Introduction
Retroperitoneal sarcomas, or retroperitoneal soft
tissue sarcomas, are rare mesenchymal tumors accounting for ~0.15%
of all malignancies (1,2). Retroperitoneal sarcomas display a vast
array of histological subtypes, among which liposarcomas are the
most common (30–50%), followed by leiomyosarcomas and malignant
fibrous histiocytomas, also referred to as undifferentiated
pleomorphic sarcomas, according to the updated World Health
Organization (WHO) classification of soft tissue tumors (1,3).
Retroperitoneal liposarcoma typically presents as advanced disease
and often carries a poor prognosis (4). However, its rarity means that its
prognostic factors are poorly understood. Furthermore, the majority
of previously published series on primary malignant retroperitoneal
tumors have included retroperitoneal liposarcomas together with
other retroperitoneal sarcomas with heterogeneous histologies, thus
preventing the independent characterization of this specific
subtype (3,5–8).
Retroperitoneal liposarcomas usually present as an
asymptomatic abdominal mass, incidentally detected during abdominal
examination or abdominal imaging studies performed for other
purposes, while others are detected due to the presence of clinical
symptoms (4). Although symptoms at
diagnosis have been identified as prognostic markers in several
malignancies (9–12), their prognostic value for
retroperitoneal liposarcoma has yet to be evaluated. In this study,
we assessed the prognostic factors in primary retroperitoneal
liposarcoma, focusing on the presence or absence of clinical
symptoms at diagnosis.
Patients and methods
Patients and clinicopathological
factors
A total of 24 consecutive patients with primary
retroperitoneal liposarcomas who underwent surgical resection with
curative intent at our institution (Graduate School of Medicine,
The University of Tokyo, Tokyo, Japan) between 1985 and 2014 were
reviewed. This study was approved by the internal Institutional
Review Board.
Regarding pathological factors, a single pathologist
(T.M.) reviewed all the slides of the surgical specimens. Tumor
size was defined as the maximum diameter of the tumor at
pathological analysis. The patients were followed up until March,
2015.
Statistical analysis
The Kaplan-Meier analysis and the log-rank test were
used to estimate progression-free survival (PFS; primary endpoint)
and sarcoma-specific survival (SSS; secondary endpoint) after
initial surgery. The effect of various clinicopathological factors,
including symptoms at diagnosis, on these two endpoints was
assessed with univariate and multivariate Cox proportional hazards
analyses. For the multivariate analysis, a backward stepwise
procedure (entry, 0.05; removal, 0.10) was selected, due to the
small sample size. All the statistical analyses were performed
using JMP Pro software, version 11.0.0 (SAS Institute, Cary, NC,
USA). P<0.05 was considered to indicate statistically
significant differences.
Results
Patient characteristics
The 24 patients included 14 men (58.3%) and 10 women
(41.7%), with a median age of 59 years (range, 40–79 years) at
initial surgery. A total of 11 patients (45.8%) developed
recurrence after the initial surgery, and 8 (33.3%) succumbed to
retroperitoneal liposarcoma during the study period, with a median
follow-up of 64 months (range, 2–225 months) (Table I).
 | Table I.Patient characteristics (n=24). |
Table I.
Patient characteristics (n=24).
| Characteristics | Values |
|---|
| Gender, no. (%) |
|
| Male | 14 (58.3) |
|
Female | 10 (41.7) |
| Median age at initial
surgery, years (range) | 59 (40–79) |
| Body mass index,
kg/m2, median (range) | 23.4 (17.1–35.6) |
| Clinical symptoms at
diagnosis, no. (%) | 16 (66.7) |
| Incidental tumor, no
(%) | 8 (33.3) |
| Median tumor size at
initial surgery, cm (range) | 19.0 (11.5–32.0) |
| Dominant histological
subtype, no. (%) |
|
|
Well-differentiated
liposarcoma | 19 (79.2) |
| Myxoid
liposarcoma | 3 (12.5) |
|
Dedifferentiated
liposarcoma | 2 (8.3) |
| Presence of
dedifferentiated components, no. (%) | 8 (33.3) |
| Positive surgical
margins, no. (%) | 17 (70.8) |
| Adjuvant
chemotherapy, no. (%) | 5 (20.8) |
| Median follow-up,
months (range) | 64 (2–225) |
Symptoms at diagnosis
A total of 16 patients (67%) had symptoms at
diagnosis, while the remaining 8 (33%) were diagnosed incidentally.
