Introduction
Colorectal cancer (CRC) is the third most common
cause of malignancy in men and women in the United States. The
prognosis is poor for patients with metastatic colorectal cancer
(mCRC) and the 5-year survival rate for them is ~12% (1). Standard first-line chemotherapy regimens
for mCRC include 5-fluorouracil (5-FU)/leucovorin/oxaliplatin
(FOLFOX) and 5-FU/leucovorin/irinotecan (FOLFIRI) (2). These two have incrementally led to
improved overall response rates (ORR), progression-free survival
(PFS) and overall survival (OS) in first-line regimens. However,
the GERCOR study, which evaluated the efficacies of FOLFOX and
FOLFIRI as first- and second-line therapies in patients with mCRC,
demonstrated that the clinical benefit was greatly reduced with
second-line treatment (3). More
effective options are required to further improve outcomes.
As a key factor of tumor growth and metastasis, the
vascular endothelial growth factor (VEGF) regulates normal and
pathological angiogenesis, and activates multiple signaling
networks that promote endothelial cell growth, migration and
vascular permeability (4). A
clinically validated therapeutic strategy to target the VEGF
signaling axis in patients has been demonstrated with advanced
mCRC. The VEGF monoclonal antibody bevacizumab (Avastin®;
Genentech, San Francisco, CA, USA), has demonstrated a clinical
benefit in patients with mCRC when combined with chemotherapy in a
randomized, phase III study, in which the addition of bevacizumab
to oxaliplatin, fluorouracil and leucovorin (FOLFOX4) significantly
prolonged PFS [7.3 vs. 4.7 months; hazard ratio (HR), 0.61;
P<0.0001] and OS (12.9 vs. 10.8 months; HR, 0.75; P=0.0011)
compared with FOLFOX4 alone (5).
VEGF receptors (VEGFR) tyrosine kinase inhibitors
(TKIs), such as cediranib and axitinib, have shown antitumor
activity in patients with mCRC. Cediranib is an oral, highly potent
VEGF TKI with activity against all three VEGFRs (6,7). A
randomized, phase III study (HORIZON II) of cediranib +
FOLFOX/CAPOX versus placebo + FOLFOX/CAPOX for mCRC demonstrated
that the addition of cediranib to chemotherapy prolonged PFS, but
did not significantly improve OS (8).
Axitinib, a potent and selective second-generation inhibitor of
VEGFRs 1–3 (9), has shown promising
single-agent activity against a variety of tumor types, including
metastatic renal cell carcinoma, melanoma, thyroid cancer and
non-small-cell lung cancer (10–14). As
opposed to bevacizumab, it specifically binds VEGF-A, and cediranib
and axitinib act directly at VEGFR 1–3 and may result in a more
complete blockade of VEGF signaling. Several RTCs have been
conducted to investigate efficacy and toxicity of VEGFR TKIs versus
bevacizumab in combination with chemotherapy in patients with mCRC.
However, the conclusions are not consistent. Therefore, the present
meta-analysis was performed to evaluate the randomized controlled
trials (RCTs) and compare the efficacy and toxicity of VEGFR TKIs
plus chemotherapy with bevacizumab plus chemotherapy in patients
with mCRC.
Materials and methods
Search criteria
PubMed, Embase and the Central Registry of
Controlled Trials of the Cochrane Library were searched for all the
relevant trials on or before September 2014, which compared
efficacy and toxicity of VEGFR TKIs with bevacizumab in combination
with chemotherapy in patients with mCRC. The following keywords
were used: ‘Advanced colorectal cancer’ OR ‘metastatic colorectal
cancer’ AND ‘randomized controlled trial’ AND ‘bevacizumab’ AND
‘VEGFR TKIs’ OR ‘cediranib’ OR ‘axitinib’ OR ‘sunitinib’. Abstracts
presented at the annual meeting of the American Society of Clinical
Oncology (ASCO) and the European Society for Medical Oncology
(ESMO) were also searched, and the reference lists of all the
identified relevant studies for this topic were manually
examined.
The inclusion criteria were as follows: i) Patients
with histologically confirmed mCRC; ii) RCTs; iii) experimental and
control groups treated by VEGFR TKI and bevacizumab respectively,
and experimental group treated by VEGFR TKI plus the chemotherapy,
while control group received bevacizumab plus the chemotherapy, and
not confounded by additional biological agents or interventions;
iv) trials should be explicit regarding numbers of cases in
experimental and control groups, as well as the cases that finished
the trials; and v) clinical index included PFS, OS, ORR and adverse
events (AEs).
The exclusion criteria were: i) Trials that included
patients with major comorbidities or second tumors were excluded;
ii) quasi-randomized studies that were considered to possess
insufficient quality; and iii) trials included adjuvant
chemotherapy within 6 months or concomitant interventions were
excluded.
Quality assessment
Quality of study methodology was scored using the
methods reported by Jadad et al (15) and Kjaergard et al (16). This is a five-point scale, with one
point awarded for each quality criterion (17).
Data extraction and statistical
analysis
Two investigators (Y.L Huang and F. Lu)
independently extracted the data from all the included studies
according to the inclusion criteria listed. Disagreements were
resolved by discussion with an independent expert (Y.L. Yang). The
following information was sought: First author, year of
publication, number of patients, number of patients eligible for
response, gender rate, mean age, ORR, median OS and PFS, and data
on AEs/toxicities, such as hypertension, vomiting, diarrhea,
fatigue and neutropenia, thrombocytopenia and peripheral
neuropathy.
