Introduction
With the exception of stage, no definitive
prognostic factors have been established for head and neck cancer.
Cancer stage is currently the most important prognostic factor for
outcome. Traditional staging of head and neck squamous cell
carcinoma (HNSCC) depends on the site of disease origin, size and
extent of the primary tumor, cervical lymph node involvement and
presence or absence of distant metastasis. This staging system
relies on conventional imaging, such as computed tomography (CT)
and magnetic resonance imaging (MRI); however, this approach is
limited by outcome heterogeneity within stage categories, hampering
accurate prognostication for individual patients (1).
Clinical characteristics, including gender, age,
weight loss and biological markers, such as p53 (2), cyclin D (3) and epidermal growth factor receptor
(4), have been investigated but
are not sufficiently accurate for individual patient prognosis.
More accurate markers would be helpful to stratify patients for
therapy and predict outcomes.
As with other types of cancer, there is great
interest in trying to identify factors predictive of outcome other
than stage and biological markers. The development of functional
imaging studies, particularly positron emission tomography (PET)
with the glucose analogue
2-(18F)-fluoro-2-deoxy-D-glucose (18F-FDG)
has emerged as a useful tool for several malignancies. PET scanning
enables non-invasive study of the physiology of cancers (5). Tumor uptake of18 F-FDG,
standardized uptake value (SUV), as measured by PET, has been
associated with various cellular characteristics, such as cell
viability (6) and proliferative
activity (7). Furthermore, SUV as
a semi-quantitative simplified measurement of tissue deoxyglucose
metabolic rate has suggested that tumor FDG uptake may be of
prognostic significance, as patients with high FDG uptake generally
have a less favorable outcome (8).
Other retrospective studies have investigated the
prognostic significance of SUV, but the majority of those reports
only included a small patient sample. Based on these
considerations, we performed a systematic review of the literature
on18 F-FDG-PET scan and local control and a
meta-analysis of the data to determine the prognostic value of
primary tumor SUV in patients with head and neck cancer.
Materials and methods
Search strategy
We conducted a search, without language
restrictions, through PubMed, Embase, the Cochrane Controlled
Trials Register and OVID databases. We employed both medical
subject headings and free-language terms for ‘HNSCC’ (i.e., ‘head
and neck cancer’, ‘head and neck carcinoma’, ‘squamous cell
carcinoma’), combined with each of the following: ‘positron
emission tomography or PET or PET imaging tomography’ and
‘FDG-F18 or FDG or18 F-fluorodeoxyglucose
or18 F-FDG’ and ‘SUV or standardized uptake value’ as
search terms. The references reported in all the identified studies
were used to complete this search, which ended in May, 2014.
Further searches were conducted by scanning the abstracts of major
ENT and nuclear medicine meetings.
Inclusion and exclusion criteria
In order to be eligible for the systematic review, a
study was required to fulfill the following criteria: i) Limited to
HNSCC, with any stage or any histological grading; ii) assessed the
association between pretherapeutic SUV and local control, at least
in a univariate analysis; iii) SUV referred to the primary tumor;
and iv) reports using all modalities of care were included.
Abstracts were excluded, as they do not provide
sufficient details to assess methodology or relative information to
perform a meta-analysis. Furthermore, we carefully examined the
possibility of patient duplication by reporting the same cohorts in
different publications. This led us to suppress one article,
although no reference to such duplicates was reported by the
authors.
