Introduction
Gastrointestinal stromal tumor (GIST), the most
common type of non-epithelial tumors, is a term used to describe a
unique group of mesenchymal neoplasms that typically arise in the
muscularis propria of the gastrointestinal tract wall, accounting
for 0.1–3% of all gastrointestinal tumors (1). GISTs may develop synchronously with
tumors originating from different cell layers, such as cancer,
carcinoid and lymphadenoma (2–4). The
synchronous development of a gastric GIST and adenocarcinoma is
relatively rare. The aim of this study was to conduct a
retrospective review of the computed tomography (CT) imaging
characteristics of 18 cases of synchronous GIST and primary gastric
cancer and evaluate the clinicopathological characteristics of
GISTs.
Patients and methods
Patients
The database of the First Affiliated Hospital of
Fujian Medical University was searched and patients who were
treated at our hospital between January, 2006 and April, 2013 were
identified. A total of 26 patients with histologically proven
synchronous GISTs and gastric cancer were investigated. Of these
patients, 8 with GISTs sized <1.0 cm were excluded, as the
preoperative CT images were difficult to characterize with
confidence. Therefore, the study cohort consisted of 18 patients
(12 men and 6 women; mean age, 69.2 years; and age range, 47–82
years). An Institutional Review Board (IRB) exemption and a waiver
of the requirement for written informed consent were obtained to
perform this retrospective study. The patient characteristics are
summarized in Table I.
 | Table I.Characteristics of patients with GISTs
(n=18). |
Table I.
Characteristics of patients with GISTs
(n=18).
| Characteristics | No. |
|---|
| Gender |
|
|
Female | 6 |
| Male | 12 |
| Age, years |
|
| Mean | 69.2 |
|
Range | 47–82 |
| Location of
GISTs |
|
| Gastric
fundus | 9 |
| Body | 3 |
|
Cardia | 2 |
|
Antrum | 2 |
|
Pylorus | 1 |
| Tumor size, cm |
|
| Mean | 2.2 |
|
Range | 1.0–6.5 |
| Stage of primary
gastric cancer |
|
| I or
II | 5 |
| III | 13 |
CT imaging and pathological
analysis
CT imaging was performed using a Toshiba Aquilion
16-slice CT scanner. A total of 18 patients underwent
contrast-enhanced CT within 1 week of the operation. After an
overnight fast, oral contrast, 750–1,000 ml water, or 2% oral
diatrizoate meglumine was administered to all the patients 30 min
prior to scanning. The scan range was determined depending on the
size of the tumor and the distance from the diaphragmatic dome to
the inferior border of the liver. Scanning was performed with 5- or
10-mm slice thickness and interval. The CT parameters were 120 kVp
and 350 mAs. Iopamidol (80–100 ml, 370 mgI/ml) was injected
intravenously at a rate of 3 ml/sec and imaging was performed with
a dual-phase technique. The technique was performed with a late
arterial (portal inflow) phase scan at 32 sec and a hepatic venous
phase scan at 102 sec. Section widths of 2 mm and reconstruction
intervals of 1.25 mm were used. Two radiologists retrospectively
reviewed all radiological studies, with final interpretations by
consensus. Each tumor was assessed for location, density, presence
of calcifications, size, margin, cyst formation, hemorrhage,
necrosis and presence of a tumor capsule. The adjacent organs, fat
planes and stomach wall were assessed for evidence of invasion,
which was suspected when there was focal enlargement of the organ
or anatomic structure in direct continuity with the tumor mass. The
tumors were also assessed for the pattern of attenuation during the
administration of the contrast material.
The medical records were subsequently reviewed to
determine the clinical presentation, therapy and patient course.
The pathology records were also reviewed to determine the
histological and immunohistochemical characteristics of the
lesions, including CD117, CD34, smooth muscle actin (SMA), S-100,
Ki-67, desmin (DES), discovered on GIST-1 (DOG1) and
platelet-derived growth factor receptor α (PDGFRA) mutation status,
when available.
Results
Clinical presentation
The demographic characteristics of 18 patients with
synchronous GISTs are presented in Table
I. The major symptoms at presentation included abdominal pain
(n=10), abdominal distention (n=9), sour regurgitation (n=7),
melena (n=7), nausea and vomiting (n=3), hematemesis (n=3), weight
loss (n=6) or unexplained anemia (n=2). Certain patients presented
with ≥1 of these symptoms. Each patient had a preoperative
histological diagnosis of gastric adenocarcinoma established by
gastroscopy and biopsy. Preoperative gastroscopy revealed a
cancerous ulcer (n=10), soft tissue mass (n=5), pyloric stenosis
and stiffness of the stomach wall (n=3). Five patients had early
and 13 patients advanced gastric cancer. Early gastric cancer is
defined as tumor confined to the mucosa or submucosa, independent
of regional lymph node (LN) metastases, irrespective of the tumor
size.
