Introduction
The treatment for advanced prostate cancer is
initially surgical or medical castration, with or without
concomitant use of antiandrogens. Once the cancer becomes
refractory to these treatments or becomes castration-resistant
prostate cancer (CRPC), second-line treatments, such as
chemotherapy, immunotherapy and molecular-targeted therapy, may be
indicated. The National Comprehensive Cancer Network and European
Association of Urology guidelines recommend docetaxel, sipuleucel-T
and novel hormonal drugs targeting endocrine pathways, including
abiraterone acetate and enzalutamide (1, 2).
Docetaxel has been one of the standard chemotherapy treatments for
CRPC since 2004, when the TAX 327 and SWOG 99–16 studies
demonstrated the superiority of docetaxel-based chemotherapy over
mitoxantrone plus prednisone (3,
4).
However, docetaxel has been associated with multiple
adverse events; grade 3 or 4 neutropenia occurred in 32% of the
cases in the TAX 327 study and 93.7% of the cases in a Japanese
phase II study (5). Alopecia and
fatigue are the most common non-hematological adverse effects noted
in ∼ 50–70% of the cases. Nausea, vomiting, diarrhea, nail changes
and neuropathy are also common, occurring in ∼ 30–40% of the cases
(3).
Intermittent treatment is one of the measures
applied to reduce these adverse events; intermittent or suspended
treatment may provide the patients with ‘drug holidays’, which may
also serve as a monitoring phase of therapeutic responses. However,
a limited number of studies have investigated intermittent
docetaxel treatment and no practical intermittency policy has been
reported (6–8). In the present study, we aimed to
establish a regimen for intermittent docetaxel treatment and
prospectively evaluate its feasibility.
Patients and methods
Patient recruitment
This study was approved by the Institutional Ethics
Committee of our hospital (no. 3124). Patients who were scheduled
to receive docetaxel chemotherapy for CRPC were recruited between
January, 2008 and October, 2012. The CRPC status was defined as
>25% increase of the serum prostate-specific antigen (PSA) level
with values ≥ 2.0 ng/ml obtained on two consecutive measurements
taken at least 4 weeks apart (9).
Four weeks were required after the termination of antiandrogen
treatment (6 weeks for bicalutamide) to exclude antiandrogen
withdrawal syndrome.
Treatment regimen
The patients were administered 75 mg/m2
docetaxel intravenously over 1 hour every 3 weeks, with concomitant
administration of 1.0-2.0 mg/day oral dexamethasone. Dexamethasone
was continued at the same dose throughout the course of the
treatment (10). Chemotherapy was
suspended when the serum PSA level decreased to < 4 ng/ml, with
a reduction rate of >50% from the baseline. Treatment was
resumed when serum PSA increased to > 2 ng/ml, with an increase
rate of >50% from the nadir.
Adverse events
The adverse events were prospectively recorded
according to the Common Terminology Criteria for Adverse Events
(CTCAE) 3.0 every 3 weeks in the outpatient clinic. Laboratory
tests included a complete blood count, alkaline phosphatase (ALP),
lactate dehydrogenase and PSA levels.
Statistical analysis
The Chi square test or the t-test were used for
analysis of the patient characteristics. The overall survival was
calculated from the initiation of docetaxel therapy. The log-rank
test and the logistic regression model were used for univariate and
multivariate analyses, respectively. P-values < 0.05 were
considered to indicate statistically significant differences. All
the analyses were performed using the StatMate III software,
version 3.07 (ATMS Co., Ltd., Tokyo, Japan).