Quality assessment
To improve quality assessment, 7 physicians and 1
biostatistician reviewed all the publications to assess their
methodological quality and extract the most important information
determining the clinical and PET characteristics. A methodological
quality scale was designed for the purpose of this study, using the
variables available in the publications (9). This score assessed the clinical and
PET reports. The clinical report included the distribution of the
expected ‘prognostic factors’ (age, gender, stage, performance
status, histological grading and weight loss), tumor stage
description, staging characteristics [definition of the size of
pathological metastasis, systematic use of the head and neck CT for
head and neck staging, systematic metastatic work-up, systematic
use of a CT or MRI for distant metastasis, histological
confirmation of metastasis and if the analysis of the association
between SUV and each expected prognostic factor was performed
without knowledge of clinical results and vice versa (double
blind)], description of results of local control and analyses
(number of patients, number of local control, follow-up duration,
number of patients lost to follow-up, univariate and multivariate
analyses, description of statistical tests, definition of local
control and SUV cut-off definition). The PET report included
patient characteristics (weight/height, glycaemia and histological
subtype),18 F-FDG-PET acquisition protocol
characteristics (injected dose of18 F-FDG, delay between
injection and data acquisition and fasting duration) and technical
parameters (investigation area, delay between head and neck CT and
PET acquisition, SUV formula, type of PET engine, duration of
emission time, duration of transmission time, attenuation and
reconstruction parameters and type of SUV). The clinical and PET
reports were scored on 21 and 14 points, respectively. A value
between 0 and 2 was attributed to each item. The scores were
expressed in percentage of the maximal theoretical value that can
be obtained. When the results of a particular study were reported
in more than one publication, only the most recent and complete
data were included in the meta-analysis.
Statistical analysis
Data were extracted by Zhang and Nie and
discrepancies were discussed and resolved by Dong. Data were
entered into the Cochrane Collaboration Review Manager program
RevMan 4.2.2. We measured the impact of SUV by relative risk (RR)
between the local control distributions of two groups. For each
trial, this RR was estimated by a method depending on the results
provided in the publication. The most accurate method was through
determining the total number of events and the number of patients
at risk in each group, allowing calculation of the RR estimation.
If the only exploitable data were in the form of graphical
representations of local control distribution, they were used to
extract the corresponding rates at specified times to reconstruct
the RR estimate and its variance, with the hypothesis that the rate
of patients censored was constant during the study follow-up. The
individual RR point estimates were combined following acceptation
of the null hypothesis of the homogeneity of the treatment effect
across the various trials, using the RevMan 4.2.2 software to
obtain a global RR estimate of the treatment effect. The RR was
calculated using the fixed-effects method. In case of significant
heterogeneity (P<0.05), the random-effects method was applied.
This impact of SUV on local control was considered as statistically
significant if the 95% confidence interval (95% CI) for the overall
RR did not overlap 1. All reported P-values were two-tailed.
Finally, funnel plot asymmetry was used to detect any publication
bias in the meta-analysis.
Results
Study characteristics
A total of 7 articles (10–16)
on SUV in HNSCC were retrieved. One study was excluded from the
analysis due to patient duplication (16) and the remaining 6 studies,
published between 2002 and 2009, were considered eligible for this
review. The sites of primary tumor in the studies included the oral
cavity, oropharynx, nasopharynx, hypopharynx, larynx and maxilla.
The follow-up time for local control, disease-free survival and
overall survival ranged between 2 and 82.8 months per study. The
principal characteristics of the 6 studies eligible for the
meta-analysis are described in Table
I. For the present study, SUV referred to the primary tumor,
except in 5 patients (3 with unknown primary tumors and 2 with T1-2
tumors), in whom only the lymph nodes exhibited increased uptake.
Consequently, for these 5 patients, the SUV of the lymph node was
used as reference for correlation with local control. The main SUV
characteristics reported in the publications are described in
Table II.
 | Table I.Characteristics of studies included in
this meta-analysis. |
Table I.
Characteristics of studies included in
this meta-analysis.
| Author (year) | Total patient
no. | SUV<threshold
patient no. | SUV>threshold
patient no. | Histology | (Refs.) |
|---|
| Brun et al
(2002) | 44 | 23 | 21 | HNSCC | (10) |
| Allal et al
(2004)a | 119 | 62 | 57 | HNSCC | (11) |
| Kim et al
(2007) | 52 | 27 | 25 | HNSCC | (12) |
| Roh et al
(2007) | 79 | 48 | 31 | HNSCC | (13) |
| Liao et al
(2009) | 109 | 97 | 12 | HNSCC | (14) |
| Torizuka et al
(2009) | 50 | 21 | 29 | HNSCC | (15) |
 | Table II.Main SUV characteristics reported in
the six publications assessable for meta-analysis. |
Table II.