CT findings
Of the 18 synchronous GISTs, 9 were found in the
gastric fundus (50%), 4 in the body (22%), 2 in the cardia (11%), 2
in the antrum (11%) and 1 in the pylorus (6%) (Table I). The median size ± standard
deviation of the GISTs was 2.2 ± 1.2 cm (range, 1.0–6.5 cm).
All 18 CT scans revealed a solitary,
well-marginated, isodense mass with an ovoid shape that was
inseparable from the adjacent stomach; the contrast-enhanced CT
scans revealed slight to moderate gradual enhancement of these
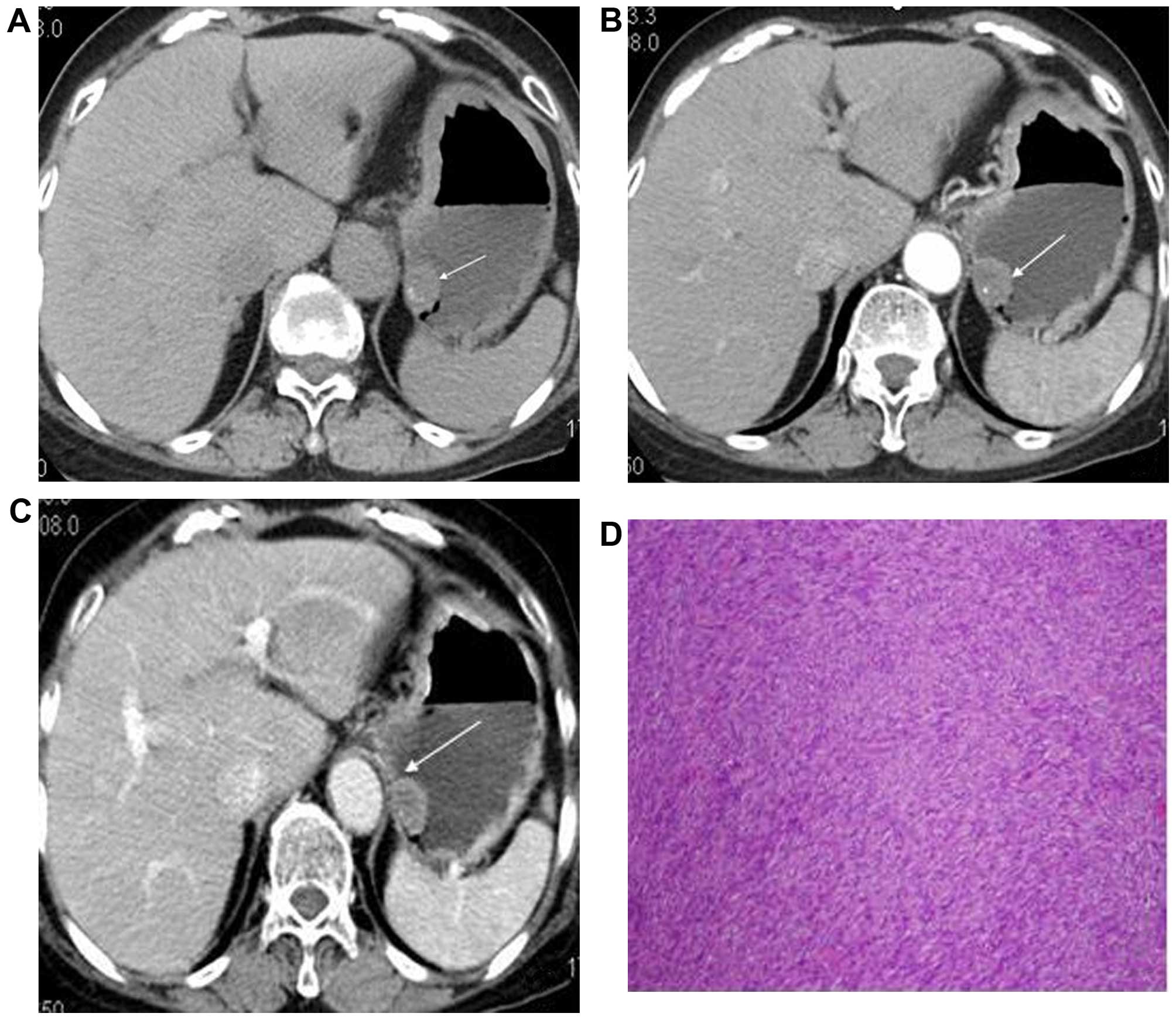
lesions (Fig. 1A–C). The mean