Results
Patients and course of treatment
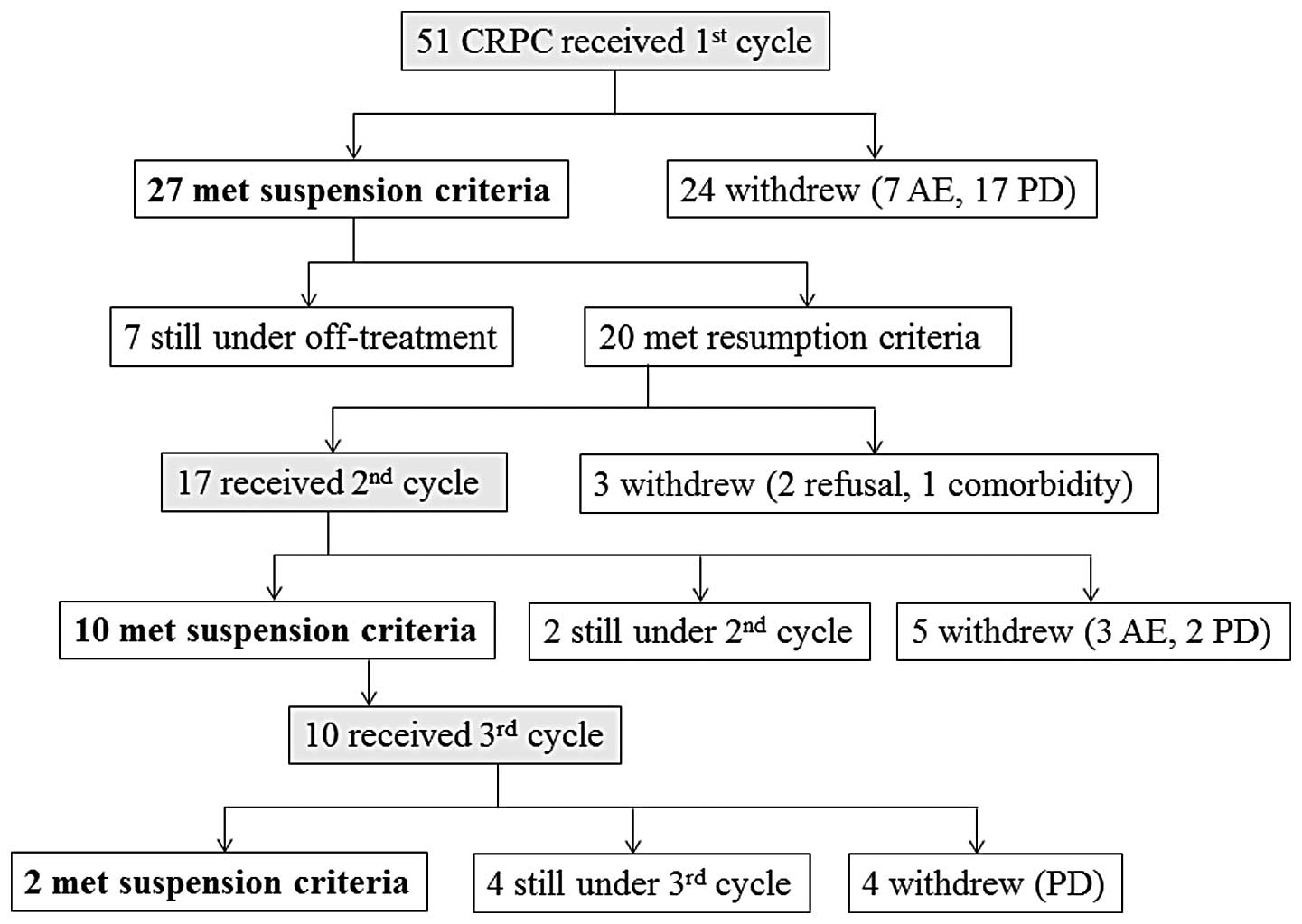
A total of 51 patients were enrolled in this study
and followed up up to January, 2014, with a median observation
period of 19.9 months (range, 0.7-48.6 months). Of the 51 patients,
27 (52.9%) qualified for treatment suspension. Of those 27 patients
who received intermittent therapy (intermittent cases) 7 remained
under off-treatment at the end of study and the remaining 20 met
the resumption criteria. Among the latter 20 subjects, 3 did not
receive docetaxel therapy due to patients' refusal or respiratory
comorbidity, whereas the remaining 17 patients received the second
course of docetaxel therapy. After the second course, 5 patients
withdrew from the regimen due to disease progression or adverse
events, 2 continued with the chemotherapy and 10 entered the second
off-treatment period. All 10 patients who met the suspension
criteria twice received the third course of treatment and
eventually met suspension criteria for the third time (n=2),
continued with the third therapy cycle (n=4) or withdrew due to
disease progression (n=4) (Fig.
