Introduction
Pancreatic cancer accounts for ~3.0% of new cancer
cases and is the fourth leading cause of cancer-related mortality,
accounting for 6.9% of all cancer deaths worldwide, with an
increasing incidence (1). The poor
prognosis of pancreatic cancer is mainly attributed to the lack of
early diagnostic tools, resulting in limited treatment options, as
tumor resectability becomes increasingly limited with advancing
cancer stage. Approximately 80% of pancreatic cancers are not
diagnosed until they are locally advanced or have already
metastasized, at which point there is no curative therapy, with a
median survival of only 8–12 or 5–8 months, respectively (2). Further research has been implemented in
identifying potential tumor markers or diagnostic tools to help
physicians recognize the potential presence of pancreatic cancer,
rather than discovering it incidentally on imaging studies at a
more advanced stage. Erythropoietin (EPO) has been found to be a
potential cancer biomarker. Ectopic EPO production has also been
reported in metastatic pancreatic carcinoid tumor (3), pancreatic ductal adenocarcinoma
(4) and polycystic pancreatic
neoplasms (5).
Case presentation
A 59-year-old male patient with a past medical
history of stage IV pancreatic cancer with metastases to the liver
and lung, a history of pulmonary embolism post-inferior vena cava
filter placement, intra-abdominal thrombosis, hypercholesterolemia
and hypertension, presented to the emergency room with complaints
of shortness of breath. The dyspnea had started a few weeks prior,
was progressive and was associated with productive cough with
yellowish sputum. The patient had no chest pain, fever, chills or
palpitations.
The patient had been diagnosed with stage IV
pancreatic cancer in August, 2013. A computed tomography (CT) scan
of the abdomen was conducted at the time for right-sided abdominal
pain, which revealed a focal hypodensity of the body and tail of
the pancreas. These findings were consistent with pancreatic
carcinoma and metastatic disease was also evident, as there were
multiple hepatic lesions. The imaging findings at the time also
included splenic, renal, and left portal vein thrombosis. The
patient underwent fine-needle aspiration of a liver mass, which was
diagnosed as adenocarcinoma; the tumor cells were positive for
cytokeratin (CK)7, CK19 and carbohydrate antigen (CA)19-9, and
negative for CD56, S100, chromogranin, synaptophysin and CK20.
These findings were consistent with a primary pancreatic tumor. The
patient underwent 26 cycles of 48-h chemotherapy over the course of
8 months, at which point he developed resistance and the therapy
was discontinued for 1 month prior to his admission. The patient
was subsequently placed on hospice 2 weeks prior to presentation to
the hospital, which was revoked at the family's request due to a
new drug (Tarceva) three days later. However, due to the cost of
this drug, therapy was put on hold.
On initial physical examination, the patient had a
blood pressure of 147/54 mmHg, a heart rate of 93 bpm, a
respiratory rate of 14/min, a temperature of 97.5°F and a
saturation of 92% on 4 l of oxygen. The patient was found to be in
moderate distress, with bilateral rhonchi that were more prominent
at the bases of the lungs. The extremities exhibited minimal
non-pitting edema. The laboratory findings revealed a mild
leukocytosis of 11.4×109/l, a hemoglobin concentration
of 11.1 g/dl, a hematocrit of 33.4 and a platelet count of
202,000/mm3. The sodium level was 134 mmol/l, potassium
5.1 mEq/L, chloride 92 mEq/L, bicarbonate 26 mmol/l, blood urea
nitrogen 67 mg/dl, and creatinine 8.3 mg/dl. The other initial
laboratory findings were non-significant. The chest X-ray revealed
a bilateral infiltrate, with bilateral pleural effusions. Due to
the presence of anemia and sudden decreases in the hemoglobin
concentration, an anemia workup was conducted. The iron level was
increased to 193 ng/ml, ferritin to 186 ng/ml, haptoglobin to 86
mg/dl, reticulocyte count to 0.4%, iron-binding capacity to 312
µg/dl, whereas the iron saturation was also increased to 62%. The
vitamin B12 level was increased to 1,532 ng/l, the folate level was
within normal limits and the EPO level was increased to 25.2 mU/ml.
On repeat CA19-9 testing, the levels had significantly increased to
2,245 U/ml from the previous value of 962 U/ml at the time of
initial diagnosis of pancreatic cancer.
The patient was admitted to the intensive care unit
due to respiratory distress and acute renal failure; he was
initiated on Zosyn and received one dose of vancomycin. Further
imaging studies revealed not only findings of liver metastasis, but
also metastasis to the lungs, resulting in respiratory distress.
