|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: National Comprehensive Cancer Network: NCCN
Clinical Practice Guidelines in Oncology: Colon cancer. J Natl
Compr Canc Netw. 7:778–831. 2009.PubMed/NCBI
|
|
3
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9:(Suppl 1).
2–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III:
Eastern Cooperative Oncology Group Study E3200: Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wedge SR, Kendrew J, Hennequin LF,
Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M,
Curwen JO, et al: AZD2171: A highly potent, orally bioavailable,
vascular endothelial growth factor receptor-2 tyrosine kinase
inhibitor for the treatment of cancer. Cancer Res. 65:4389–4400.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drevs J, Medinger M, Mross K, et al: Phase
I clinical evaluation of AZD2171, a highly potent VEGF receptor
tyrosine kinase inhibitor, in patients with advanced tumors. J Clin
Oncol. 23:192s2005.
|
|
8
|
Hoff PM, Hochhaus A, Pestalozzi BC,
Tebbutt NC, Li J, Kim TW, Koynov KD, Kurteva G, Pintér T, Cheng Y,
et al: Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX
in patients with previously untreated metastatic colorectal cancer:
A randomized, double-blind, phase III study (HORIZON II). J Clin
Oncol. 30:3596–3603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin
ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu
EY, et al: Nonclinical antiangiogenesis and antitumor activities of
axitinib (AG-013736), an oral, potent, and selective inhibitor of
vascular endothelial growth factor receptor tyrosine kinases 1, 2,
3. Clin Cancer Res. 14:7272–7283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen EE, Rosen LS, Vokes EE, Kies MS,
Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, et
al: Axitinib is an active treatment for all histologic subtypes of
advanced thyroid cancer: Results from a phase II study. J Clin
Oncol. 26:4708–4713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fruehauf J, Lutzky J, McDermott D, Brown
CK, Meric JB, Rosbrook B, Shalinsky DR, Liau KF, Niethammer AG, Kim
S, et al: Multicenter, phase II study of axitinib, a selective
second-generation inhibitor of vascular endothelial growth factor
receptors 1, 2, and 3, in patients with metastatic melanoma. Clin
Cancer Res. 17:7462–7469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Wilding G, Hudes G, Stadler WM,
Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L and Dutcher JP: Phase
II study of axitinib in sorafenib-refractory metastatic renal cell
carcinoma. J Clin Oncol. 27:4462–4468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rixe O, Bukowski RM, Michaelson MD,
Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo
J, et al: Axitinib treatment in patients with cytokine-refractory
metastatic renal-cell cancer: A phase II study. Lancet Oncol.
8:975–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schiller JH, Larson T, Ou SH, Limentani S,
Sandler A, Vokes E, Kim S, Liau K, Bycott P, Olszanski AJ, et al:
Efficacy and safety of axitinib in patients with advanced
non-small-cell lung cancer: Results from a phase II study. J Clin
Oncol. 27:3836–3841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kjaergard LL, Villumsen J and Gluud C:
Reported methodologic quality and discrepancies between large and
small randomized trials in meta-analyses. Ann Intern Med.
135:982–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomised trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
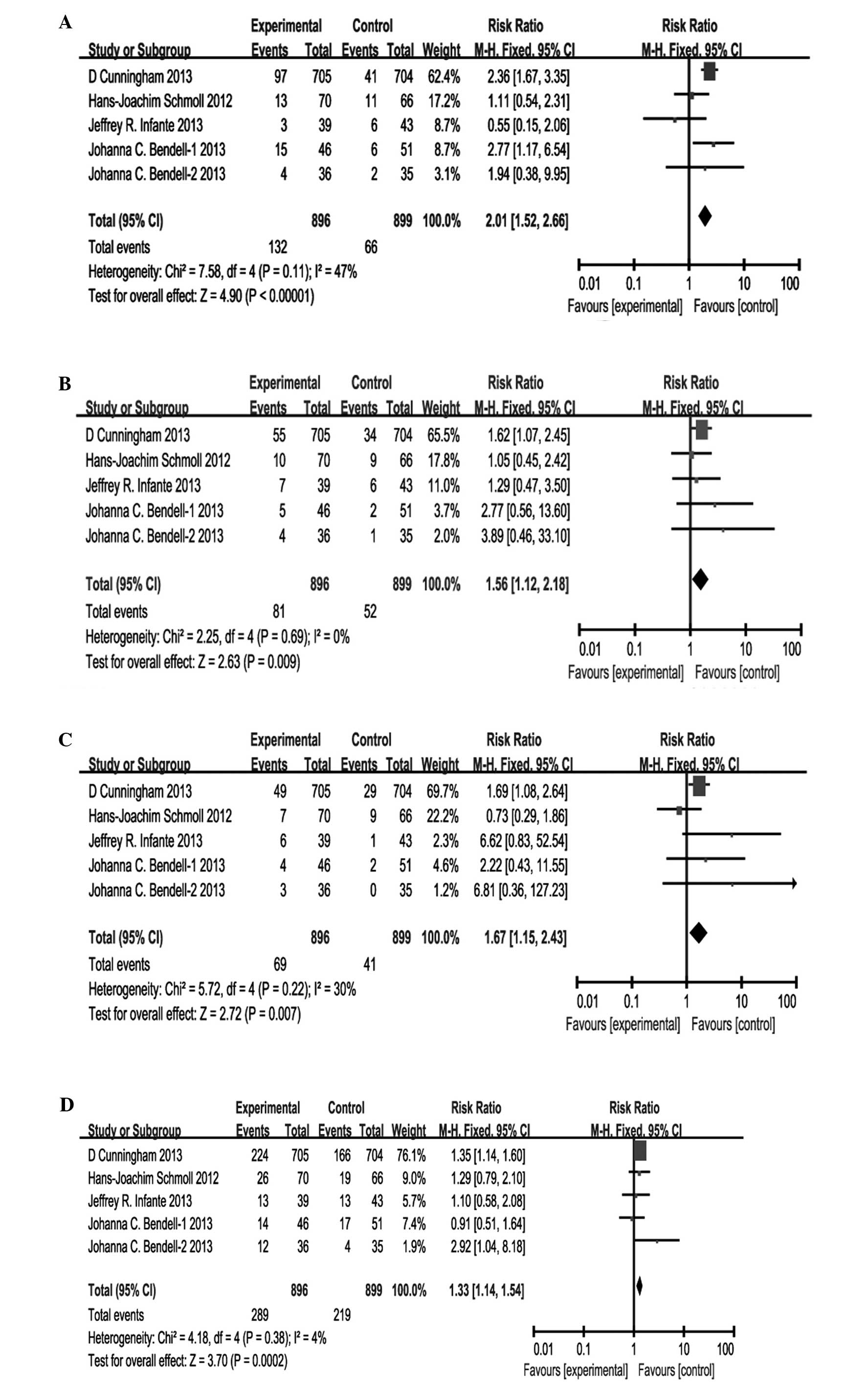
Cunningham D, Wong RP, D'Haens G,
Douillard JY, Robertson J, Stone AM and Van Cutsem E: HORIZON I
