Introduction
Since Marx (1) first
reported bisphosphonate (BP)-related osteonecrosis of the jaw
(BRONJ), several cases have been reported worldwide (2). Anti-receptor activator of nuclear factor
κ-B ligand (RANKL) antibodies, including denosumab or
antiangiogenic agents, are also known to cause ONJ (2). Accordingly, the American Association of
Oral and Maxillofacial Surgeons (AAOMS) changed the defined term
BRONJ to medication-related ONJ (MRONJ) in 2014 (2). AAOMS basically recommends conservative
treatment for the majority of MRONJ cases, excluding those of stage
3 disease or those exhibiting a well-defined sequestrum. However,
the optimal treatment strategy remains controversial. In recent
years, previous studies described the effectiveness of extensive
surgery in the early stages of MRONJ (3,4). Our
previous study also observed good outcomes of extensive surgery for
MRONJ (5).
The histopathological findings of BRONJ have been
evaluated in several previous studies (6–8), which
revealed that the viable osteoclasts exhibit the feature of
multinucleated giant cells. These giant osteoclasts are detached
from the smooth bone surface and have lost their resorptive
function (6–8). Furthermore, these abnormal osteoclasts
may persist on the site (9).
Denosumab-related ONJ was first reported in 2010 (10), with only a few previous reports
regarding this being published since then (11–18).
However, reports of ONJ caused by single application of denosumab
are scarce. In addition, none of the above mentioned reports have
described the histopathological features of this condition. Even if
histopathological analysis was performed, viable osteoclasts and
other bone remodeling-related cells, including osteoblasts and
osteocytes were not described, since only the sequestrum, which has
no viable cells, was surgically resected, according to the AAOMS
recommendations for MRONJ (18).
The present study described the clinical and
histopathological features of ONJ caused by single application of
denosumab in two patients who were subsequently treated by
extensive surgery.
Clinical findings