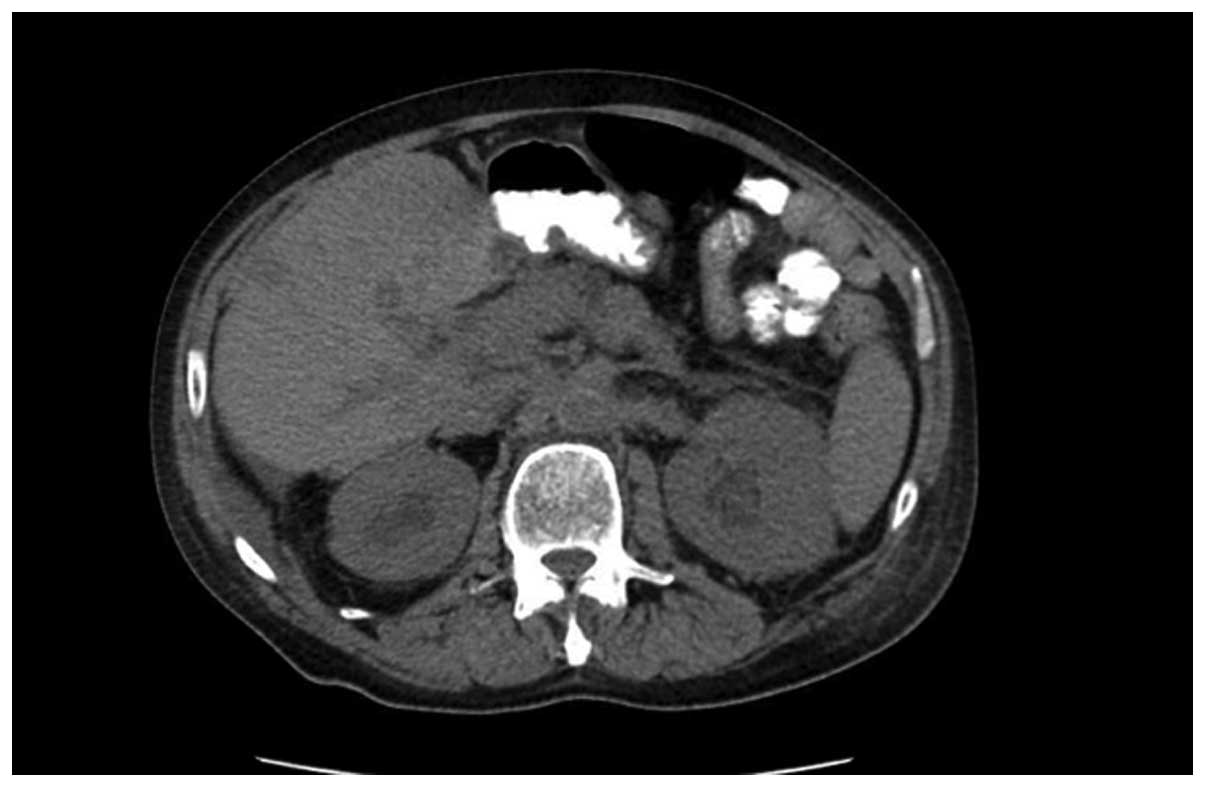
Repeat CT imaging of the chest-abdomen-pelvis two days later
revealed moderate bilateral hydronephrosis and bilateral
perinephric stranding, indicating ureteral obstruction, possibly
associated with metastatic disease; however, a stone or obstructing
mass could not be excluded at the time (Fig. 1). The lungs exhibited bilateral
pulmonary metastatic disease and bilateral pneumonia.
On the following day, the patient had a right
nephrostomy tube placed and the renal function began to improve
steadily. During his hospital course, however, the patient's
condition continued to deteriorate and the patient eventually went
into respiratory failure and required intubation. After a
discussion with the patient's family regarding his prognosis
following failure to wean off the ventilator for 5 days, the family
placed a do-not-resuscitate order and the patient was returned to
hospice care. The patient was transferred to a long-term care
facility where he eventually succumbed to the disease after 1
month.
Discussion
Unlike the mortality rates of other cancers, such as
cancer of the stomach, lungs, colon and prostate, which have been
declining over the past 40 years, the rate of pancreatic cancer has
remained unchanged or increased in elderly patients, signifying
that it is one of the deadliest malignancies (6). Surgically resectable pancreatic cancer
has been shown to be associated with an improved prognosis, as it
is commonly diagnosed at an early stage. However, an early
diagnosis is not always possible, as most symptoms associated with
pancreatic cancer are relatively unspecific. Such symptoms include,
but are not limited to, abdominal pain, weight loss, new-onset
diabetes, jaundice, nausea and vomiting. As it is either
incidentally diagnosed or found at a later stage, when the symptoms
become progressively worse, only 20% of the patients with
pancreatic cancer are candidates for a potentially curative
resection (7).
Pancreatic cancer may develop through several stages
of pancreatic intraepithelial neoplastic lesions that may progress
from benign to malignant. There have been different gene changes
during these phase changes, including K-ras in the early stage, p16
in the intermediate, and p53 in the late stage (8). Although these genetic alterations have
been extensively investigated, their diagnostic significance and
etiology remain unclear. Exosomes and telomeres are promising
markers for early diagnosis; however their clinical utility is
still under investigation (9,10).
As mentioned earlier, tumor markers suitable for
screening are lacking and the available markers are not recommended
for initial testing, even when underlying malignancy is a concern.