Meta-analysis was carried out by RevMan 5.0 provided
by the Cochrane Collaboration (Oxford, UK). HR for PFS and OS,
relative risks (RR) for ORR and AEs with 95% confidence intervals
(CI) were calculated. To test the statistical heterogeneity for
each trial, a Cochrane's Q test was performed, and if P<0.1, the
assumption of homogeneity was considered invalid and the random
effect model was used. HR>1 for PFS and OS indicated that the
anti-VEGF TKI treatment group derived more progression or
fatalities. RR>1 for ORR and AEs indicated that the anti-VEGF
TKI treatment group derived more overall response or more
toxicities. The potential presence of publication bias was
evaluated visually by inspecting funnel plots and statistically
using the Egger's test.
Results
Included studies
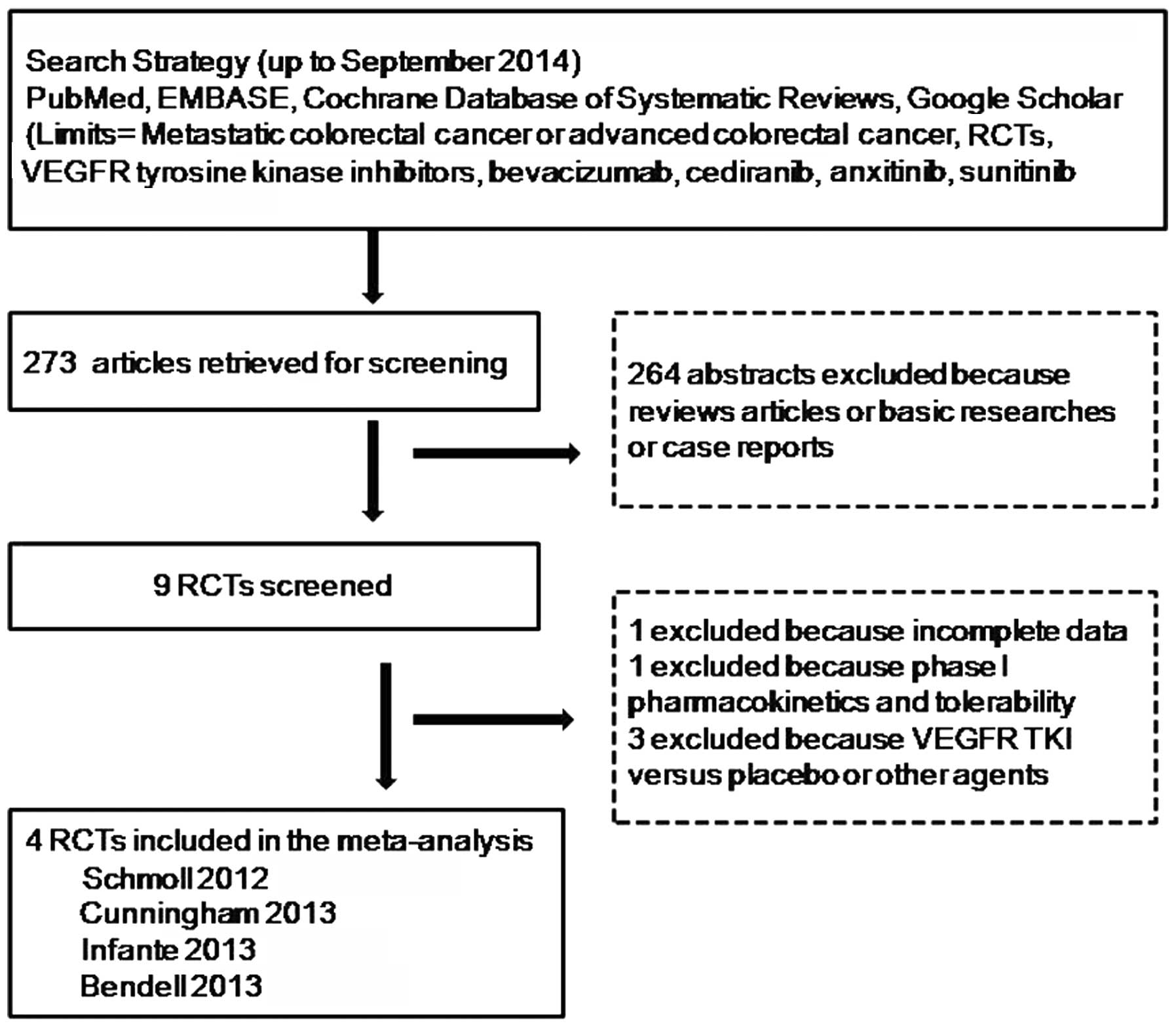
A total of 273 potentially relevant citations were
reviewed, and following exclusion of 264 as they were reviews
studies, basic researches or case reports, 9 potential RCTs were
identified and the full text for each study was screened. Of these,
5 studies were excluded due to incomplete data, phase I
pharmacokinetics and tolerability or irrelevant data to compare
VEGFR TKI with bevacizumab in mCRC. Finally, 4 randomized trials
were eligible for inclusion in the meta-analysis. The study search
process is shown in Fig. 1. Four
trials with a total of 1,929 intent-to-treat patients included in
the meta-analysis were RCTs, and the full text was published in
English. Two were double-blind, phase III RCTs (18,19) and 2
were open-label, phase II RCTs (20,21). The
main characteristics of all the eligible RCTs are listed in
Table I. The trial conducted by
Cunningham et al (18)
compared cediranib (20 and 30 mg once daily) plus mFOLFOX6 with
bevacizumab plus mFOLFOX6, respectively. Only the comparative data
between the cediranib 20-mg group and the bevacizumab group were
included in the analysis in order to reduce heterogeneity. The
trial conducted by Bendell et al (21) compared axitinib/FOLFIRI and
axitinib/mFOLFOX6 with FOLFIRI alone and mFOLFOX6 alone,
respectively. Therefore, the trial was included in the analysis as
2 independent studies (Bendell-1 2013 and Bendell-2 2013).
