Introduction
Anaplastic carcinoma of the pancreas (ACP) is a rare
undifferentiated tumor accounting for 2–7% of all exocrine
pancreatic tumors (1). The prognosis
of ACP is poorer compared with that of common pancreatic ductal
adenocarcinoma (PDAC), with a median overall survival of 5.2 months
and a 3-year survival rate of only 3% (1). Conversely, intraductal papillary
mucinous neoplasm (IPMN), which was recently recognized as an
epithelial exocrine neoplasm, is associated with a more favorable
prognosis compared with common PDAC (2,3). Shon
et al (4) previously reported
on IPMNs with associated ACP. However, to the best of our
knowledge, no case of ACP arising in an IPMN has been reported to
date. Herein, we report a unique case of a patient with ACP arising
in an IPMN.
Case report
A 68-year-old Japanese woman was admitted to
Shiroyama Hospital complaining of fatigue in November, 2013. The
laboratory tests showed impaired liver function (aspartate
aminotransferase, 147 IU/l; alanine aminotransferase, 189 IU/l;
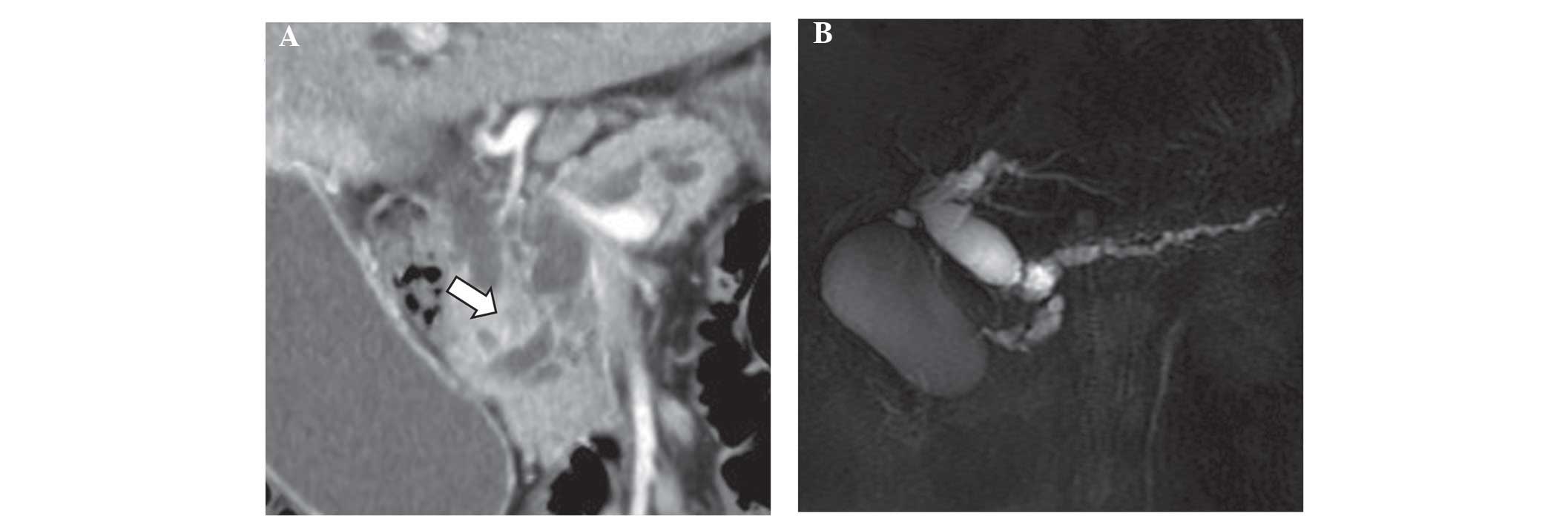
serum amylase, 166 IU/l). A horizontal section on a computed
tomography (CT) scan revealed an irregular mass in the pancreatic
head exhibiting lower enhancement relative to non-tumor pancreatic
parenchyma in the arterial-dominant phase. A coronal section
revealed the presence of cysts in the inferior part of the mass,
and main pancreatic duct distension by ~11 mm (Fig. 1A). Magnetic resonance
cholangiopancreatography revealed stenosis of the main pancreatic
and common bile ducts, caused by the mass-neighboring cysts
(Fig. 1B). The mass at the pancreatic
head displayed high-signal intensity on diffusion-weighted magnetic
resonance imaging (MRI). The CT and MRI scans revealed no evidence
of local or distant metastases, and the superior mesenteric vessels
were not infiltrated by the tumor. The patient was diagnosed with
pancreatic cancer and underwent pancreaticoduodenectomy.