The symptoms included palpability of the tumor in 8 patients;
abdominal pain/fullness in 3; flank pain/fullness in 2; lower
extremity pain in 1; testicular pain due to varicocele in 1; and
discomfort on urination in 1 patient. Regarding the last 3 cases,
the lower extremity pain was attributed to neural invasion by the
tumor; varicocele to tumor infiltration of the left gonadal vein;
and discomfort on urination to tumor infiltration of the urinary
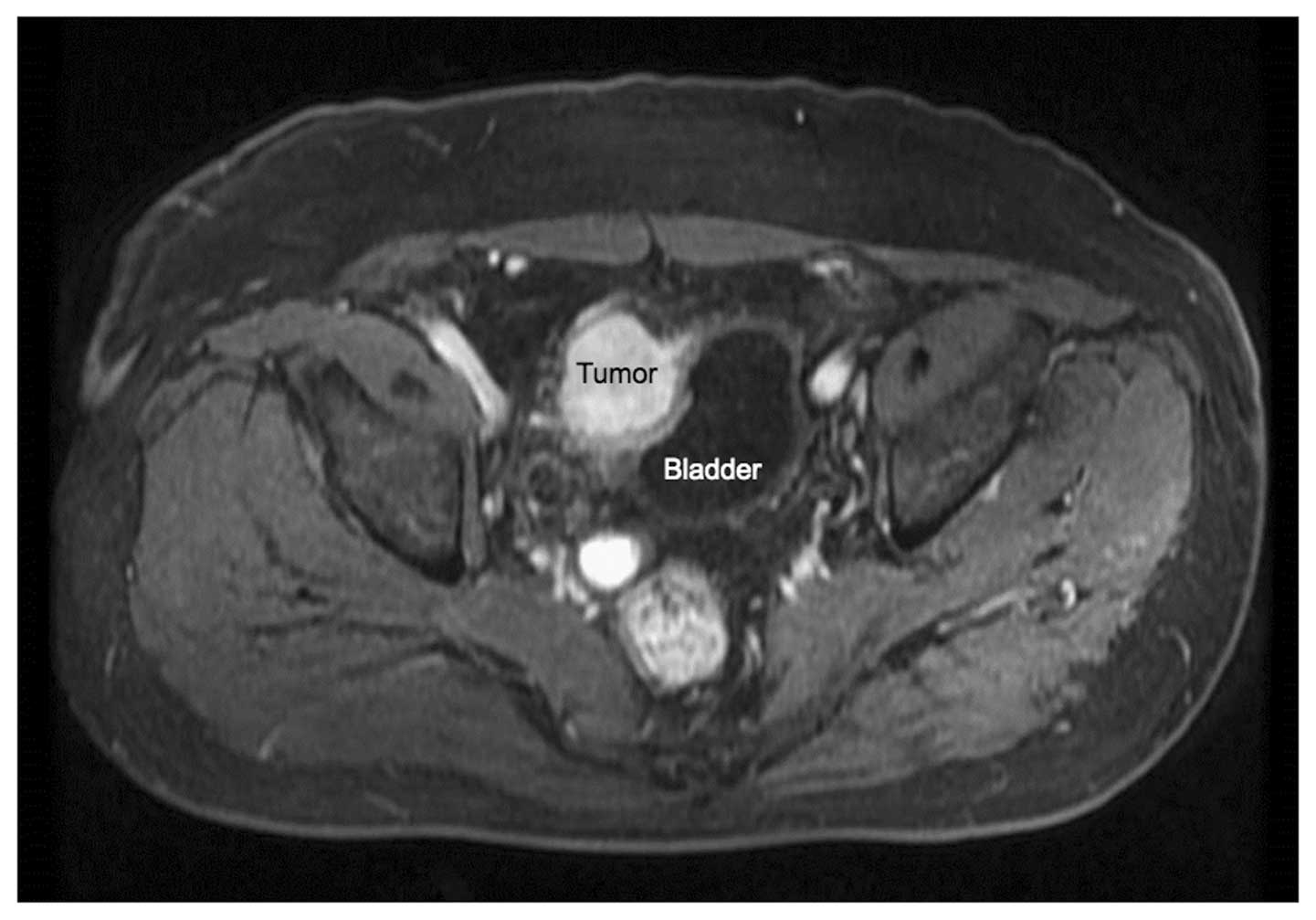
bladder. Preoperative dynamic contrast-enhanced magnetic resonance
imaging in the last patient is shown in Fig. 1, demonstrating tumor infiltration of
the urinary bladder, which may have caused the discomfort on
urination. Of the 8 asymptomatic patients, 5 were incidentally
diagnosed with retroperitoneal liposarcoma during a follow-up visit
for another condition, and 3 were diagnosed during a periodic
check-up for cancer.
Tumor characteristics
The median tumor size was 19.0 cm (range, 11.5–32.0
cm). All the patients had tumors sized >10 cm, and all patients