 | Table I.Characteristics of patients in RCTs
included in this meta-analysis. |
Table I.
Characteristics of patients in RCTs
included in this meta-analysis.
| First author,
year | Phase | Patients in control
arm | Patients in
experiment arm | Therapies | Males, %/median age,
years | PS 0–1, % exp
vs.control | PFS, exp vs.
control | OS, exp vs.
control | ORR, % exp vs.
control | (Refs.) |
|---|
| Cunningham, 2013 | II | Be, n=66 | Ce (20 mg), n=71 | Ce (20 mg) + mFOLFOX6
vs. Be + mFOLFOX6 | 64.2/55.6 | 97 vs. 97 | 5.8 vs. 7.8 months
(20 mg Ce); HR, 1.28 (P=0.29) | 14.3 vs. 19.6 months
(20 mg Ce); HR, 1.39 (P=0.10) | 18.3 vs. 27.3 | (18) |
|
|
|
| Ce (30 mg), n=73 | Ce (30 mg) + mFOLFOX6
vs. Be + mFOLFOX6 |
| 97 vs. 97 | 7.2 vs. 7.8 months
(30 mg Ce); HR, 1.17 (P=0.79) | 16.8 vs. 19.6 months
(30 mg Ce); HR, 1.00 (P=0.88) | 19.2 vs. 27.3 |
|
| Schmoll, 2012 | III | Be, n=713 | Ce (20 mg),
n=709 | Ce (20 mg) + mFOLFOX6
vs. Be + mFOLFOX6 | 58.0/59.5 | 100 vs. 100 | 9.9 vs. 10.3 months;
HR, 1.10 (P=0.12) | 22.8 vs. 21.3 months;
HR, 0.94 (P=0.55) | 46.0 vs. 47.0 | (19) |
| Infante, 2013 | II | Be, n=43 | Ax, n=42 | Ax + mFOLFOX6 vs. Be
+ mFOLFOX6 | 62.7/61.3 | 91 vs. 93 | 11.0 vs. 15.9 months;
HR, 1.08 (P=0.57) | 18.1 vs. 21.6 months;
HR, 1.16 (P=0.69) | 28.6 vs. 48.8 | (20) |
|
|
|
| Ax + Be, n=41 | Ax + Be + mFOLFOX6
vs. Be + mFOLFOX6 |
| 95 vs. 93 | 12.5 vs. 15.9
months; HR, 1.49 (P=0.87) | 19.7 vs. 21.6
months; HR, 0.94 (P=0.41) | 28.6 vs. 48.8 |
|
| Bendell, 2013 | II | Be + FOLFIRI,
n=51 | Ax + FOLFIRI,
n=49 | Ax + FOLFIRI vs. Be
+ FOLFIRI | 57.3/58.9 | 100 vs. 100 | 5.7 vs. 6.9 months;
HR, 1.27 (P=0.83) | 12.9 vs. 15.7
months; HR, 1.36 (P=0.88) | 24.5 vs. 23.5 | (21) |
|
|
| Be + mFOLFOX6,
n=35 | Ax + mFOLFOX6,
n=36 | Ax + mFOLFOX6 vs.
Be + mFOLFOX6 |
| 100 vs. 100 | 7.6 vs. 6.4 months;
HR, 1.04 (P=0.55) | 17.1 vs. 14.1
months; HR, 0.69 (P=0.12) | 19.4 vs. 20.0 |
|
The mean Jadad score was 3.3 for the included
studies (Table II). All the trials
were randomized, but only 1 described the methods of randomization.
Two were double-blind, phase III trials and 2 were open-label,
phase II trials; all 4 trials reported their withdrawals and
dropouts.
 | Table II.Jadad score calculation for included
studies. |
Table II.
Jadad score calculation for included
studies.
| Jadad score
calculation parameters | Cunningham et
al (18) | Schmoll et
al (19) | Infante et
al (20) | Bendell et
al (21) |
|---|
| Was the study
described as randomized (this includes words such as randomly,
random and randomization)? | 1 | 1 | 1 | 1 |
| Was the method used
to generate the sequence of randomization described and appropriate
(such as table of random numbers and computer-generated)? | 0 | 0 | 1 | 0 |
| Was the study
described as double blind? | 1 | 1 | 0 | 0 |
| Was the method of
double blinding described and appropriate (such as identical
placebo, active placebo and dummy)? | 1 | 1 | 0 | 0 |
| Was there a
description of withdrawals and dropouts? | 1 | 1 | 1 | 1 |
| Total | 4 | 4 | 3 | 2 |
All the studies included in the meta-analysis were
approved by the institutional review board or independent ethics
committee of each participating center, followed the guiding
principles of the Declaration of Helsinki and good clinical
practice, and complied with all local laws and regulations. All the
patients provided written informed consent prior to enrolment.