1). In total, 17 patients underwent one additional course (two
courses in total) of docetaxel chemotherapy and 10 patients
received two additional courses (three courses in total). The
median duration of the off-treatment interval was 266 days for the
first, 129.5 days for the second and 146.5 days for the third
suspension period.
Characteristics of intermittent and
non-intermittent cases
Intermittent cases tended to be younger at diagnosis
(median age, 67.8 vs. 69.4 years, P=0.058) and less likely to be
diagnosed as Gleason score 8–10 (51.9 vs. 79.2%, P=0.081) compared
to patients who did not meet the suspension criteria
(non-intermittent cases) (Table
I). During prior androgen-deprivation therapy, intermittent
cases had been less frequently exposed to steroids (22.2 vs. 50.0%,
P=0.038) or estrogen (14.8 vs. 41.7%, P=0.032) and attained a lower
nadir of serum PSA (median 0.30 vs. 0.82 ng/ml, P=0.029). Upon
chemotherapy, the serum PSA level decreased to a significantly
lower nadir in intermittent cases (median 8.37 vs. 107.6 ng/ml,
P=0.0004). The dose of docetaxel and relative dose intensity tended
to be higher in the intermittent cases (72.0 vs. 69.3
mg/m2, P=0.087 and 91.5 vs. 84.0%, P=0.064,
respectively).
 | Table I.Characteristics of patients (n=51)
with castration-resistant prostate cancer (CRPC). |
Table I.
Characteristics of patients (n=51)
with castration-resistant prostate cancer (CRPC).
| Characteristics | Intermittent cases
(n=27) | Non-intermittent
cases (n=24) | P-value |
|---|
| Prior to docetaxel
therapy |
| Initial
PSA (ng/ml) | 57.0 (5.80-2,
750) | 217.2 (14.1-5,
407) | 0.422 |
| Age at
diagnosis (years) | 67.8 (54.4-77.7) | 69.4 (53.6-82.0) | 0.058 |
| Gleason
score | 8–10 14 (51.9) | 19 (79.2) | 0.081 |
| Radical
prostatectomy or radiotherapy | 7 (25.9) | 5 (20.8) | 0.923 |
| PSA at
the start of ADT | 42.2 (1.70-2,
750) | 217.8 (0.88-5,
407) | 0.477 |
|
Estramustine use | 11 (40.7) | 13 (54.2) | 0.338 |
| Estrogen
use | 4 (14.8) | 10 (41.7) | 0.032 |
| Steroid
use | 6 (22.2) | 12 (50.0) | 0.038 |
| Nadir PSA
(ng/ml) during ADT | 0.30 (0.00-9.65) | 0.82 (0.00-16.7) | 0.029 |
| PSA
response to prior ADT (%) | 99.7
(79.1-100.0) | 99.1
(88.4-100.0) | 0.933 |
| Time from
diagnosis to CRPC status (months) | 18.2
(0.53-163.4) | 24.2
(5.47-106.3) | 0.408 |
| PSA
doubling time (months) | 1.88 (0.23-16.3) | 2.67 (0.72-22.1) | 0.560 |
| PSA
velocity (ng/ml/months) | 1.38
(0.00-315.5) | 16.6
(0.00-234.8) | 0.112 |
| Time from
diagnosis to docetaxel therapy (months) | 50.4 (7.7-184.7) | 37.5
(15.3-111.4) | 0.190 |
| Upon docetaxel
therapy |
| Age
(years) | 72.0 (55.9-82.5) | 73.9 (59.5-87.0) | 0.281 |
| Karnofsky
performance status ≥ 80% | 26 (96.3) | 23 (95.8) | 0.524 |
| PSA
(ng/ml) | 8.37
(1.80-173.6) | 107.6
(8.21-794.6) | 0.0004 |
| Lactate
dehydrogenase (IU/l) | 219 (116–907) | 266.5 (166-1,
200) | 0.090 |
| Alkaline
phosphatase (IU/l) | 253 (46-4, 348) | 227 (115-3, 462) | 0.905 |
|
Hemoglobin (g/dl) | 12.1 (9.4-14.4) | 11.2 (8.1-14.2) | 0.142 |
| Bone
metastasis | 21 (77.8) | 20 (83.3) | 0.884 |
| Lymph
node metastasis | 17 (63.0) | 15 (62.5) | 0.973 |
|
Analgesics use | 5 (18.5) | 9 (37.5) | 0.669 |
| Docetaxel
therapy |
| Dose
(mg/m2) | 72.0 (52.3-77.8) | 69.3 (26.2-74.9) | 0.087 |
| Relative
dose intensity (%) | 91.5
(62.8-104.7) | 84.0
(32.8-101.8) | 0.064 |
The multivariate analysis incorporating possible
variables indicated low baseline PSA level (<median, 30.55
ng/ml; odds ratio = 16.95, P=0.010) and low Gleason score at
diagnosis (≤ 7; odds ratio = 8.621, P=0.040) as significant factors
for receiving intermittent therapy (Table II).