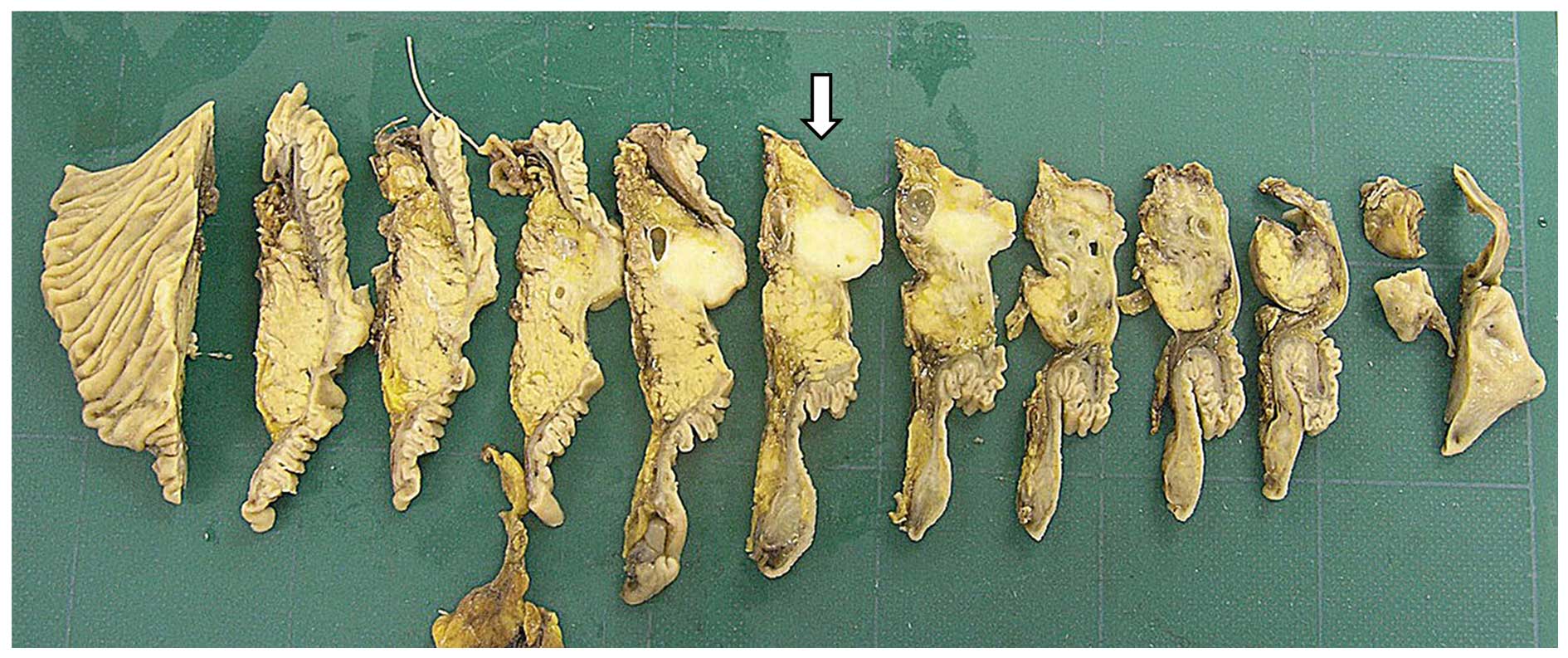
Macroscopically, the cut surface of the invasive tumor was solid
and whitish yellow, measuring 19 mm in diameter (Fig. 2). The histopathological and
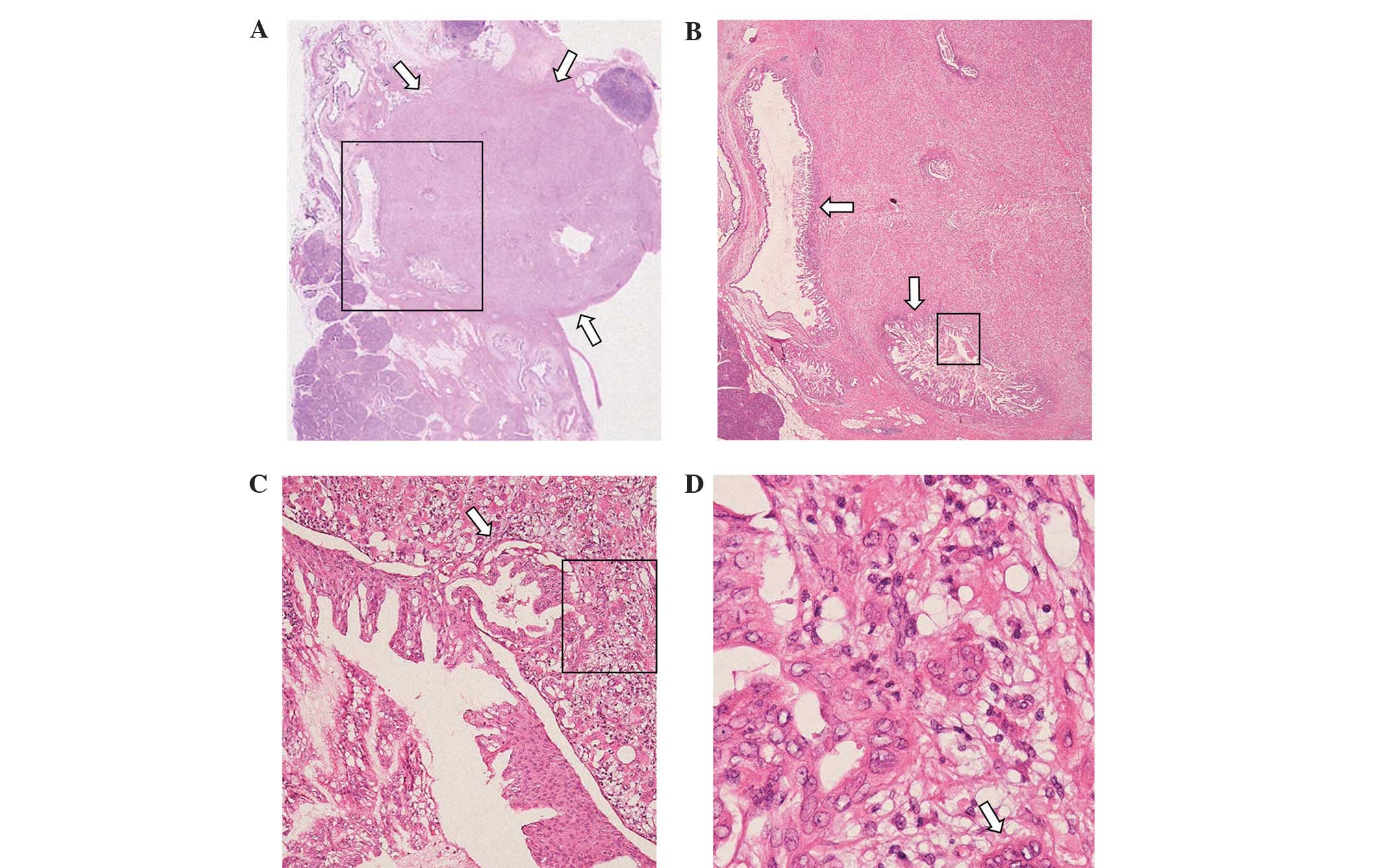
immunohistochemical findings are summarized in Figs. 3 and 4,
respectively. As shown in Fig. 3A and
B, the focus of the invasive carcinoma was located at the
periphery of the IPMN. The pathological diagnosis of the invasive
carcinoma was anaplastic giant-cell carcinoma. The IPMN cells
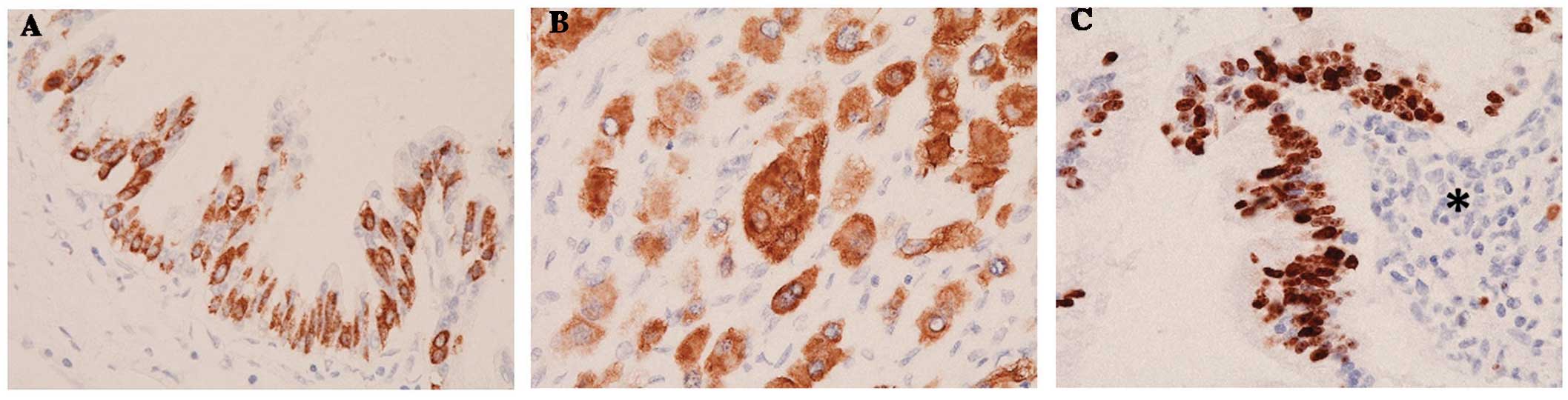
exhibited mucin 2 immunoreactivity (anti-MUC2 monoclonal mouse
antibody; dilution, 1:20; cat. no., 555926; BD Biosciences)
(Fig. 4A). The mono- and multinuclear
cells of the ACP exhibited similar immunoprofiles (monoclonal mouse
antibody against cytokeratin 7; dilution, 1:100; cat. no., M7014;
Dako) (Fig. 4B). The mindbomb E3
ubiquitin protein ligase 1 (MIB-1; Ki-67) mean labeling index was
~5% around the IPMN (Fig. 4C), and
~25% in sites distant from the IPMN (MIB-1 monoclonal mouse
antibody; dilution, 1:150; cat. no., M7240; Dako). However, a part
of the IPMN with high-grade dysplasia exhibited continuous
transition to invasive carcinoma, lacked polarity, and displayed