Case 1
A 50-year-old patient was referred to Nagasaki
University Hospital (Nagasaki, Japan). The patient had undergone
extraction of a fractured mandibular left second premolar 1 year
previously. Three weeks after the extraction, treatment with 120 mg
denosumab was administered subcutaneously for bone metastasis from
breast cancer. The serum calcium level prior to denosumab treatment
was 9.1 mg/dl, while that at the first visit to our department was
8.0 mg/dl. The patient had never received BP treatment. The
extracted socket was already covered with oral mucosa and had
remained asymptomatic for a while. However, the patient began
experiencing pain with bone exposure in the left mandible 1 month
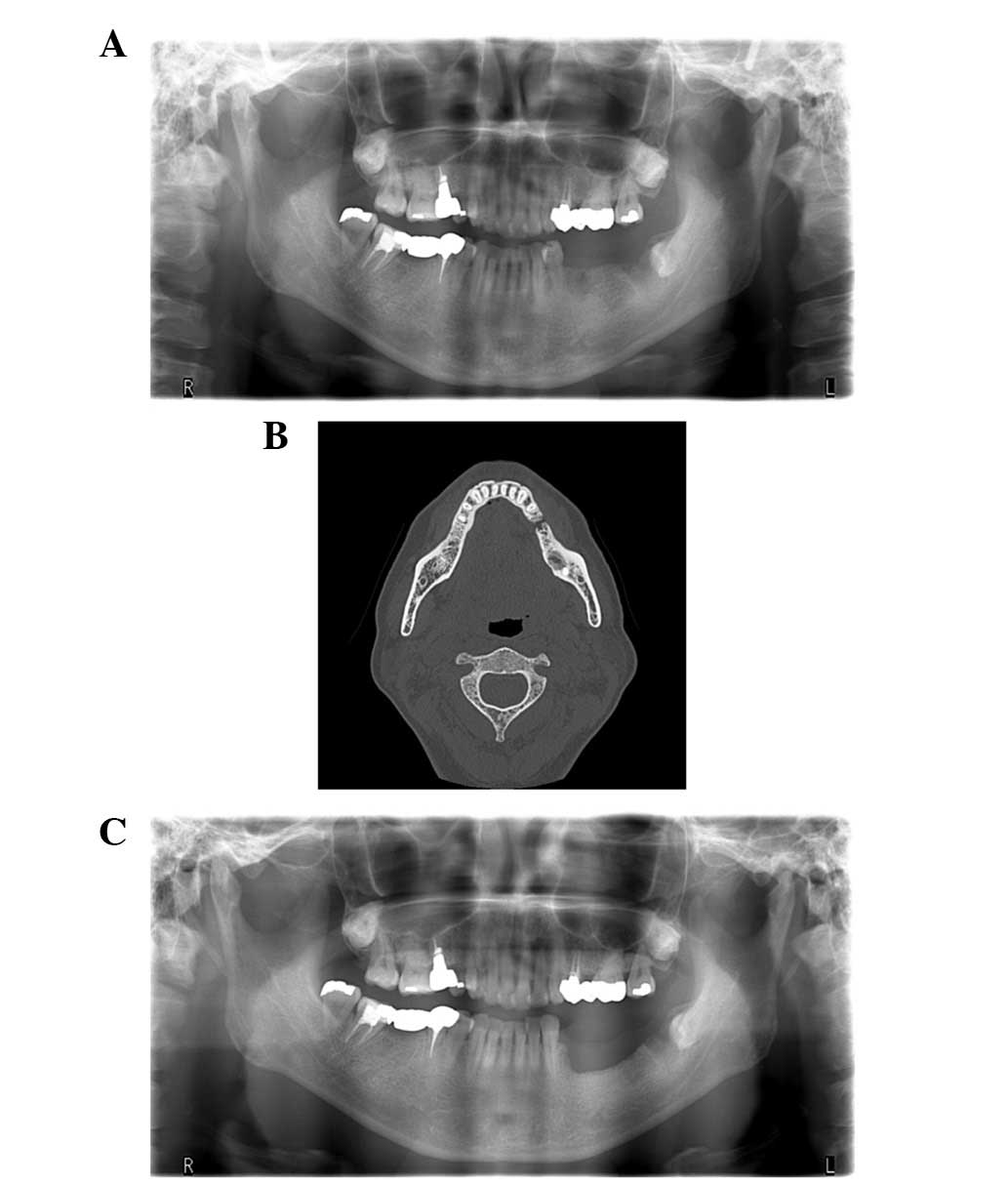
prior to presentation at our department. Panoramic radiographs
showed a bone defect at the site of the mandibular left second
premolar. Computed tomography (CT) revealed bone sclerosis and
sequestrum formation (Fig. 1A and B).
Although penicillin antibiotics were administered for 2 weeks, the
symptoms persisted. A final diagnosis of stage 2 MRONJ was made,
and following consultation with the oncologist, marginal resection,
including the sequestrum, a mandibular left first premolar, and
viable bone around the sequestrum, was performed under general
anesthesia. Denosumab was discontinued for 1 month prior to
surgery. No recurrence occurred during a follow-up period of 7
months following the surgery (Fig.
1C).
Case 2
A 76-year-old patient was referred to Nagasaki
University Hospital. Treatment with 120 mg denosumab was
administered subcutaneously for bone metastasis from prostate
cancer and was initiated 2 years previously. The serum calcium
level prior to denosumab treatment was 8.5 mg/dl, while that at the
first visit to our department was 8.7 mg/dl. The patient had never
received BP treatment and had undergone root canal treatment in the
maxillary left second molar 3 months previously. Although treatment
was completed, the pain and swelling persisted. Therefore, the
patient was referred to our department for further investigations.