Main SUV characteristics reported in
the six publications assessable for meta-analysis.
| Study (year) | Type of SUV | Correction of
SUV | SUV threshold
definition | SUV threshold | (Refs.) |
|---|
| Brun et al
(2002) |
SUVmean | Weight | Median |
9.0 | (10) |
| Allal et al
(2004)a |
SUVmax | Weight | Median |
4.76 | (11) |
| Kim et al
(2007) |
SUVmax | Lean body weight | Median |
6.0 | (12) |
| Roh et al
(2007) |
SUVmax | Weight | Best
cut-offb |
8.0 | (13) |
| Liao et al
(2009) |
SUVmax | Weight | Best
cut-offb |
19.3 | (14) |
| Torizuka et al
(2009) |
SUVmax | Weight | Best
cut-offb |
7.0 | (15) |
Study quality
The methodological quality of the studies was
moderate. Overall, the median quality score was 64%, ranging
between 53 and 70%. The respective median values for the clinical
and PET reports were 62 % (range, 55–69%) and 66% (range, 50–82%),
respectively.
High FDG uptake is a marker of poor
outcome in HNSCC
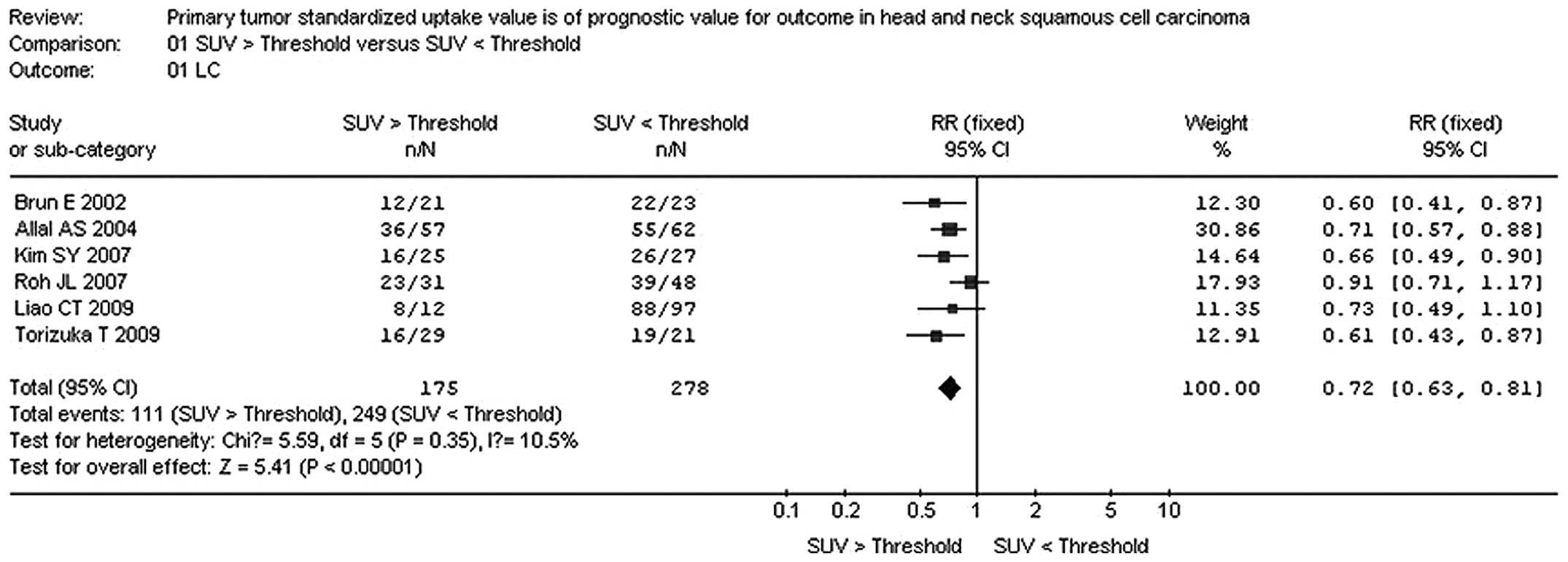
The combined RR from the 6 reports for local control
was 0.72 (95% CI: 0.63-0.81). The combined RR was calculated using
the fixed-effects method. The combined RR confirmed that high FDG
uptake on PET is a marker for poor outcome in primary HNSCC. The
results are detailed in Fig. 1.