 | Table II.Multivariate analysis of significant
factors for chemotherapy suspension. |
Table II.
Multivariate analysis of significant
factors for chemotherapy suspension.
| Factors | Odds ratio (95%
CI) | P-value |
|---|
| Age
<68.5a years at
diagnosis | 4.367
(0.690-27.78) | 0.117 |
| Gleason score ≤
7 | 8.621
(1.105-66.67) | 0.040 |
| History of steroid
use | 0.291
(0.044-1.928) | 0.201 |
| History of estrogen
use | 0.429
(0.067-2.741) | 0.371 |
| PSA nadir <0.52
ng/mla during
ADT | 1.047
(0.192-5.714) | 0.957 |
| PSA <30.55
ng/mla at the start
of docetaxel | 16.95
(1.953-142.9) | 0.010 |
| LDH <251
IU/mla at the start
of docetaxel | 2.584
(0.425-15.63) | 0.302 |
| Relative dose
intensity <89.6%a | 0.274
(0.026-2.888) | 0.281 |
Adverse events
During the off-treatment period, leukopenia,
thrombopenia, appetite loss, diarrhea, alopecia, nail changes and
fatigue persisted in none (0.0%), 2 (7.4%), 1 (3.7%), 1 (3.7%), 3
(11.1%), 2 (7.4%) and 3 (11.1%) of the 27 intermittent cases,
respectively (Table III).
However, sensory and/or motor neuropathy persisted in 12 of the 27
cases (44.4%). There were no observed grade 3/4 adverse events
persisting during the chemotherapy-free period.
 | Table III.Adverse events. |
Table III.
Adverse events.
|
| On-treatment
period | Off-treatment
period |
|---|
|
|
|
|
|---|
| Adverse events | All grades, no.
(%) | Grades 3, 4, no.
(%) | Persisting AEs, no.
(%) |
|---|
| Leukopenia | 24 (88.9) | 14 (51.9) | 0 (0.0) |
| Thrombopenia | 6 (22.2) | 0 (0.0) | 2 (7.4) |
| Appetite loss | 6 (22.2) | 0 (0.0) | 1 (3.7) |
| Diarrhea | 4 (14.8) | 0 (0.0) | 1 (3.7) |
| Alopecia | 14 (51.9) | 0 (0.0) | 3 (11.1) |
| Nail changes | 11 (40.7) | 0 (0.0) | 2 (7.4) |
| Fatigue | 6 (22.2) | 1 (3.7) | 3 (11.1) |
| Neuropathy (sensory,
motor) | 14 (51.9) | 0 (0.0) | 12 (44.4) |
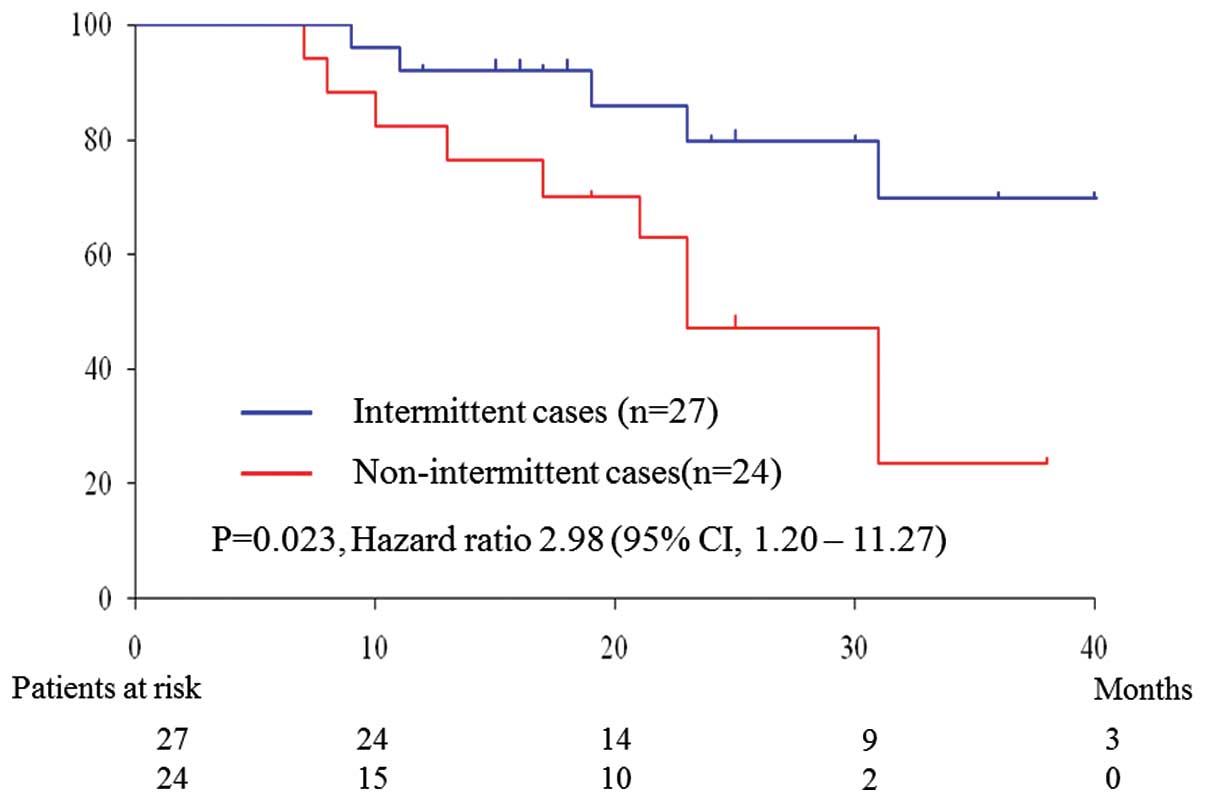
Survival
During a median follow-up period of 19.9 months, 15
patients succumbed to prostate cancer (6 intermittent and 9