with symptoms at diagnosis had tumors sized >15 cm. The
predominant histological subtypes were well-differentiated
liposarcoma (atypical lipomatous tumor) in 19 (79.2%); myxoid
liposarcoma in 3 (12.5%); and dedifferentiated liposarcoma in 2
patients (8.3%). Pathological analysis was performed according to
the updated WHO classification of soft tissue tumors. A total of 8
patients (33.3%) had dedifferentiated sarcoma components in their
surgical specimens and 17 patients (70.8%) had microscopically
positive resection margins.
PFS and SSS in patients with and
without symptoms at diagnosis
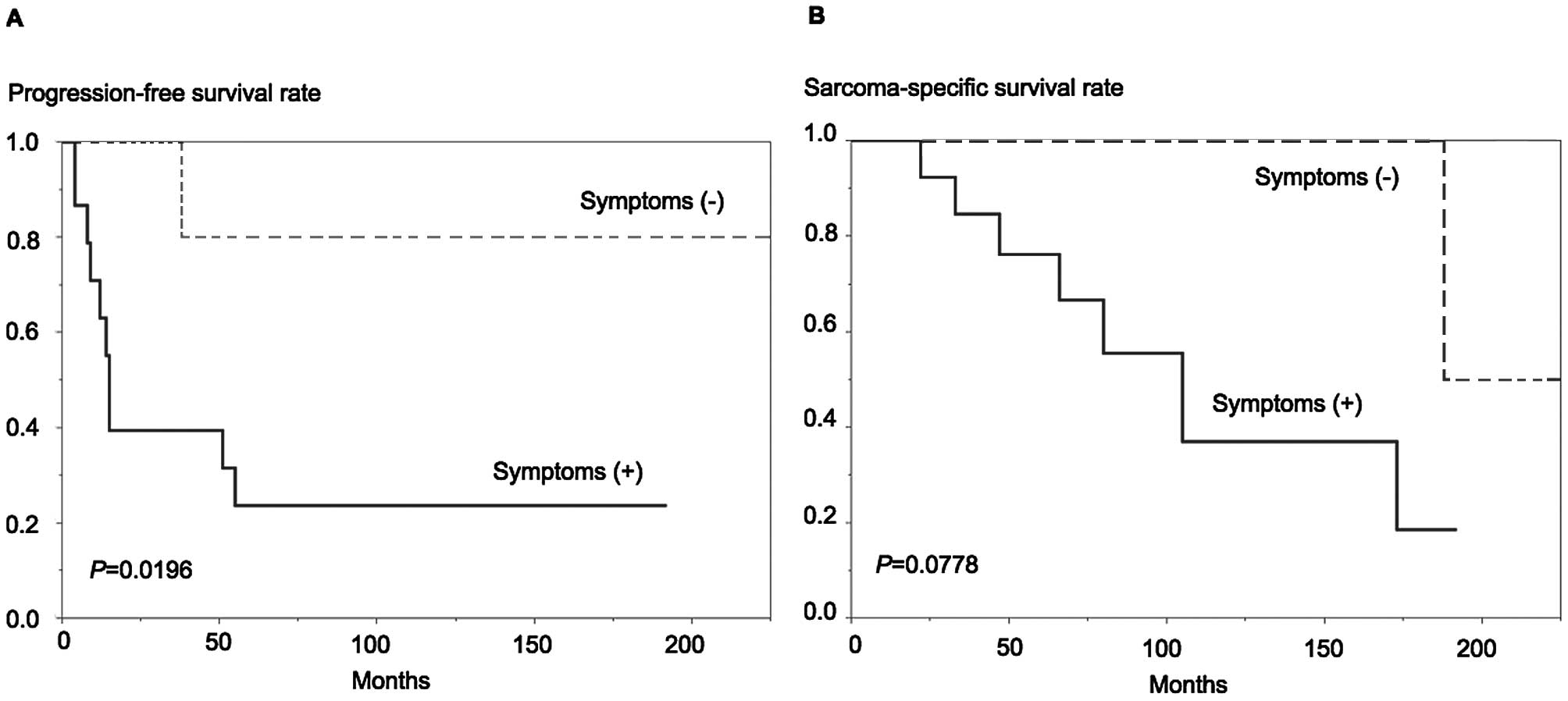
The Kaplan-Meier curves depicting PFS and SSS in
patients with and without symptoms at diagnosis are presented in
Fig. 2. The patients with symptoms at
diagnosis were significantly more likely to develop recurrence
(log-rank test, P=0.0196, Fig. 2A)
and more likely to succumb to sarcoma (P=0.0778, Fig. 2B) compared with asymptomatic
patients.
Effect of clinicopathological factors
on PFS
The univariate analysis demonstrated that symptoms
at diagnosis, dedifferentiated components and positive surgical
margins were all associated with poor PFS, whereas in the
multivariate analysis, the presence of symptoms at diagnosis
[hazard ratio (HR)=6.134, P=0.0347] and dedifferentiated components
(HR=4.809, P=0.0337) were identified as independent predictors of
poor PFS (Table II). The details of
this multivariate analysis using the backward stepwise procedure
were as follows: For the first step, all three univariately
significant factors (symptoms at diagnosis, dedifferentiated
components and surgical margins) were entered in the multivariate
analysis, and surgical margins, which had the highest P-value
(0.2139) were eliminated. Subsequently, the remaining two factors
were entered in the analysis, and this was considered as the final
model, as both had statistically significant P-values
(<0.05).
 | Table II.Univariate and multivariate analyses
of the effect of clinicopathological factors on progression-free
survival after the initial surgery. |
Table II.