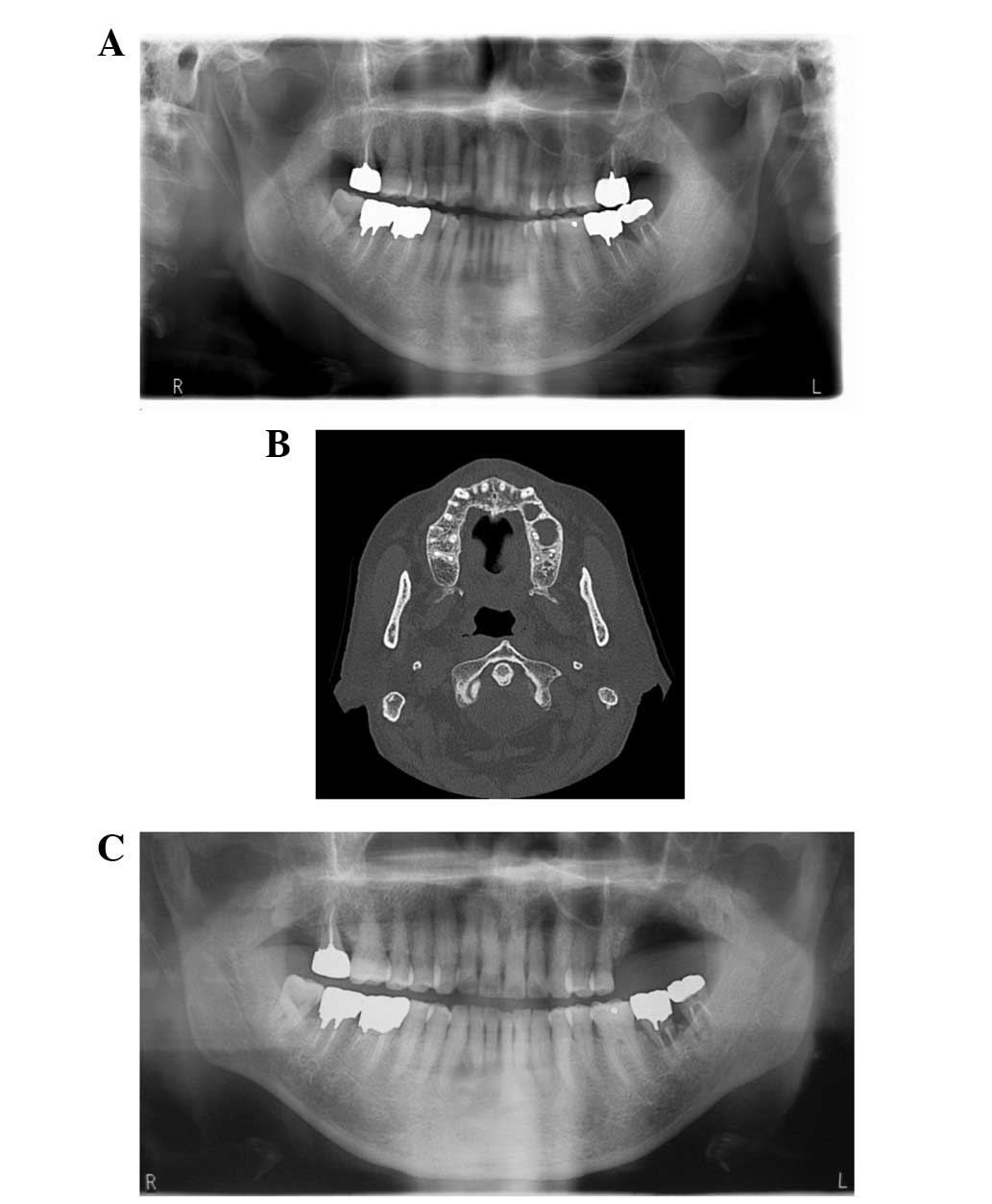
The maxillary left second molar was mobile and there was sequestrum
formation around the tooth. Panoramic radiographs and CT revealed
sequestrum separation and bone sclerosis in the left maxilla, and
thickening of the mucous membrane of the maxillary sinus (Fig. 2A and B). A final diagnosis of stage 2
MRONJ was made, and following consultation with the oncologist,
partial resection was performed under general anesthesia. The
maxillary left first and second molars were extracted, and the
sequestrum and surrounding viable bone were resected. There was no
recurrence during a 6-month follow-up period after surgery
(Fig. 2C).