unenhanced value was 36 HU (range, 23–45 HU; n=18) and the mean
contrast-enhanced value was 54 HU (range, 30–68 HU) in the late
arterial (portal inflow) phase; the calculated mean enhancement was
22 HU. The mean contrast-enhanced value was 61 HU (range, 37–77 HU)
in the hepatic venous phase; the calculated mean enhancement was 12
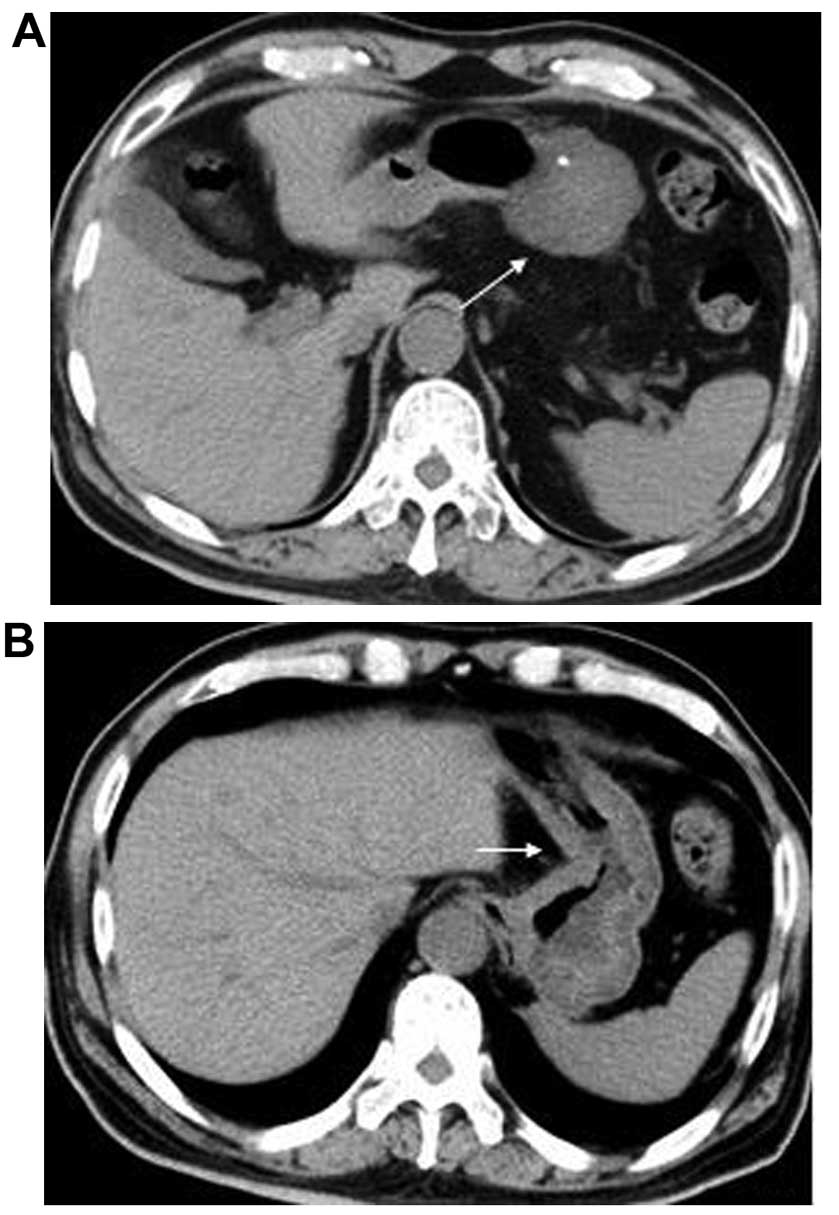
HU. Two GISTs displayed punctate calcifications (Figs. 1A and 2A); the other GISTs were invariably
homogeneous, irrespective of the tumor size. No evidence of
necrosis, cystic degeneration or hemorrhage was observed in any of
the tumors.