The markers that have been implemented include carcinoembryonic
antigen and CA19-9; however these have relatively insignificant
sensitivity and specificity for diagnostic purposes. CA19-9 has
been used to monitor the progression of pancreatic cancer but is
not typically used as an initial diagnostic tool.
As the characteristics and symptoms of pancreatic
cancer are non-specific, identifying sensitive and specific
biomarkers is crucial. An incidental finding of elevated EPO levels
may suggest an underlying malignancy or may be correlated with
pancreatic adenocarcinoma. However, further investigation is
required to correlate the elevated levels of EPO to pancreatic
cancer or any other malignancy.
As seen with a few other documented case
presentations, our patient displayed increased EPO levels as an
incidental finding during a routine anemia workup. EPO is a
glycoprotein produced by peritublar fibroblasts in the renal cortex
and is a regulator of red blood cell formation. The level of EPO is
usually low; however, when there is hypoxia or loss of red blood
cells, it may increase within a few hours due to increased
production by the kidneys. An EPO receptor has been identified in a
number of cells and tissues in the body. However, ectopic forms of
EPO have been shown to be associated with certain malignancies,
which include not only the kidney, but the liver and cerebellum as
well. These malignancies include renal cell carcinoma,
hepatocellular carcinoma, cerebellar hemangioblastoma, ovarian
cancer and pheochromocytoma (5,11). EPO
receptors have also been reported to occur in tumor cells and, as
EPO enhances cell proliferation through cell signaling, an EPO
receptor antagonist may be a potential tool for reducing the tumor
growth. Recently, an elevated EPO level has been found in
pancreatic malignancies, including multicystic neoplasms of the
pancreas (5), metastatic pancreatic
carcinoid tumor (3) and in pancreatic
ductal adenocarcinoma (4). In a
previous study, EPO receptors were identified in breast cancer
cells, while adjacent normal cells did not display any such
receptors (12).
This brings into question whether testing for EPO
receptors should also be implemented in the follow-up, or even the
initial diagnostic workup of other malignancies, such as pancreatic
cancer. Through this case presentation, an association was
demonstrated between elevated EPO level and pancreatic
adenocarcinoma, suggesting that EPO may be used as an early
diagnostic measure or used similarly to CA19-9 to monitor tumor
progression and prognosis. This association between EPO and the
pancreas remains to be fully elucidated, and further investigation
is required to determine its role in tumor growth and its potential
use as a cancer biomarker.
Glossary
Abbreviations
Abbreviations:
References
|
1
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, Lewis DR, Chen HS, Feuer EJ and Cronin KA: SEER Cancer
Statistics Review, 1975–2012, National Cancer Institute. Bethesda,
MD: http://seer.cancer.gov/csr/1975_2012/based on
November 2014 SEER data submission, posted to the SEER web site.
April;2015.
|
|
2
|
Sultana A, Smith CT, Cunningham D,
Starling N, Neoptolemos JP and Ghaneh P: Meta-analysis of
chemotherapy for locally advanced and metastatic pancreatic cancer.
J Clin Oncol. 25:2607–2615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samyn I, Fontaine C, Van Tussenbroek F,
Pipeleers-Marichal M and De Grève J: Paraneoplastic syndromes in
cancer: Case 1. Polycythemia as a result of ectopic erythropoietin
production in metastatic pancreatic carcinoid tumor. J Clin Oncol.
22:2240–2242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawai H, Kojima M, Yokota M, Iguchi H,
Wakasugi H, Jimi A and Funakoshi A: Erythropoietin-producing
pancreatic ductal adenocarcinoma. Pancrease. 21:427–429. 2000.
View Article : Google Scholar
|
|
5
|
Nai Q, Regeti K, Arshed S, Hossain MA,
Zhang P, Luo H, Singh S, Mathew T, Islam M, Sen S, et al: Elevated
erythropoietin and multicystic neoplasm of the pancreas. Case Rep
Oncol. 8:148–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur S, Baine M, Jain M, Sasson AR and
Batra SK: Early diagnosis of pancreatic cancer: Callenges and new
developments. Biomark Med. 6:597–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeo CJ and Cameron JL: Improving results
of pancreaticoduodenectomy for pancreatic cancer. World J Surg.
23:907–912. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maitra A, Kern SE and Hruban RH: Molecular
pathogenesis of pancreatic cancer. Best Pract Res Clin
Gastroenterol. 20:211–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform cell-independent microRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou L, Joyce BT, Gao T, Liu L, Zheng Y,
Penedo FJ, Liu S, Zhang W, Bergan R, Dai Q, et al: Blood telomere
length attrition and cancer development in the normative aging
study cohort. EBioMedicine. 2:591–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bunn HF: Erythropoeitin. Cold Spring Harb
Perspect Med. 3:a0116192013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acs G, Zhang PJ, Rebbeck TR, Acs P and
Verma A: Immunhistochemical expression of erythropoietin and
erythropoietin receptor in breast carcinoma. Cancer. 95:969–981.
2002. View Article : Google Scholar : PubMed/NCBI
|