This study was approved by the institutional review
board of Nagasaki University Hospital and each patient provided
informed consent for publication of this report.
Histopathological findings
The resected surgical segment was subjected to
histopathological analysis, which revealed nearly identical
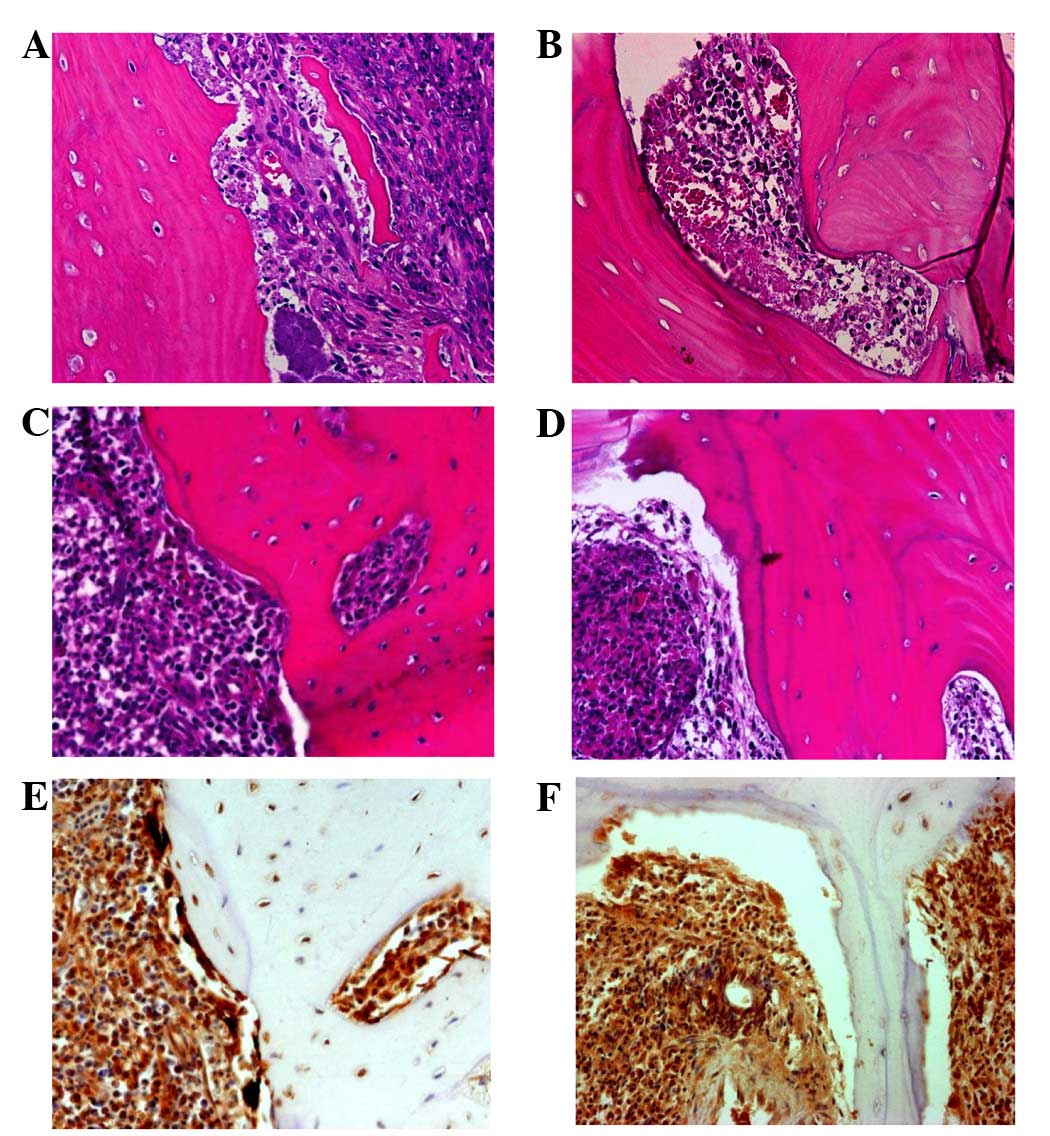
findings in each specimen. Hematoxylin and eosin (HE) staining
revealed sequestrum without viable cells, granulation tissue and
viable bone with inflammation (Fig.