Univariate and multivariate analyses
of the effect of clinicopathological factors on progression-free
survival after the initial surgery.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender |
|
|
|
|
| Male vs.
female | 1.506
(0.449–5.813) | 0.5119 |
|
|
| Age at initial
surgery |
|
|
|
|
| >59
vs. ≤59 yearsa | 0.695
(0.182–2.310) | 0.5572 |
|
|
| Body mass index |
|
|
|
|
| >23.4
vs. ≤23.4 kg/m2a | 0.782
(0.223–2.638) | 0.6886 |
|
|
| Symptoms at
diagnosis |
|
|
|
|
| Yes vs.
no | 7.782
(1.481–143.0) | 0.0114b | 6.134
(1.118–114.4) | 0.0347b |
| Tumor size |
|
|
|
|
| >19
vs. ≤19 cma | 2.418
(0.726–9.271) | 0.1506 |
|
|
| Dedifferentiated
components |
|
|
|
|
| Yes vs.
no | 5.890
(1.501–28.87) | 0.0111b | 4.809
(1.126–27.25) | 0.0337b |
| Surgical margins |
|
|
|
|
| Positive
vs. negative | 9.220
(1.685–172.1) | 0.0070b |
|
|
| Adjuvant
chemotherapy |
|
|
|
|
| Yes vs.
no | 2.528
(0.656–8.481) | 0.1647 |
|
|
| Leukocyte count | 1.059
(0.314–3.719) | 0.9255 |
|
|
| >6,200
vs. ≤6,200 cells/µla |
|
|
|
|
|
Neutrophil-to-lymphocyte ratio | 1.371
(0.410–4.782) | 0.6035 |
|
|
| >2.3
vs. ≤2.3a |
|
|
|
|
|
Lymphocyte-to-monocyte ratio | 1.425
(0.428–5.462) | 0.5684 |
|
|
| >3.6
vs. ≤3.6a |
|
|
|
|
| Hemoglobin |
|
|
|
|
| >13
vs. ≤13 g/dla | 1.037
(0.298–3.453) | 0.9525 |
|
|
| Albumin |
|
|
|
|
| >3.9
vs. ≤3.9 g/dla | 0.590
(0.153–1.980) | 0.3964 |
|
|
| Lactate
dehydrogenase |
|
|
|
|
| Using
actual values (per 10 IU/l increase) | 0.929
(0.811–1.061) | 0.2744 |
|
|
| Alkaline
phosphatase |
|
|
|
|
| Using
actual values (per 10 IU/l increase) | 1.050
(0.906–1.222) | 0.5159 |
|
|
| C-reactive
protein |
|
|
|
|
| >0.3
vs. ≤0.3 mg/dl | 0.873
(0.250–2.919) | 0.8229 |
|
|
Effect of clinicopathological factors
on SSS
Dedifferentiated components and positive surgical
margins were associated with poor SSS in the univariate analyses,
whereas positive surgical margins were found to be an independent
predictor of poor SSS in the multivariate analysis (Table III). However, the HR for positive
surgical margins did not converge, possibly due to few events;
therefore this result is only suitable for reference purposes. The
presence of symptoms at diagnosis exhibited a non-significant trend
for poor SSS in the univariate analysis (P=0.0577).
 | Table III.Univariate and multivariate analyses
of the effect of clinicopathological factors on sarcoma-specific
survival after the initial surgery. |
Table III.
Univariate and multivariate analyses
of the effect of clinicopathological factors on sarcoma-specific
survival after the initial surgery.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender |
|
|
|
|
| Male
vs. female | 1.893
(0.455–9.381) | 0.3804 |
|
|
| Age at initial
surgery |
|
|
|
|
| >59
vs. ≤59 yearsa | 0.608
(0.088–2.693) | 0.5319 |
|
|
| Body mass
index |
|
|
|
|
|
>23.4 vs. ≤23.4
kg/m2a | 1.306
(0.300–5.650) | 0.7118 |
|
|
| Symptoms at
diagnosis |
|
|
|
|
| Yes vs.
no | 5.691
(0.952–109.0) | 0.0577 |
|
|
| Tumor size |
|
|
|
|
| >19
vs ≤19 cma | 3.215
(0.649–23.67) | 0.1568 |
|
|
| Dedifferentiated
components |
|
|
|
|
| Yes vs.