Efficacy of VEGFR TKIs plus
chemotherapy versus bevacizumab plus chemotherapy in metastatic
colorectal cancer
PFS
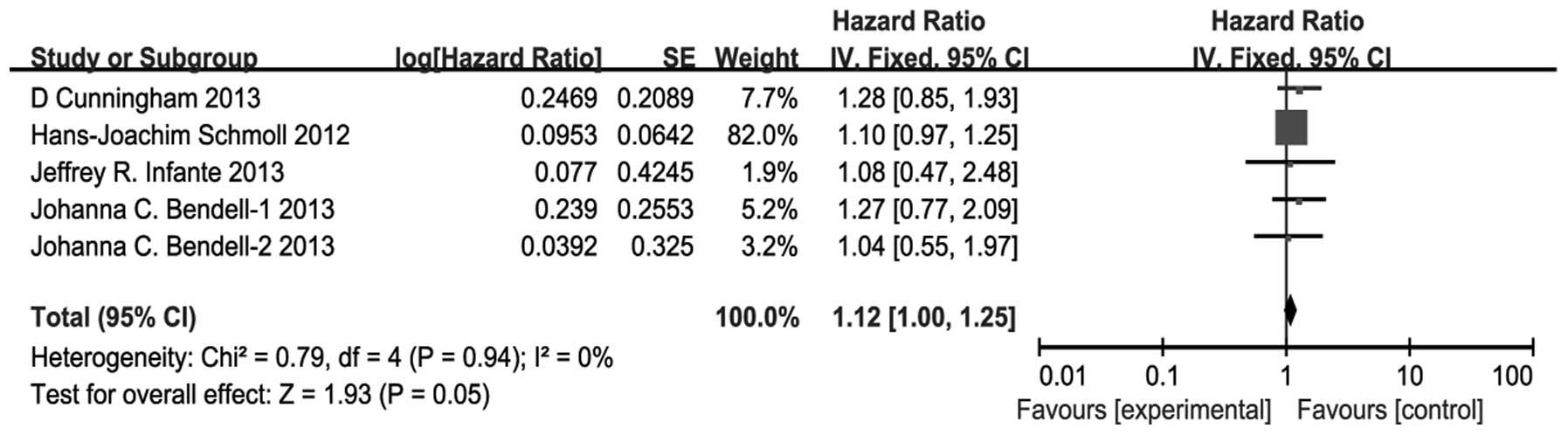
The combination of VEGFR TKIs and chemotherapy
resulted in a significant decline in PFS compared with bevacizumab
plus chemotherapy (HR, 1.12; 95% CI, 1.00–1.25; P=0.05) (Fig. 2). There was no significant
heterogeneity (P=0.94, I2=0%), and the pooled HR for PFS
was performed using the fixed-effect model.
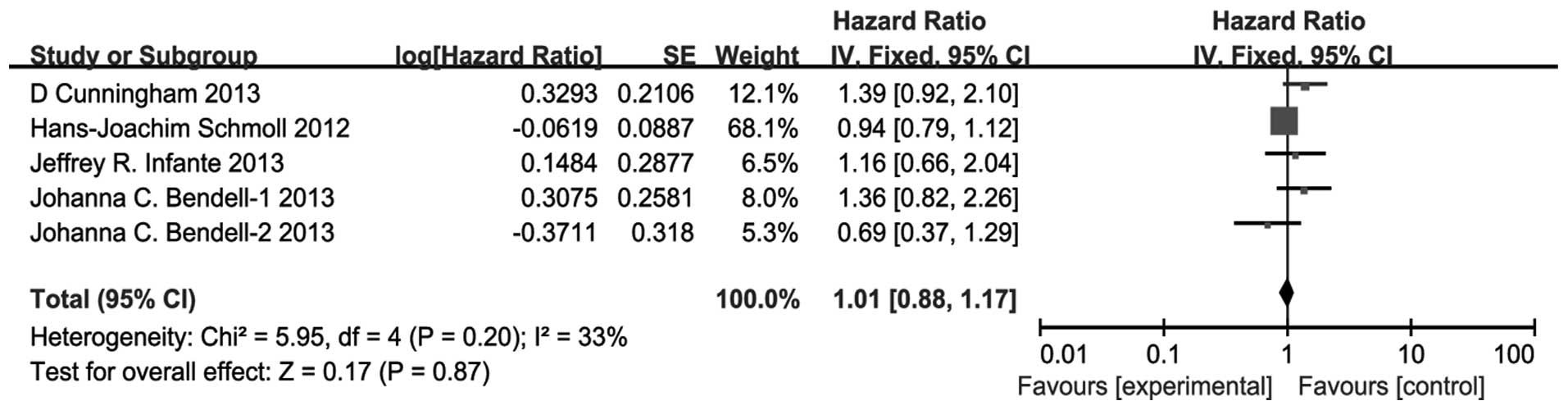
OS
There was no significant difference between the
VEGFR TKIs and bevacizumab groups for the pooled HR for OS (HR,
1.01; 95% CI, 0.88–1.17; P=0.87) (Fig.