3A–D). In the necrotic bone, granulation tissue, containing
neutrophils, lymphocytes and plasma cells, was observed. A
bacterial mass was attached to the sequestrum, which revealed no
osteoclasts, osteoblasts and osteocytes, with completely necrotic
bone and empty osteocytic lacunae as characteristic findings
(Fig. 3A and B). By contrast, in the
viable bone, osteocytic lacunae, including viable osteocytes were
observed, indicating the viability of the bone in this region
(Fig. 3C and D). Bone resorption
cavities were observed on the surface. However, the surrounding
osteoclasts exhibited specific features, including being few in
number despite the presence of bone resorption cavities. In case 2
in particular, barely any osteoclasts were observed. Furthermore,
the existing osteoclasts had very few nuclei, giving a
morphologically immature appearance. It was occasionally difficult
to identify osteoclasts using HE staining. Immunohistochemistry
using cathepsin K, which is regarded as a marker for osteoclasts
(19), confirmed the findings of the
HE staining. The cathepsin K-positive cells with very few nuclei
that existed along, or were detached from, the bone surface were
observed, predominantly in the case 1 specimen (Fig. 3E and F).
Discussion
Reportedly, the risk ratio for MRONJ in patients who
receive anti-RANKL inhibitors for cancer treatment ranges between
0.7 and 1.9% (20,21), which is equivalent to that reported
for patients who receive zoledronate treatment (22,23).
Although certain previous case reports on denosumab-related ONJ
have been published (10–18), only one described the
histopathological features (18),
which revealed complete osteonecrosis with empty osteocytic lacunae
and no osteocytes, osteoblasts or osteoclasts. The authors
concluded that the histological features of denosumab-associated
ONJ were similar to those of BP-associated ONJ. However, the
authors only evaluated the necrotic sequestrum, since only this
portion was surgically resected, according to the AAOMS
recommendations for MRONJ (2). By
contrast, in the present report, viable bone with cells responsible
for bone remodeling was observed since extensive surgery was
performed, which involves resection of not only the sequestrum, but
also viable, inflamed bone (3–5).
Furthermore, osteocytic lacunae with osteocytes were clearly
observed, permitting distinction between viable and necrotic bone.
The osteoclasts in this viable region were few and revealed a
decrease in the number of nuclei. It was hypothesized that the
maturation of immature osteoclasts around the sequestrum in
patients treated with denosumab is inhibited. Although a few
immature osteoclasts were observed in the case 1 specimen, there
were barely any in the case 2 specimen. This was probably a result
of case 2 being older than case 1; therefore, the bone turnover
rate was higher in case 1 compared with in case 2. Weinstein et
al (6) performed a transiliac
bone biopsy in patients who received BP treatment and suggested
that this treatment is associated with an increase in the number of
osteoclasts, which include distinct, giant, hypernucleated and
detached osteoclasts that are undergoing protracted apoptosis.
Additionally, Cho et al (8)
observed a notable number of osteoclasts, which were detached from
the bony trabeculae in patients with stage 3 BRONJ, treated by
partial mandibulectomy. These results suggested that osteoclasts
generally remain viable following BP treatment. Therefore,
evaluation of viable bone containing cells, including osteoclasts,
is important for assessing the effects of denosumab on bone
cells.