no | 15.26
(2.108–307.6) | 0.0062b | 7.088
(0.989–143.1) | 0.0525 |
| Surgical
margins |
|
|
|
|
|
Positive vs. negative | NC | 0.0011b | NC |
0.0084b |
| Adjuvant
chemotherapy |
|
|
|
|
| Yes vs.
no | 3.510
(0.647–19.07) | 0.1370 |
|
|
| Leukocyte
count | 0.376
(0.565–13.98) | 0.2103 |
|
|
|
>6,200 vs. ≤6,200
cells/µla |
|
|
|
|
|
Neutrophil-to-lymphocyte ratio | 1.954
(0.438–13.53) | 0.3968 |
|
|
| >2.3
vs. ≤2.3a |
|
|
|
|
|
Lymphocyte-to-monocyte ratio | 0.532
(0.124–2.284) | 0.3815 |
|
|
| >3.6
vs. ≤3.6a |
|
|
|
|
| Hemoglobin |
|
|
|
|
| >13
vs. ≤13 g/dla | 0.805
(0.164–3.316) | 0.7661 |
|
|
| Albumin |
|
|
|
|
| >3.9
vs. ≤3.9 g/dla | 0.622
(0.126–2.571) | 0.5130 |
|
|
| Lactate
dehydrogenase |
|
|
|
|
| Using
actual values (per 10 IU/l increase) | 0.932
(0.798–1.090) | 0.3669 |
|
|
| Alkaline
phosphatase |
|
|
|
|
| Using
actual values (per 10 IUl increase) | 1.130
(0.942–1.368) | 0.1853 |
|
|
| C-reactive
protein |
|
|
|
|
| >0.3
vs. ≤0.3 mg/dl | 1.306
(0.301–5.644) | 0.7117 |
|
|
Discussion
In the present study, the presence of symptoms at
diagnosis in patients with primary retroperitoneal liposarcoma was
an independent predictor of poor PFS, and tended to be associated
with poor SSS. To the best of our knowledge, this is the first
assessment of the prognostic value of symptoms at diagnosis in
patients with retroperitoneal liposarcoma.
The rarity of retroperitoneal liposarcoma means that
its prognostic factors have yet to be clearly determined.
Furthermore, the majority of previous studies on primary malignant
retroperitoneal tumors have included patients with other
retroperitoneal sarcomas with heterogeneous histologies together
with retroperitoneal liposarcomas, thus preventing independent
characterization (3,5–8). However,
a limited number of studies have specifically investigated
retroperitoneal liposarcomas. Singer et al (13) retrospectively reviewed 177 patients
with primary retroperitoneal liposarcoma and demonstrated that
dedifferentiated histology (HR=4, P<0.0001) and contiguous organ
resection (HR=2, P=0.04) were significantly associated with PFS
according to the Cox regression analysis, while dedifferentiated
histology (HR=6, P<0.0001), gross positive margins (HR=4,
P<0.0001), contiguous organ resection (HR=2, P=0.05) and age
(HR=1.03, P=0.03) were significantly associated with SSS. A recent
comprehensive review of retroperitoneal liposarcomas also reported
that the most consistent prognostic factor was completeness of
surgical resection, with negative margins (4). Our results supported these findings,
given that the univariate analysis revealed an association of
dedifferentiated components and positive surgical margins with both
PFS and SSS, while the multivariate analysis identified the
presence of positive surgical margins as an independent predictor
of SSS.
Regarding the presence of symptoms, we were only
able to find one study that evaluated the prognostic impact of
symptoms on survival in retroperitoneal sarcoma. In a review of 49
patients with primary malignant retroperitoneal tumors, including
25 (51%) with liposarcomas (6), 44
patients (90%) had symptoms, but these were not associated with
overall survival (P=0.282, log-rank test).
By contrast, several studies have investigated the
prognostic value of tumor size in malignant retroperitoneal tumors
(5–8,13), mostly
using a cut-off value of 10 cm, particularly in studies conducted
over a decade ago (5–7). A relatively small-scale (n=49) study
reported a prognostic value of tumor size >10 cm (6), whereas another larger (n=500) study did
not report such an association (7).