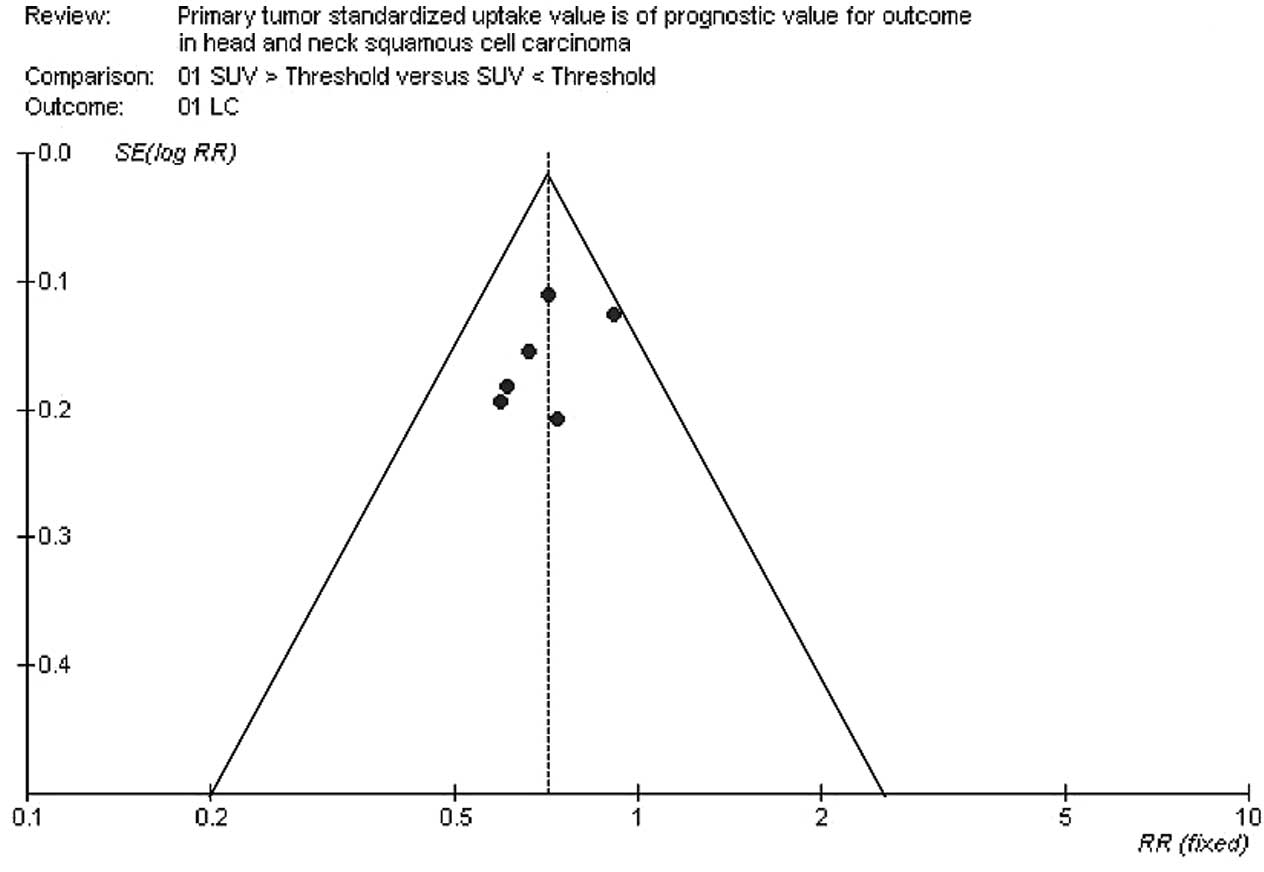
The funnel plot revealed a symmetrical distribution, indicating no
evidence of substantial publication bias. The results are detailed
in Fig. 2.