stratified and pleomorphic nuclei (Fig.
3C); these lesions had an MIB-1 index of ~80%. The final
pathological diagnosis was IPMN (intestinal type, involving the
main and branch duct system), with an associated invasive carcinoma
of the anaplastic giant-cell type. The tumor was classified as
stage III (5). The patient was
prescribed S-1 for 6 months following surgery, and her
postoperative course was uneventful. The patient remains
disease-free at 18 months postoperatively.
Discussion
ACPs are rare and highly aggressive tumors (1), with common symptoms including weight
loss, fatigue, loss of appetite, abdominal pain, nausea and
vomiting. Three histological variants of anaplastic carcinoma,
namely the spindle-, pleomorphic- and giant-cell types, have been
described (5). These carcinomas are
classified as undifferentiated (anaplastic), whereas anaplastic
carcinoma with osteoclast-like giant cells (OGCs) is classified as
a subtype of invasive ductal carcinoma (6). Preoperative diagnosis of ACP is
difficult; on imaging studies, ACPs are usually detected as large,
moderately hypervascular, exophytic tumors, with large areas of
necrosis (1). Anaplastic foci may be
identified in ductal adenocarcinomas and in ectopic pancreatic
tissue, although they are not the dominant pattern of growth
(7). It was previously demonstrated
that OGCs are positive for the histiocytic marker CD68, without
reactivity for epithelial markers (8). In the present case, the tumor giant
cells were positive for cytokeratin 7 and negative for CD68,
suggesting an epithelial origin.
ACP coexisting with IPMN is extremely rare, with
only 6 previously reported cases (4).
However, those cases, in which there were no foci of IPMN
dedifferentiation to ACP, were considered to be ACP coexisting with
IPMN rather than ACP originating in IPMN. PDAC may develop in a
pancreatic duct independently from an IPMN (9,10). When
PDAC originates in the vicinity of an IPMN, the distinction between
PDAC derived from the IPMN and PDAC concomitant with the IPMN may
occasionally be difficult. Definitions of these conditions were
recommender by the Japan Pancreas Society, mainly with regard to
the topological association and histological transition between
IPMN and PDAC (5,10). Due to the evidence of ‘budding and
intruding’ of the IPMN into the ACP, our case is considered to be
an ACP originating in an IPMN. In our case, the foci where the IPMN
dedifferentiated to ACP were located at the periphery of the IPMN.