3). There was no significant heterogeneity (P=0.20,
I2=33%) and the pooled HR for OS was also performed
using the fixed-effect model.
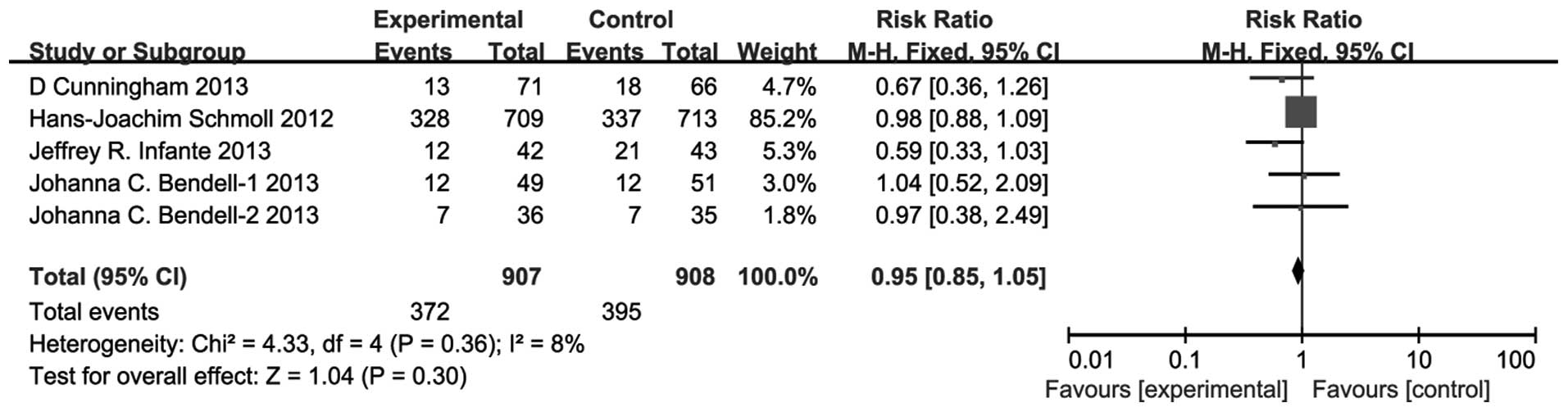
ORR
There was no significant difference between the
VEGFR TKIs group and bevacizumab group for the pooled RR for ORR
(RR, 0.95; 95% CI, 0.85–1.05; P=0.30) (Fig. 4). There was no significant
heterogeneity (P=0.36, I2=8%) and the pooled HR for OS
was also performed using the fixed-effect model.
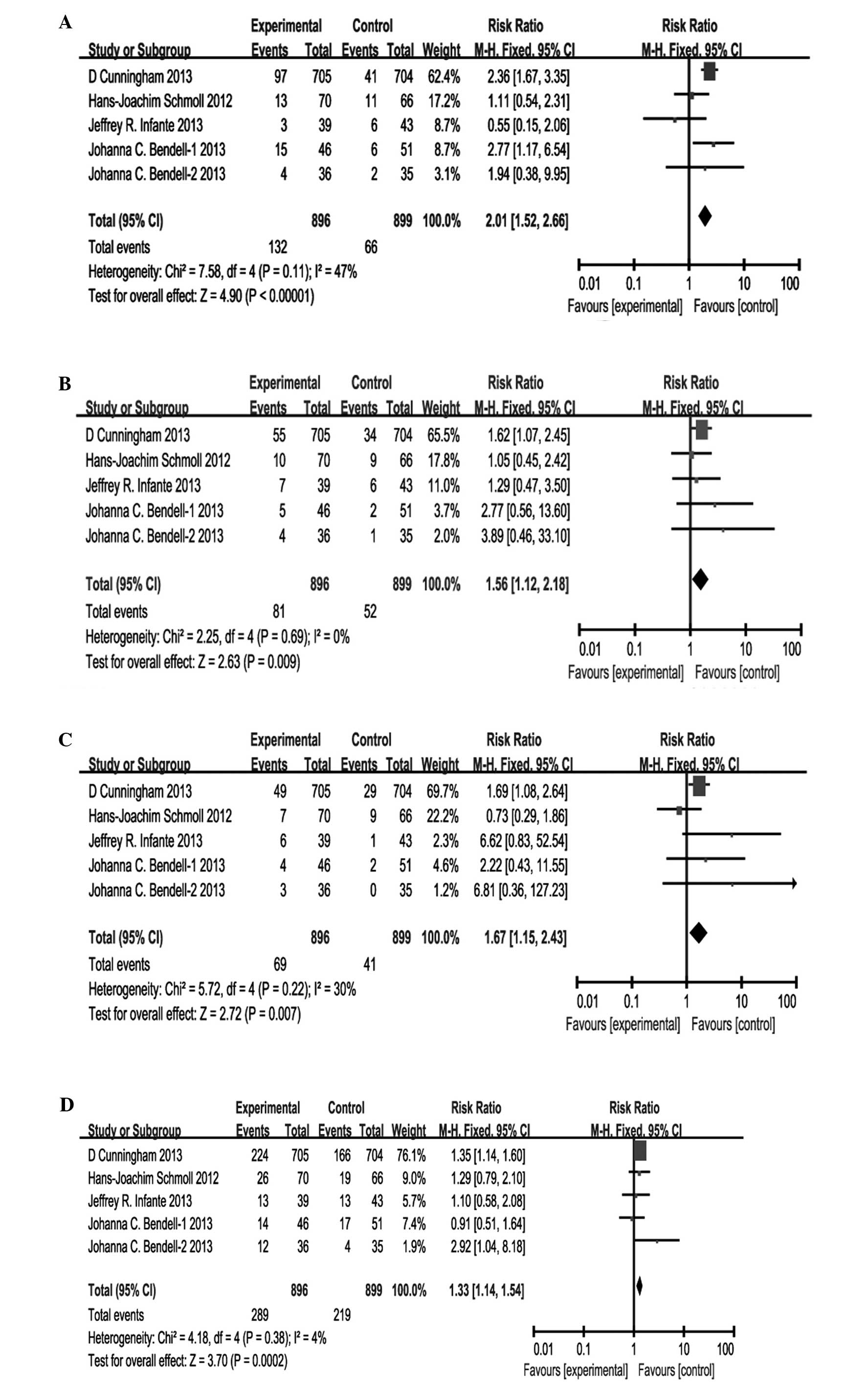
Toxicities of anti-VEGFR TKIs plus
chemotherapy versus bevacizumab plus chemotherapy in metastatic
colorectal cancer
Reported grade-III/IV adverse reactions in these 4
studies included diarrhea, fatigue, hypertension, neutropenia,
peripheral neuropathy, thrombocytopenia, vomiting and abdominal
pain. All studies reported diarrhea, fatigue, hypertension,
neutropenia, thrombocytopenia and peripheral neuropathy in
patients, while 4 reported vomiting and abdominal pain. The
comparison of grade-III/IV adverse reactions between the VEGFR TKIs
and bevacizumab groups are shown in Fig.
5. The statistically significant differences in pooled
estimates suggest a higher incidence of grade-III/IV diarrhea (RR,
2.01; 95% CI, 1.52–2.66; P<0.00001), grade-III/IV fatigue (RR,
1.56; 95% CI, 1.12–2.18; P=0.009), grade-III/IV hypertension (RR,
1.67; 95% CI, 1.15–2.43; P=0.007), grade-III/IV neutropenia (RR,
1.33; 95% CI, 1.14–1.54; P=0.0002) and grade-III/IV
thrombocytopenia (RR, 2.44; 95% CI, 1.48–4.02; P=0.0005) associated
with the anti-VEGFR TKI group, particularly for diarrhea and
thrombocytopenia. However, the statistically significant
differences in pooled estimates suggest a higher incidence of
grade-III/IV peripheral neuropathy (RR, 0.72; 95% CI, 0.52–0.99;
P=0.05) associated with the bevacizumab group. No statistically
significant differences were noted in the incidence of grade-III/IV
vomiting (RR, 0.96; 95% CI, 0.41–2.25; P=0.92) and grade-III/IV
abdominal pain (RR, 1.64; 95% CI, 0.62–4.34; P=0.32).
Discussion
Agents targeting the angiogenic pathway have been
the cornerstone of mCRC treatment in recent years. The survival