study group: Cediranib with mFOLFOX6 vs. bevacizumab with mFOLFOX6
in previously treated metastatic colorectal cancer. Br J Cancer.
108:493–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmoll HJ, Cunningham D, Sobrero A,
Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky
G, Mainwaring P, et al: Cediranib with mFOLFOX6 versus bevacizumab
with mFOLFOX6 as first-line treatment for patients with advanced
colorectal cancer: A double-blind, randomized phase III study
(HORIZON III). J Clin Oncol. 30:3588–3595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Infante JR, Reid TR, Cohn AL, Edenfield
WJ, Cescon TP, Hamm JT, Malik IA, Rado TA, McGee PJ, Richards DA,
et al: Axitinib and/or bevacizumab with modified FOLFOX-6 as
first-line therapy for metastatic colorectal cancer: A randomized
phase 2 study. Cancer. 119:2555–2563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bendell JC, Tournigand C, Swieboda-Sadlej
A, Barone C, Wainberg ZA, Kim JG, Pericay C, Pastorelli D, Tarazi
J, Rosbrook B, et al: Axitinib or bevacizumab plus FOLFIRI or
modified FOLFOX-6 after failure of first-line therapy for
metastatic colorectal cancer: A randomized phase II study. Clin
Colorectal Cancer. 12:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colucci G, Gebbia V, Paoletti G, Giuliani
F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione
L, et al: Gruppo Oncologico Dell'Italia Meridionale: Phase III
randomized trial of FOLFIRI versus FOLFOX4 in the treatment of
advanced colorectal cancer: A multicenter study of the Gruppo
Oncologico Dell'Italia Meridionale. J Clin Oncol. 23:4866–4875.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Cutsem E, Bajetta E, Valle J, Köhne
CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T, et al:
Randomized, placebo-controlled, phase III study of oxaliplatin,
fluorouracil, and leucovorin with or without PTK787/ZK 222584 in
patients with previously treated metastatic colorectal
adenocarcinoma. J Clin Oncol. 29:2004–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hecht JR, Trarbach T, Hainsworth JD, Major
P, Jäger E, Wolff RA, Lloyd-Salvant K, Bodoky G, Pendergrass K,
Berg W, et al: Randomized, placebo-controlled, phase III study of
first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an
oral vascular endothelial growth factor receptor inhibitor, in
patients with metastatic colorectal adenocarcinoma. J Clin Oncol.
29:1997–2003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schutz FA, Je Y and Choueiri TK:
Hematologic toxicities in cancer patients treated with the
multi-tyrosine kinase sorafenib: A meta-analysis of clinical
trials. Crit Rev Oncol Hematol. 80:291–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carrato A, Swieboda-Sadlej A,
Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, Bondarenko I,
Jonker DJ, Sun Y, De la Cruz JA, et al: Fluorouracil, leucovorin,
and irinotecan plus either sunitinib or placebo in metastatic
colorectal cancer: A randomized, phase III trial. J Clin Oncol.
31:1341–1347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabernero J, Garcia-Carbonero R, Cassidy
J, Sobrero A, Van Cutsem E, Köhne CH, Tejpar S, Gladkov O,
Davidenko I, Salazar R, et al: Sorafenib in combination with
oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as
first-line treatment of metastatic colorectal cancer: The RESPECT
trial. Clin Cancer Res. 19:2541–2550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmoll H, Hoff PM, Robertson JD, et al:
Association of baseline CEA, VEGF, and soluble VEGF receptor-2 with
treatment outcomes in two randomized phase III trials of cediranib
in metastatic colorectal cancer (mCRC). J Clin Oncol. 29:(Suppl).
35902011.
|