The primary focus of malignant invasive tumors is usually located
in the center of the developed mass; however, in the present case,
several other foci were present, which were also suspected as
lesions where IPMN dedifferentiated to ACP. Therefore, the
dedifferentiation was possibly multicentric. At the site distant
from the IPMN, the anaplastic cancer cells became larger and
exhibited a higher MIB-1 index, suggesting further
dedifferentiation with increased proliferation and infiltration of
the tumor cells. In addition, the frequent giant cells positive for
cytokeratin 7 support the hypothesis of an epithelial origin and
dedifferentiation of the IPMN.
In conjunction with the finding that a subset of
IPMN-associated invasive carcinomas tend to have a better prognosis
compared with adenocarcinomas arising in the setting of pancreatic
intraepithelial neoplasia (PanIN), an emerging consensus suggests
that there are two major pathways leading to the development of
invasive carcinoma in the pancreas: One that is more aggressive
(via PanIN and pancreatobiliary-type IPMN precursors) and a second
that is more indolent (via intestinal-type IPMN precursors)
(6). It is possible that these major
pathways to invasive carcinoma are applicable to ACP as well. Thus,
the prognosis of ACP derived from IPMN is not necessarily poor.
In conclusion, ACP is associated with a poorer
survival compared with invasive PDAC. Irrespective of the
treatment, the prognosis for this type of tumor remains grave, due
to its aggressive nature and rapid recurrence. IPMNs in the
clinical field of invasive pancreatic carcinoma should not be
overlooked and, in cases of ACP derived from IPMN,
immunohistochemistry should be applied to determine whether they
are of the intestinal type histologically. The case presented
herein may enable a better understanding of the pathogenesis of
ACP.
Acknowledgements
We would like to thank Japan Clinical Laboratories,
Inc. for fostering constructive discussions and scientific
understanding with different medical backgrounds. All the authors
have read and approved this manuscript.
References
|
1
|
Paal E, Thompson DL, Frommelt RA,
Przygodzki RM and Heffes CS: A clinicopathologic and
immunohistochemical study of 35 anaplastic carcinomas of the
pancreas with review of the literature. Ann Diagn Pathol.
5:129–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schnelldorfer T, Sarr MG, Nagorney DM,
Zhang L, Smyrk TC, Qin R, Chari ST and Farnel MB: Experience with
208 resections for intraductal papillary mucinous neoplasm of the
pancreas. Arch Surg. 143:639–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka M, Fernández-del Castillo C, Adsay
V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB,
Schmidt CM, et al: International consensus guidelines 2012 for the
management of IPMN and MCN of the pancreas. Pancreatology.
12:183–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shon TA, Yeo CJ, Cameron JL, Hruban RH,
Fukushima N, Campbell KA and Lillemoe KD: Intraductal papillary
mucinous neoplasms of the pancreas: An updated experience. Ann
Surg. 239:788–797; discussion 797–799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Japan Pancreas Society: General Rules for
the Study of Pancreatic Cancer (6th). Tokyo: Kanehara Shuppan.
1–13. 2013.
|
|
6
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumors of the digestive system.
WHO Classification of Tumours. 3:(4th). (Lyon). IARC Press.
2010.
|
|
7
|
Roshe J, Del Buono E, Domenico D and
Colturi TJ: Anaplastic carcinoma arising in ectopic pancreas
located in the distal esophagus. J Clin Gastroenterol. 22:242–244.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molberg KH, Heffes C, Delgado R and
Albores-Saavedra J: Undifferentiated carcinoma with osteoclast-like
giant cells of the pancreas and periampullary region. Cancer.
82:1279–1287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka M: Controversies in the management
of pancreatic IPMN. Nat Rev Gastroenterol Hepatol. 8:56–60. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano
K and Tanaka M: Intraductal papillary-mucinous tumor of the
pancreas concomitant with ductal carcinoma of the pancreas.
Pancreatology. 2:484–490. 2002. View Article : Google Scholar : PubMed/NCBI
|