More recently, in a review of 1,091 patients with soft tissue
sarcoma of any primary site (including extremities, trunk and
retroperitoneum), Lahat et al (14) demonstrated that patients with tumors
sized >15 cm were at increased risk of developing distant
recurrence and exhibited higher disease-specific mortality compared
with those with smaller tumors; they suggested that tumor size
should be revised in the current American Joint Committee on Cancer
(AJCC) staging system used for soft tissue sarcomas. The AJCC
system currently uses a 5-cm threshold, which is better suited for
extremity sarcomas, but has limited discriminative power for
retroperitoneal liposarcomas, almost all of which are >5 cm
(4,15). Indeed, all the patients in the present
study had tumors >10 cm (median, 19.0 cm), and tumor size was
not associated with PFS or SSS.
The mechanisms underlying the association between
symptoms at diagnosis and poor outcome in patients with
retroperitoneal liposarcomas may be associated with the fact that
an aggressive tumor may be recognized by the host more easily than
an indolent one. The most common symptoms (13/16, 81%) in the
present study were palpability of the tumor and pain/fullness of
the abdomen/flank, as previously reported (3). In the case of two tumors of the same
size but with different growth rates, the faster-growing tumor
(i.e., more aggressive tumor) would be more likely to cause
pain/fullness compared with the slower-growing tumor, due to the
greater change. However, tumor size itself is also an important
factor, which is supported by the fact that all the symptomatic
patients in our cohort had tumors sized >15 cm. In addition to
growth rate and tumor size, local invasion of the retroperitoneal
structures may cause neurological, musculoskeletal and
urinary/bowel symptoms (4), which are
also considered to be specific characteristics of aggressive
tumors. Of the 16 (19%) symptomatic patients in our cohort, 1 had
lower extremity pain due to neural invasion, 1 had testicular pain
due to varicocele as a consequence of tumor infiltration of the
left gonadal vein, and 1 presented with discomfort on urination due
to tumor infiltration of the urinary bladder. Since several
inflammatory markers, such as neutrophil-to-lymphocyte ratio,
lymphocyte-to-monocyte ratio and C-reactive protein, have been
reported to be prognostic factors in soft tissue sarcomas (16–19), we
also evaluated certain laboratory parameters; however, none were
found to be associated with prognosis in the present study
(Tables II and III).
This study was limited by its retrospective design
and small sample size. Therefore, further confirmatory studies with
larger populations are required to validate these results. However,
these preliminary results suggest that the presence of symptoms at
diagnosis may be an easily available, useful prognostic factor to
complement existing markers in retroperitoneal liposarcoma.
In conclusion, retroperitoneal liposarcomas
diagnosed by clinical symptoms are associated with a poorer
prognosis compared with incidentally diagnosed retroperitoneal
liposarcomas. Furthermore, the presence of symptoms at diagnosis
was found to be an independent predictor of PFS, which may prove to
be a useful additional prognostic factor in primary retroperitoneal
liposarcoma.
References
|
1
|
Mettlin C, Priore R, Rao U, Gamble D, Lane
W and Murphy P: Results of the national soft-tissue sarcoma
registry. J Surg Oncol. 19:224–227. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer S, Maki RG and O'Sullivan B: Soft
tissue sarcoma. Cancer: Principles and Practice of Oncology Primer
of the Molecular Biology of Cancer (9th). DeVita VT Jr, Lawrence TS
and Rosenberg SA: (Philadelphia, PA). Lippincott Williams &
Wilkins. 1533–1577. 2011.
|
|
3
|
Fernández-Ruiz M, Rodríguez-Gil Y,
Guerra-Vales JM, Manrique-Municio A, Moreno-González E and
Colina-Ruizdelgado F: Primary retroperitoneal liposarcoma Clinical
and histological analysis of ten cases. Gastroenterol Hepatol.