Histopathology
All the GISTs exhibited a spindle cell pattern,
without necrosis or cystic changes on histological examination
(Fig. 1D). The histopathological
examination revealed mitotic rates ranging from 0 to 5 mitoses/50
high-power fields (hpf). All 18 tumors were considered of low or
very low malignant potential (Table
II). On immunohistochemical examination, all the tumors
expressed CD34 and the c-kit protein (CD117, + to +++), which is a
type III tyrosine kinase receptor encoded by the c-kit
proto-ongogene. Five synchronous GISTs (28%) exhibited weak focal
SMA positivity, but the remaining 13 (72%) were SMA-negative. No
GISTs were S-100-positive; 4 GISTs were DOG1-positive and only 1
GIST exhibited DES positivity. The Ki-67 expression varied from
0.01 to 0.05 in 16 patients. Three of the resected specimens
underwent molecular genotyping and two PDGFRA mutations were
identified.
 | Table II.Immunohistochemistry and risk
stratification of GISTs. |
Table II.
Immunohistochemistry and risk
stratification of GISTs.
| Case | Size (cm) | Mitoses (/50
hpf) | CD117 | CD34 | DOG1 | PDGFRA | SMA | S-100 | Des | Ki-67 (%) | Risk |
|---|
| 1 | 1.8 | 1 | + | + | + | | + | – | – | +1 | Very low |
| 2 | 1.0 | 0 | + | + | | | + | – | | +1 | Very low |
| 3 | 2.7 | <5 | + | + | + | | – | – | – | +2 | Very low |
| 4 | 1.2 | 0 | – | + | | | – | – | | – | Low |
| 5 | 3.5 | 0 | +++ | +++ | +++ | | – | – | | +2–3 | Very low |
| 6 | 1.2 | 1 | + | + | + | | + | – | | +2 | Very low |
| 7 | 1.9 | 5 | + | + | | | – | – | – | – | Very low |
| 8 | 4.2 | 0 | – | + | | | + | – | + | +2 | Very low |
| 9 | 1.0 | <5 | + | + | | – | – | – | – | +2 | Low |
| 10 | 6.5 | <5 | + | + | | + | – | | | +1 | Low |
| 11 | 1.9 | <5 | + | + | | + | – | – | | +1 | Low |
| 12 | 1.5 | 0 | +++ | +++ | | | + | – | | +1 | Very low |
| 13 | 1.0 | <5 | + | + | | | – | – | – | +5 | Low |
| 14 | 1.3 | Rare | + | + | | | – | – | – | +1 | Very low |
| 15 | 2.8 | 0 | + | + | | | – | – | – | +2 | Very low |
| 16 | 1.0 | 0 | + | + | | | – | – | | +1 | Very low |
| 17 | 1.0 | <5 | + | + | | | – | – | | +1 | Very low |
| 18 | 4.0 | 3 | + | + | | | – | – | | +2 | Low |
Discussion
The coexistence of gastric cancer and GIST is
relatively rare, and GISTs are occasionally detected in the gastric
serosa or mucosa during surgery (3,5–7). Gastric adenocarcinoma (Fig. 2B) and GISTs are different neoplasms,
originating from different cell layers; an accurate pre- and
postoperative diagnosis is important. However, when the GIST is
subserosal or submucosal, the gastric mucosa has not yet been
invaded and endoscopic biopsies may come back as normal.
In the available literature, no case of coexistence
of adenocarcinoma and GIST has been detected preoperatively
(2,3,7), and there
has been no report of CT imaging characteristics of the
simultaneous development of adenocarcinoma and GIST in the stomach.
In fact, in our cases, the preoperative gastroscopy biopsy
fragments only showed adenocarcinoma, and most GISTs were detected
in the resected stomach. Only in 18 patients (18/26, 69%) were the
GISTs identified on preoperative CT.
GISTs <1.0 cm could not be detected on
preoperative CT, as the tumors are difficult to differentiated from
perigastric metastatic LNs and may only confirmed on
histopathological examination. The individual CT characteristics of
GISTs and regional LN enlargement, which is a significantly more
common finding, overlap; therefore, CT cannot reliably
differentiate between the two based on the size or pattern of their
contour alone. Imaging characteristics that may be associated with
LN metastases include multiple lesions, strong enhancement and
hypodense areas.
No evidence of necrosis, hemorrhage, or cystic
degeneration was found in any of the patients, irrespective of the
tumor size (>5 or <5 cm). These CT imaging characteristics
were different from those of conventional GISTs (8). The tumor exhibited homogeneous slight or
moderate enhancement and it was clinically more common in men
compared with women; the median age at the time of diagnosis was
69.2 years in our patients, as previously reported by Fletcher
et al (9).