benefit of adding bavacizumab to chemotherapy has been demonstrated
in a number of randomized clinical studies, and consequently, the
combination of bevacizumab with FOLFOX is the preferred front-line
regimen amongst US clinicians (22).
Since then, additional randomized studies have shown the antitumor
activity of other VEGF-targeted therapies (23). Recently, several RCTs comparing the
efficacy and toxicity of VEGFR TKIs against bevacizumab have been
reported, but the majority have shown inadequate results. In the
HORIZON III trial, cediranib in combination with mFOLFOX6 showed
comparable clinical activity to bevacizumab plus mFOLFOX6 in
first-line mCRC but failed to meet the predefined boundary for
cediranib PFS non-inferiority. Similarly, in the HORIZON I trial,
cediranib had antitumor activity in patients with previously
treated mCRC, with no statistically significant differences
observed in PFS, OS and ORR comparisons with bevacizumab. However,
Infante et al (20)
demonstrated that neither the addition of continuous axitinib nor
the axitinib/bevacizumab combination to FOLFOX-6 improved ORR, PFS
or OS compared with bevacizumab as first-line treatment of mCRC. In
another study, Bendell et al (21) showed that axitinib did not improve
outcomes when added to second-line chemotherapy compared with
bevacizumab for mCRC. The present meta-analysis focused on the RCTs
comparing the efficacy and toxicity of VEGFR TKIs against
bevacizumab to identify the potentially significant benefit with
the combined treatment regimens.
The results confirmed that VEGFR TKIs plus
chemotherapy significantly resulted in a modest but significantly
shorter PFS (HR, 1.12; P=0.05) compared with bevacizumab plus
chemotherapy. However, there were no statistically significant
differences in OS (HR, 1.10; 95% CI, 0.88–1.17; P=0.87) and ORR
(RR, 0.95; 95% CI, 0.85–1.05; P=0.30) between the treatment arms.
As for the safety profile, the VEGFR TKIs group showed a less
favorable AE profile compared with bevacizumab, with higher rates
of grade-III/IV diarrhea, fatigue, hypertension, neutropenia and
thrombocytopenia, whereas a higher incidence of peripheral
neuropathy was associated with the bevacizumab group.