non-intermittent cases). The overall survival was significantly
longer in the intermittent cases by the log-rank test (hazard ratio
= 2.98, P=0.023) (Fig. 2).
Discussion
The present study prospectively investigated the
feasibility of a protocol of intermittent docetaxel chemotherapy
for CRPC. Chemotherapy was suspended when serum PSA level decreased
to < 4 ng/ml and the reduction rate was > 50%. The suspension
was maintained as long as serum PSA level remained < 2 ng/ml or
the increase rate from the nadir was < 50%. Once the suspension
criteria were no longer fulfilled, docetaxel chemotherapy was
resumed. The protocol was applicable to 27 of the 51 CRPC patients
(52.9%), with a median off-treatment period of 266 days. During the
drug holidays, the majority of the adverse events, apart from
neuralgia, subsided. The overall survival was significantly longer
among patients who met the suspension criteria.
Previous studies indicated that intermittent
docetaxel chemotherapy was feasible in approximately half of the
cases, although the patients' backgrounds and the criteria
determining docetaxel suspension and resumption differed from the
ones in our study (6–8). Although the planned dose intensities
were similar among the studies (∼ 25 mg/m2/week), the
serum PSA level at the initiation of docetaxel therapy was higher
in the study of Beers et al (median, 104.65 ng/ml) (7), which may explain the lower treatment
suspension rate (16.0%) observed in their study.
Our multivariate analysis identified lower baseline
PSA level as a significant factor for receiving intermittent
therapy. Beer et al (7)
also reported that intermittent cases had a lower PSA level, a
better performance status, a higher hemoglobin concentration and a
lower serum ALP level. These results suggest that cases with less
advanced disease may achieve a better response to docetaxel therapy
and, thus, have a higher chance to undergo intermittent
chemotherapy.