Immunostaining for CD34, c-kit, SMA and S-100 may be
diagnostically useful. Rabin et al (1) reported that 40–70% of GISTs were
positive for CD34, 20–30% were positive for SMA, and 10% were
positive for S-100 protein; In our study, 100% of the tumors were
positive for CD34 and CD117, and 28% were positive for SMA. The
positivity for CD34 and CD117 markers was higher compared with what
was previously reported.
The biological behavior of GISTs is often difficult
to predict on MSCT. Tumor size and mitotic rate are most frequently
used to predict malignant behavior (10). However, as benign-appearing GISTs
(small size and absent mitoses or low mitotic rate) may recur or
metastasize, a recently published consensus statement suggests
classifying GISTs as very low-, low-, intermediate- and high-risk,
rather than as benign or malignant (9). All the tumors in our study exhibited ≤5
mitoses/50 hpf and were classified as being of low or very low
malignant potential.
In summary, the synchronous occurrence of GISTs and
other primary tumors may be more common than what was previously
considered and they are usually discovered incidentally during
surgery performed for the primary malignancy. Simple coincidence
appears to be the most likely explanation, although gene mutations
or neighboring gastric tissues affected by the same carcinogen are
other hypotheses reported in the literature (2,11,12). A combined genetic deregulation appears
to be involved in the pathogenesis of these two entities. Further
studies are required to elucidate the molecular and genetic
mechanisms underlying carcinogenesis and cancer progression
associating GIST and other synchronous tumors.
Acknowledgements
This study was supported by a grant from the
scientific research programs of Fujian provincial Health and Family
Planning Commission for young scholars (2014-01-45). We are
particularly grateful to Dr Shi Sumeng, for her advice and support
at various stages of the project.
References
|
1
|
Rabin I, Chikman B, Lavy R, Sandbank J,
Maklakovsky M, GoldDeutch R, Halpren Z, Wassermann I and Halevy A:
Gastrointestinal stromal tumors: A 19 year experience. Isr Med
Assoc J. 11:98–102. 2009.PubMed/NCBI
|
|
2
|
Maiorana A, Fante R, Maria Cesinaro A and
Adriana Fano R: Synchronous occurrence of epithelial and stromal
tumors in the stomach: A report of 6 cases. Arch Pathol Lab Med.
124:682–686. 2000.PubMed/NCBI
|
|
3
|
Rauf F, Ahmad Z, Muzzafar S and Hussaini
AS: Synchronous occurrence of gastrointestinal stromal tumor and
gastric adenocarcinoma: A case report. J Pak Med Assoc. 56:184–186.
2006.PubMed/NCBI
|
|
4
|
Al Brahim N, Radhi J and Gately J:
Synchronous epithelioid stromal tumour and lipoma in the stomach.
Can J Gastroenterol. 17:374–375. 2003.PubMed/NCBI
|
|
5
|
Bircan S, Candir O, Aydin S, Başpinar S,
Bülbül M, Kapucuoğlu N, Karahan N and Ciriş M: Synchronous primary
adenocarcinoma and gastrointestinal stromal tumor in the stomach: A
report of two cases. Turk J Gastroenterol. 15:187–191.
2004.PubMed/NCBI
|
|
6
|
Liu SW, Chen GH and Hsieh PP: Collision
tumor of the stomach: a case report of mixed gastrointestinal
stromal tumor and adenocarcinoma. J Clin Gastroenterol. 35:332–334.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto D, Hamada Y, Tsubota Y, Kawakami
K, Yamamoto C and Yamamoto M: Simultaneous development of
adenocarcinoma and gastrointestinal stromal tumor (GIST) in the
stomach: Case report. World J Surg Oncol. 10:62012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ulusan S, Koc Z and Kayaselcuk F:
Gastrointestinal stromal tumours: CT findings. Br J Radiol.
81:618–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, OLeary TJ, Remotti
H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A
consensus approach. Int J Surg Pathol. 10:81–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miettinen M, El Rifai WHL, Sobin L and
Lasota J: Evaluation of malignancy and prognosis of
gastrointestinal stromal tumors: A review. Hum Pathol. 33:478–483.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YL, Tzeng JE, Wei CK and Lin CW: Small
gastrointestinal stromal tumor concomitant with early gastric
cancer: A case report. World J Gastroenterol. 12:815–817.
2006.PubMed/NCBI
|
|
12
|
Andea AA, Lucas C, Cheng JD and Adsay NV:
Synchronous occurrence of epithelial and stromal tumors in the
stomach. Arch Pathol Lab Med. 125:318–319. 2001.PubMed/NCBI
|