Denosumab is a fully human monoclonal antibody that
targets RANKL (24). Generally, RANKL
activates osteoclast differentiation by binding to RANK, a single
transmembrane receptor expressed in osteoclast lineage cells. RANKL
inhibition prevents the fusion of monocytes and macrophages to form
multinucleated osteoclasts. Denosumab prevents RANKL from binding
to RANK and subsequently inhibits osteoclast formation, function
and survival. By contrast, BPs bind to bone minerals and these are
taken up by mature osteoclasts at sites of bone resorption. These
osteoclasts subsequently lose their resorptive function and persist
(9). Denosumab was found to result in
nearly complete disappearance of osteoclasts in an ovariectomized
human-RANKL mouse model (25). From
this perspective, the histopathological findings of
denosumab-related ONJ in the two cases reported in the present
study are acceptable. Denosumab is considered to exhibit a faster
offset of action compared with BP, and its effects on bone
remodeling are mostly diminished within 6 months of treatment
cessation (26). Denosumab must be
discontinued prior to surgery in patients with MRONJ, if systemic
conditions permit. However, the effects of discontinuation remain
to be elucidated. The necrotic sequestrum and inflamed bone formed
in patients with ONJ are different from normal bone, and it remains
unclear how they exhibit the identical metabolism. In the present
study, the serum calcium level was decreased or maintained low by
denosumab treatment, indicating the effects of this drug on bone
metabolism. Since it was impossible to discontinue denosumab for a
long duration in these cases, the present study performed extensive
surgery 1 month following discontinuation. The prognosis of each
case was good during the postoperative follow-up.
In conclusion, the present study described the
clinical and histopathological features of denosumab-related ONJ in
two patients. More data should be collected to describe the bone
metabolism at the site of denosumab-related ONJ and to elucidate
the mechanisms underlying denosumab-related ONJ.
References
|
1
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: A
growing epidemic. J Oral Maxillofac Surg. 61:1115–1117. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruggiero SL, Dodson TB, Fantasia J,
Goodday R, Aghaloo T, Mehrotra B and O'Ryan F; American Association
of Oral and Maxillofacial Surgeons: American association of oral
and maxillofacial surgeons position paper on medication-related
osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg.
72:1938–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rupel K, Ottaviani G, Gobbo M, Contardo L,
Tirelli G and Vescovi P: DiL enarda R and Biasotto M: A systematic
review of therapeutical approaches in bisphosphonates-related
osteonecrosis of the jaw (BRONJ). Oral Oncol. 50:1049–1057. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fliefel R, Tröltzsch M, Kühnisch J,
Ehrenfeld M and Otto S: Treatment strategies and outcomes of
bisphosphonate-related osteonecrosis of the jaw (BRONJ) with
characterization of patients, A systematic review. Int J Oral
Maxillofac Surg. 44:568–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayashida S, Uda A, Yamada S, Yanamoto S,
Kawasaki G, Kakehashi H, Asahina I and Umeda M: A study of
treatment for bisphosphonate-related osteonecrosis of the jaw
(BRONJ)-Indication and method of surgery in patients with stage II
BRONJ-J. Jpn Stomatol Soc. 64:18–27. 2015.
|
|
6
|
Weinstein RS, Roberson PK and Manolagas
SC: Giant osteoclast formation and long-term oral bisphosphonate
therapy. N Engl J Med. 360:53–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jain N and Weinstein RS: Giant osteoclasts
after long-term bisphosphonate therapy: Diagnostic challenges. Nat
Rev Rheumatol. 5:341–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho YA, Yoon HJ, Lee JI, Hong SP and Hong
SD: Histopathological features of bisphosphonate-associated
osteonecrosis, Findings in patients treated with partial
mandibulectomies. Oral Surg Oral Med Oral Pathol Oral Radiol.