The efficacy outcomes of bevacizumab in the included
studies, in terms of PFS and OS, were consistent with the earlier
studies of this agent in combination with chemotherapy in first- or
second-line mCRC. Median PFS in the first-line setting [10.3
months, Schmoll et al (19);
15.9 months, Infante et al (20)] was comparable to that observed with
bevacizumab plus FOLFOX4/CAPOX [9.4 months; Van Cutsem et al
(24)], PFS in the second-line
setting [7.8 months, Cunningham et al (18); 6.9 months and 6.4 months, Bendell
et al (21)] was also
comparable to that observed with vatalanib (PTK787/ZK) plus
mFOLFOX6 [5.6 months; Van Cutsem et al (24)]. OS data were similar to the earlier
studies.
Cediranib and axitinib are highly potent VEGFR TKIs
with in vivo activity against all three VEGF receptors and
may result in more complete blockade of VEGF to additive antitumor
activity compared with bevacizumab, however, it was confirmed that
neither the addition of cediranib nor axitinib combination to
chemotherapy improved ORR, PFS or OS compared with bevacizumab as
first- or second-line treatment of mCRC. Furthermore, AEs leading
to discontinuation, dose reduction or dose interruption were
reported more frequently for VEGFR TKIs treatment arms, which may
lead to fewer cycles and lower dose intensity of chemotherapy. The
meta-analysis showed that VEGFR TKIs plus chemotherapy
significantly increased the risk of progression by 12% compared
with bevacizumab (HR 1.12; P=0.05). More tolerability issues and
lower dose intensity of chemotherapy may also be important factors
to consider.
In the present meta-analysis, patients who received
TKIs had higher incidences of grade-III/IV diarrhea, fatigue,
hypertension, neutropenia and thrombocytopenia, which were
consistent with the safety profiles of other VEGFR TKIs (25–27). The
pooled analysis showed that the rate of diarrhea and
thrombocytopenia were more than twice as high with the addition of
VEGFR TKI to chemotherapy, which may be associated with the
antitumor activities of 5-FU/LV. Hypertension may be a class and
common effect of angiogenesis inhibitors although the mechanisms of
hypertension are unclear. There was a higher incidence of
peripheral neuropathy in the bevacizumab group and it may account
for more cycles and a higher dose intensity of chemotherapy
compared with VEGFR TKI. In general, it appears that VEGFR TKIs
plus chemotherapy were not as well-tolerated as bevacizumab-based
regimens and may result in early discontinuations and decreased
dose intensity of all the agents.
Although the previous studies conducted with
additional VEGFR TKIs to chemotherapy have been unsuccessful to
date (24,25,28,29), the
potential use of oral VEGFR TKIs continues to be investigated.
Recently, it was demonstrated that the oral VEGFR TKI regorafenib
improved OS and PFS compared with the placebo in patients with
advanced CRC who had received previous treatment with oxaliplatin
and irinotecan-based chemotherapy (30), which showed promise for treatment of
mCRC. Further investigation with this class of agent is warranted
in pursuit of effective clinical treatment. Markers that are
predictive for response to VEGFR therapy have not yet been
identified but are undergoing investigation, which may reveal a
benefit in select patient populations in the future (31).
In conclusion, based on the results of the present
meta-analysis, VEGFR TKIs plus chemotherapy significantly resulted
in a modest but significantly shorter PFS compared with bevacizumab
plus chemotherapy but not in OS and ORR. VEGFR TKI treatment showed
a less favorable AE profile compared with bevacizumab, with higher
rates of grade-III/IV diarrhea, fatigue, hypertension, neutropenia
and thrombocytopenia, whereas a higher incidence of peripheral
neuropathy was associated with bevacizumab.
Acknowledgements
The authors wish to acknowledge Professor Xuejun Lao
and, in particular, Dr Fang Lu, Dr Yongliang Huang and Dr Junjie
Liang for their support in the development of the study, and Dr
Yiling Yang for the revision of the manuscript.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: National Comprehensive Cancer Network: NCCN
Clinical Practice Guidelines in Oncology: Colon cancer. J Natl
Compr Canc Netw. 7:778–831. 2009.PubMed/NCBI
|
|
3
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9:(Suppl 1).
2–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III:
Eastern Cooperative Oncology Group Study E3200: Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wedge SR, Kendrew J, Hennequin LF,
Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M,
Curwen JO, et al: AZD2171: A highly potent, orally bioavailable,
vascular endothelial growth factor receptor-2 tyrosine kinase
inhibitor for the treatment of cancer. Cancer Res. 65:4389–4400.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drevs J, Medinger M, Mross K, et al: Phase
I clinical evaluation of AZD2171, a highly potent VEGF receptor
tyrosine kinase inhibitor, in patients with advanced tumors. J Clin
Oncol. 23:192s2005.
