japanese patients with epidermal growth factor
receptor (EGFR) mutation-positive advanced non-small-cell lung
cancer (NSCLC). Lung Cancer. 82:109–114. 2013. View Article : Google Scholar : PubMed/NCBI
|