114:785–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baron R, Ferrari S and Russell RG:
Denosumab and bisphosphonates, Different mechanisms of action and
effects. Bone. 48:677–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor KH, Middlefell LS and Mizen KD:
Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J
Oral Maxillofac Surg. 48:221–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aghaloo TL, Felsenfeld AL and Tetradis S:
Osteonecrosis of the jaw in a patient on denosumab. Br J Oral
Maxillofac Surg. 48:221–223. 2010.PubMed/NCBI
|
|
12
|
Malan J, Ettinger K, Naumann E and Beirne
OR: The relationship of denosumab pharmacology and osteonecrosis of
the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 114:671–676.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pichardo SE, Kuypers SC and van Merkesteyn
JP: Denosumab osteonecrosis of the mandible. A new entity? A case
report. J Craniomaxillofac Surg. 41:e65–e69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otto S, Baumann S, Ehrenfeld M and Pautke
C: Successful surgical management of osteonecrosis of the jaw due
to RANK-ligand inhibitor treatment using fluorescence guided bone
resection. J Craniomaxillofac Surg. 41:694–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neuprez A, Coste S, Rompen E, Crielaard JM
and Reginster JY: Osteonecrosis of the jaw in a male osteoporotic
patient treated with denosumab. Osteoporos Int. 25:393–395. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olate S, Uribe F, Martinez F, Almeida A
and Unibazo A: Osteonecrosis of the jaw in patient with denosumab
therapy. Int J Clin Exp Med. 7:3707–3709. 2014.PubMed/NCBI
|
|
17
|
O'Halloran M, Boyd NM and Smith A:
Denosumab and osteonecrosis of the jaws-the pharmacology,
pathogenesis and a report of two cases. Aust Dent J. 59:516–519.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aghaloo TL, Dry SM, Mallya S and Tetradis
S: Stage 0 osteonecrosis of the jaw in a patient on denosumab. J
Oral Maxillofac Surg. 72:702–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Littlewood-Evans A, Kokubo T, Ishibashi O,
Inaoka T, Wlodarski B, Gallagher JA and Bilbe G: Localization of
cathepsin K in human osteoclasts by in situ hybridization and
immunohistochemistry. Bone. 20:81–86. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi WX, Tang LN, He AN, Yao Y and Shen Z:
Risk of osteonecrosis of the jaw in cancer patients receiving
denosumab, A meta-analysis of seven randomized controlled trials.
Int J Clin Oncol. 19:403–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scagliotti GV, Hirsh V, Siena S, Henry DH,
Woll PJ, Manegold C, Solal-Celigny P, Rodriguez G, Krzakowski M,
Mehta ND, et al: Overall survival improvement in patients with lung
cancer and bone metastases treated with denosumab versus zoledronic
acid: Subgroup analysis from a randomized phase 3 study. J Thorac
Oncol. 7:1823–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henry DH, Costa L, Goldwasser F, Hirsh V,
Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A,
Vadhan-Raj S, et al: Randomized, Double-blind study of denosumab
versus zoledronic acid in the treatment of bone metastases in
patients with advanced cancer (excluding breast and prostate
cancer) or multiple myeloma. J Clin Oncol. 29:1125–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McClung MR, Lewiecki EM, Cohen SB,
Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD,
Lederman SN, Chesnut CH, et al: Denosumab in postmenopausal women
with low bone mineral density. N Engl J Med. 354:821–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller PD, Bolognese MA, Lewiecki EM,
McClung MR, Ding B, Austin M, Liu Y and San Martin J: Amg Bone Loss
Study Group: Effect of denosumab on bone density and turnover in
postmenopausal women with low bone mass after long-term continued,
discontinued and restarting of therapy. A randomized blinded phase
2 clinical trial. Bone. 43:222–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pierroz DD, Bonnet N, Baldock PA, Ominsky
MS, Stolina M, Kostenuik PJ and Ferrari SL: Are osteoclasts needed
for the bone anabolic response to parathyroid hormone? A study of
intermittent parathyroid hormone with denosumab or alendronate in
knock-in mice expressing humanized RANKL. J Biol Chem.
285:28164–28173. 2010. View Article : Google Scholar : PubMed/NCBI
|