As regards the quality of life (QOL) during the
off-treatment period, Mountzios et al analyzed 35 cases,
using a questionnaire covering several aspects of QOL assessment
and found that all three domains of QOL assessment (general
condition, daily activities and symptom-oriented evaluation)
improved during the chemotherapy-free interval, concluding that the
intermittent docetaxel therapy was a clinically viable therapeutic
strategy (8). The authors of that
study also reported that sensory neuropathy occurred in 18% of
their cases, but only mentioned that all treatment-related
toxicities subsided during the chemotherapy-free period (8).
Our study prospectively monitored adverse events
using CTCAE and confirmed that all the adverse events, apart from
motor and sensory neuropathy, fully subsided in almost all the
cases. When reviewing studies on docetaxel therapy for breast
cancer, Alken et al concluded that peripheral neuropathy may
only be managed with appropriate dose interruptions and scheduling
(11).
Our study was performed in a single institution and
our sample number was limited. Further prospective studies of a
larger size or with comparative arms are required to determine the
clinical feasibility and significance of the intermittent therapy
for CRPC patients.
Our intermittent regimen of docetaxel chemotherapy
was found to be feasible for CRPC, since it may reduce adverse
events without compromising the oncological outcome.
References
|
1
|
http://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccess
date. 20th–November. 2014
|
|
2
|
http://www.uroweb.org/guidelines/Access date.
20th–November. 2014
|
|
3
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I,
Rosenthal MA and Eisenberger MA: Role: TAX 327
InvestigatorsDocetaxel plus prednisone or mitoxantrone plus
prednisone for advanced prostate cancer. N Engl J Med.
351:1502–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
Benson MC, Small EJ, Raghavan D and Crawford ED: Docetaxel and
estramustine compared with mitoxantrone and prednisone for advanced
refractory prostate cancer. N Engl J Med. 351:1513–1520. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naito S, Tsukamoto T, Koga H, Harabayashi
T, Sumiyoshi Y, Hoshi S and Akaza H: Docetaxel plus prednisolone
for the treatment of metastatic hormone-refractory prostate cancer:
a multicenter p hase II trial in Japan. Jpn J Clin Oncol.
38:365–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soga N, Kato M, Nishikawa K, Hasegawa Y,
Yamada Y, Kise H, Arima K and Sugimura Y: Intermittent docetaxel
therapy with estramustine for hormone-refractory prostate cancer in
Japanese patients. Int J Clin Oncol. 14:130–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beer TM, Ryan CW, Venner PM, Petrylak DP,
Chatta GS, Ruether JD, Chi KN, Young J and Henner WDASCENT (AIPC
Study of Calcitriol Enhancing Taxotere) Investigators: Intermittent
chemotherapy in patients with metastatic androgen-independent
prostate cancer: results from ASCENT, a double-blinded, randomized
comparison of high-dose calcitriol plus docetaxel with placebo plus
docetaxel. Cancer. 112:326–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mountzios I, Bournakis E, Efstathiou E,
Varkaris A, Wen S, Chrisofos M, Deliveliotis C, Alamanis C,
Anastasiou I, Constantinides C, Karadimou A, Tsiatas M,
Papadimitriou C, Bamias A and Dimopoulos MA: Intermittent docetaxel
chemotherapy in patients with castrate-resistant prostate cancer.
Urology. 77:682–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
The Japanese Urological Association, the
Japanese Society of Pathology and Japan Radiological Society, .
General rule for Clinical and Pathological Studies on Prostate
Cancer. 4th. Kanehara & Co., Ltd.; Tokyo: 2010
|
|
10
|
Kume H, Suzuki M, Fujimura T, Fukuhara H,
Enomoto Y, Nishimatsu H, Ishikawa A and Homma Y: Docetaxel as a
vital option for corticosteroid-refractory prostate cancer. Int
Urol Nephrol. 43:1081–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alken S and Kelly CM: Benefit risk
assessment and update on the use of docetaxel in the management of
breast cancer. Cancer Manag Res. 5:357–365. 2013.PubMed/NCBI
|