Discussion
Over the last decade, FDG-PET has become an
important tool used to stage patients with HNSCC. Furthermore,
in vivo imaging of human tumors with FDG-PET is a clinical
extension of classical studies on carbohydrate metabolism. It was
demonstrated that a high rate of glycolysis contributes to tumor
growth, which may be exploited for malignancy grading with PET
(17). The specific goal of this
study was to evaluate the potential of SUV as a prognostic marker.
The present meta-analysis confirmed that increased SUV of the
primary tumor is a poor prognostic factor in patients with
HNSCC.
Under the balanced circumstances of glucose
metabolism,18 F-FDG is phosphorylated. The retention
products of FDG metabolism are consistent with the amount of
glucose consumed in the cell. Therefore,18F-FDG may
reflect the utilization of glucose in vivo. SUV is a
semi-quantitative index that shows the characteristics of
the18 F-FDG tracer uptake, hence approximating the
glucose metabolic rate. Commonly, some factors affecting the SUV
include outlining of the region of interest, focus size, system
resolution, reconstruction algorithm, patient factors (body weight,
lean body weight and body surface area), non-tumor uptake caused by
activating and acquisition time following drug injection. The time
between injection and PET data acquisition in the eligible articles
ranged between 45 and 60 min; that makes SUV closer to the glucose
metabolic rate. However, SUV estimates suffer from poor
reproducibility between centers, due to the lack of standardization
of the acquisition and processing protocols for its assessment. In
our research, this poor reproducibility was proven by the broad
range of threshold values that have been used in the literature to
distinguish between patients with low and high survival. In order
for it to be used as a functional prognostic factor in routine
practice, a single SUV threshold distinguishing between patients
should be agreed on, or an optimization method, used to determine
the threshold for each center, should be established. To set a
unanimous threshold, most variable factors affecting the SUV
estimations should be defused or at least controlled. Reducing the
large variability currently affecting SUV estimates is likely to
enhance the prognostic value of SUV. In our research, we did not
take into consideration the variable conditions under which SUV was
obtained, due to the poor quality scores of the PET reports.
Despite variability in the abovementioned factors, we were able to
demonstrate that SUV was correlated with patient local control.
Indeed, our research design calculated a RR for each study center
to control the deviation of data, based on the SUV threshold used
in the corresponding study, which somehow cancelled the difference
in threshold factors used in different centers. By doing so, we
were able to ignore the difference in SUV values between different
centers and demonstrated that SUV is certainly worth considering as
a prognostic factor.
Literature-based meta-analyses have the advantage of
including published trials immediately available for analysis, with
results that can be readily checked. In our meta-analysis, some
biases may have occured. Although the funnel plot did not indicate
publication bias, bias cannot definitely be ruled out, due to the
small number of the studies and the low power of the test used to
detect publication bias. Certain studies were not included, as
separate data for head and neck cancer patients could not be
obtained. Furthermore, one limitation of the present study is the
lack of data from multicenter and large-scale perspective studies.
To avoid some of the biases of literature-based meta-analyses, we
aim to confirm our results in a meta-analysis of individual patient
data, incorporating unpublished trials and updating results.
In conclusion, this meta-analysis provided evidence
regarding the potential value of FDG uptake, as measured by SUV, in
predicting local control in head and neck carcinomas. We are
currently planning a meta-analysis based on individual patient data
that will potentially reduce biases associated with
literature-based meta-analyses.
Acknowledgements
The authors are indebted to the authors of the
primary studies.
References
|
1
|
Lydiatt WM, Shah JP and Hoffman HTHead
Neck Sites Task Force. American Joint Committee on Cancer: AJCC
stage groupings for head and neck cancer: should we look at
alternatives? A report of the Head and Neck Sites Task Force. Head
Neck. 23:607–612. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osman I, Sherman E, Singh B, et al:
Alteration of p 53 pathway in squamous cell carcinoma of the head
and neck: impact on treatment outcome in patients treated with
larynx preservation intent. J Clin Oncol. 20:2980–2987. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Izzo JG, Papadimitrakopoulou VA, Liu DD,
et al: Cyclin D1 genotype, response to biochemoprevention and
progression rate to upper aerodigestive tract cancer. J Natl Cancer
Inst. 95:198–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ang KK, Berkey BA, Tu X, et al: Impact of
epidermal growth factor receptor expression on survival and pattern
of relapse in patients with advanced head and neck carcinoma.