|
|
8
|
Hoff PM, Hochhaus A, Pestalozzi BC,
Tebbutt NC, Li J, Kim TW, Koynov KD, Kurteva G, Pintér T, Cheng Y,
et al: Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX
in patients with previously untreated metastatic colorectal cancer:
A randomized, double-blind, phase III study (HORIZON II). J Clin
Oncol. 30:3596–3603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin
ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu
EY, et al: Nonclinical antiangiogenesis and antitumor activities of
axitinib (AG-013736), an oral, potent, and selective inhibitor of
vascular endothelial growth factor receptor tyrosine kinases 1, 2,
3. Clin Cancer Res. 14:7272–7283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen EE, Rosen LS, Vokes EE, Kies MS,
Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, et
al: Axitinib is an active treatment for all histologic subtypes of
advanced thyroid cancer: Results from a phase II study. J Clin
Oncol. 26:4708–4713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fruehauf J, Lutzky J, McDermott D, Brown
CK, Meric JB, Rosbrook B, Shalinsky DR, Liau KF, Niethammer AG, Kim
S, et al: Multicenter, phase II study of axitinib, a selective
second-generation inhibitor of vascular endothelial growth factor
receptors 1, 2, and 3, in patients with metastatic melanoma. Clin
Cancer Res. 17:7462–7469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Wilding G, Hudes G, Stadler WM,
Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L and Dutcher JP: Phase
II study of axitinib in sorafenib-refractory metastatic renal cell
carcinoma. J Clin Oncol. 27:4462–4468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rixe O, Bukowski RM, Michaelson MD,
Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo
J, et al: Axitinib treatment in patients with cytokine-refractory
metastatic renal-cell cancer: A phase II study. Lancet Oncol.
8:975–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schiller JH, Larson T, Ou SH, Limentani S,
Sandler A, Vokes E, Kim S, Liau K, Bycott P, Olszanski AJ, et al:
Efficacy and safety of axitinib in patients with advanced
non-small-cell lung cancer: Results from a phase II study. J Clin
Oncol. 27:3836–3841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kjaergard LL, Villumsen J and Gluud C:
Reported methodologic quality and discrepancies between large and
small randomized trials in meta-analyses. Ann Intern Med.
135:982–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomised trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cunningham D, Wong RP, D'Haens G,
Douillard JY, Robertson J, Stone AM and Van Cutsem E: HORIZON I
study group: Cediranib with mFOLFOX6 vs. bevacizumab with mFOLFOX6
in previously treated metastatic colorectal cancer. Br J Cancer.
108:493–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmoll HJ, Cunningham D, Sobrero A,
Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky
G, Mainwaring P, et al: Cediranib with mFOLFOX6 versus bevacizumab
with mFOLFOX6 as first-line treatment for patients with advanced
colorectal cancer: A double-blind, randomized phase III study
(HORIZON III). J Clin Oncol. 30:3588–3595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Infante JR, Reid TR, Cohn AL, Edenfield
WJ, Cescon TP, Hamm JT, Malik IA, Rado TA, McGee PJ, Richards DA,
et al: Axitinib and/or bevacizumab with modified FOLFOX-6 as
first-line therapy for metastatic colorectal cancer: A randomized
phase 2 study. Cancer. 119:2555–2563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bendell JC, Tournigand C, Swieboda-Sadlej
A, Barone C, Wainberg ZA, Kim JG, Pericay C, Pastorelli D, Tarazi
J, Rosbrook B, et al: Axitinib or bevacizumab plus FOLFIRI or
modified FOLFOX-6 after failure of first-line therapy for
metastatic colorectal cancer: A randomized phase II study. Clin
Colorectal Cancer. 12:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colucci G, Gebbia V, Paoletti G, Giuliani
F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione
L, et al: Gruppo Oncologico Dell'Italia Meridionale: Phase III
randomized trial of FOLFIRI versus FOLFOX4 in the treatment of
advanced colorectal cancer: A multicenter study of the Gruppo
Oncologico Dell'Italia Meridionale. J Clin Oncol. 23:4866–4875.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Cutsem E, Bajetta E, Valle J, Köhne
CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T, et al:
Randomized, placebo-controlled, phase III study of oxaliplatin,
fluorouracil, and leucovorin with or without PTK787/ZK 222584 in
patients with previously treated metastatic colorectal
adenocarcinoma. J Clin Oncol. 29:2004–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hecht JR, Trarbach T, Hainsworth JD, Major
P, Jäger E, Wolff RA, Lloyd-Salvant K, Bodoky G, Pendergrass K,
Berg W, et al: Randomized, placebo-controlled, phase III study of
first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an
oral vascular endothelial growth factor receptor inhibitor, in
patients with metastatic colorectal adenocarcinoma. J Clin Oncol.
29:1997–2003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schutz FA, Je Y and Choueiri TK:
Hematologic toxicities in cancer patients treated with the
multi-tyrosine kinase sorafenib: A meta-analysis of clinical
trials. Crit Rev Oncol Hematol. 80:291–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carrato A, Swieboda-Sadlej A,
Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, Bondarenko I,
Jonker DJ, Sun Y, De la Cruz JA, et al: Fluorouracil, leucovorin,
and irinotecan plus either sunitinib or placebo in metastatic
colorectal cancer: A randomized, phase III trial. J Clin Oncol.
31:1341–1347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabernero J, Garcia-Carbonero R, Cassidy
J, Sobrero A, Van Cutsem E, Köhne CH, Tejpar S, Gladkov O,
Davidenko I, Salazar R, et al: Sorafenib in combination with
oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as
first-line treatment of metastatic colorectal cancer: The RESPECT
trial. Clin Cancer Res. 19:2541–2550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmoll H, Hoff PM, Robertson JD, et al:
Association of baseline CEA, VEGF, and soluble VEGF receptor-2 with
treatment outcomes in two randomized phase III trials of cediranib
in metastatic colorectal cancer (mCRC). J Clin Oncol. 29:(Suppl).
35902011.
|