Cancer Res. 62:7350–7356. 2002.PubMed/NCBI
|
|
5
|
Rohren EM, Turkington TG and Coleman RE:
Clinical applications of PET in oncology. Radiology. 231:305–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minn H, Clavo AC, Grenman R and Wahl RL:
In vitro comparison of cell proliferation kinetics and uptake of
tritiated fluorodeoxyglucose and L-methionine in squamous-cell
carcinoma of the head and neck. J Nucl Med. 36:252–258.
1995.PubMed/NCBI
|
|
7
|
Haberkorn U, Strauss LG, Reisser C, et al:
Glucose uptake, perfusion, and cell proliferation in head and neck
tumors: relation of positron emission tomography to flow cytometry.
J Nucl Med. 32:1548–1555. 1991.PubMed/NCBI
|
|
8
|
Vansteenkiste JF, Stroobants SG, Dupont
PJ, De Leyn PR, Verbeken EK, Deneffe GJ, Mortelmans LA and Demedts
MG: Prognostic importance of the standardized uptake value
on18F-fluoro-2-deoxy-glucose-positron emission tomography scan in
non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung
Cancer Group. J Clin Oncol. 17:3201–3206. 1999.PubMed/NCBI
|
|
9
|
Berghmans T, Dusart M, Paesmans M, et al
European Lung Cancer Working Party for the IASLC Lung Cancer
Staging Project: Primary tumor standardized uptake value (SUVmax)
measured on fluorodeoxyglucose positron emission tomography
(FDG-PET) is of prognostic value for survival in non-small cell
lung cancer (NSCLC): a systematic review and meta-analysis (MA) by
the European Lung Cancer Working Party for the I ASLC Lung Cancer
Staging Project. J Thorac Oncol. 3:6–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brun E, Kjellen E, Tennvall J, et al: FDG
PET studies during treatment: prediction of therapy outcome in head
and neck squamous cell carcinoma. Head Neck. 24:127–135. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allal AS, Slosman DO, Kebdani T, Allaoua
M, Lehmann W and Dulguerov P: Prediction of outcome in
head-and-neck cancer patients using the standardized uptake value
of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys.
59:1295–1300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SY, Roh JL, Kim MR, et al: Use
of18F-FDG PET for primary treatment strategy in patients with
squamous cell carcinoma of the oropharynx. J Nucl Med. 48:752–757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roh JL, Pae KH, Choi SH, et al:
2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography as
guidance for primary treatment in patients with advanced-stage
resectable squamous cell carcinoma of the larynx and hypopharynx.
Eur J Surg Oncol. 33:790–795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao CT, Chang JT, Wang HM, et al:
Pretreatment primary tumor SUVmax measured by FDG-PET and
pathologic tumor depth predict for poor outcomes in patients with
oral cavity squamous cell carcinoma and pathologically positive
lymph nodes. Int J Radiat Oncol Biol Phys. 73:764–771. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torizuka T, Tanizaki Y, Kanno T,
Futatsubashi M, Naitou K, Ueda Y and Ouchi Y: Prognostic value
of18F-FDG PET in patients with head and neck squamous cell cancer.
AJR Am J Roentgenol. 192:W156–W160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allal AS, Dulguerov P, Allaoua M,
Haenggeli CA, EI-Ghazi el A, Lehmann W and Slosman DO: Standardized
uptake value of 2-[18F] fluoro-2-deoxy-D-glucose in predicting
outcome in head and neck carcinomas treated by radiotherapy with or
without chemotherapy. J Clin Oncol. 20:1398–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burk D, Woods M and Hunter J: On the
significance of glucolysis for cancer growth, with special
reference to Morris rat hepatomas. J Natl Cancer Inst. 38:839–863.
1967.PubMed/NCBI
|