33:370–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vijay A and Ram L: Retroperitoneal
liposarcoma: A comprehensive review. Am J Clin Oncol. 38:213–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singer S, Corson JM, Demetri GD, Healey
EA, Marcus K and Eberlein TJ: Prognostic factors predictive of
survival for truncal and retroperitoneal soft-tissue sarcoma. Ann
Surg. 221:185–195. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
An JY, Heo JS, Noh JH, Sohn TS, Nam SJ,
Choi SH, Joh JW and Kim SJ: Primary malignant retroperitoneal
tumors, Analysis of a single institutional experience. Eur J Surg
Oncol. 33:376–382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lewis JJ, Leung D, Woodruff JM and Brennan
MF: Retroperitoneal soft-tissue sarcoma, Analysis of 500 patients
treated and followed at a single institution. Ann Surg.
228:355–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gronchi A, Miceli R, Shurell E, Eilber FC,
Eilber FR, Anaya DA, Kattan MW, Honoré C, Lev DC, Colombo C, et al:
Outcome prediction in primary resected retroperitoneal soft tissue
sarcoma: Histology-specific overall survival and disease-free
survival nomograms built on major sarcoma center data sets. J Clin
Oncol. 31:1649–1655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa T, Hara T, Kawahara T, Ogata Y,
Nakanishi H, Komiyama M, Arai E, Kanai Y and Fujimoto H: Prognostic
risk stratification of patients with urothelial carcinoma of the
bladder with recurrence after radical cystectomy. J Urol.
189:1275–1281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szendroi A, Tabák A, Riesz P, Szucs M,
Nyírády P, Majoros A, Haas G and Romics I: Clinical symptoms
related to renal cell carcinoma are independent prognostic factors
for intraoperative complications and overall survival. Int Urol
Nephrol. 41:835–842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reyes-Gibby CC, Anderson KO, Merriman KW,
Todd KH, Shete SS and Hanna EY: Survival patterns in squamous cell
carcinoma of the head and neck, Pain as an independent prognostic
factor for survival. J Pain. 15:1015–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MJ, Choi SB, Han HJ, Park PJ, Kim WB,
Song TJ, Suh SO and Choi SY: Clinicopathological analysis and
survival outcome of duodenal adenocarcinoma. Kaohsiung J Med Sci.
30:254–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singer S, Antonescu CR, Riedel E and
Brennan MF: Histologic subtype and margin of resection predict
pattern of recurrence and survival for retroperitoneal liposarcoma.
Ann Surg. 238:358–370; discussion 370–371. 2003.
|
|
14
|
Lahat G, Tuvin D, Wei C, Anaya DA, Bekele
BN, Lazar AJ, Pisters PW, Lev D and Pollock RE: New perspectives
for staging and prognosis in soft tissue sarcoma. Ann Surg Oncol.
15:2739–2748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on Cancer (AJCC)
Cancer Staging Manual (7th). New York, NY: Springer. 2010.
|
|
16
|
Szkandera J, Absenger G, Liegl-Atzwanger
B, Pichler M, Stotz M, Samonigg H, Glehr M, Zacherl M, Stojakovic
T, Gerger A and Leithner A: Elevated preoperative
neutrophil/lymphocyte ratio is associated with poor prognosis in
soft-tissue sarcoma patients. Br J Cancer. 108:1677–1683. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: The combined use of the
neutrophil-lymphocyte ratio and C-reactive protein level as
prognostic predictors in adult patients with soft tissue sarcoma. J
Surg Oncol. 108:481–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi ES, Kim HS and Han I: Elevated
preoperative systemic inflammatory markers predict poor outcome in
localized soft tissue sarcoma. Ann Surg Oncol. 21:778–785. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szkandera J, Gerger A, Liegl-Atzwanger B,
Absenger G, Stotz M, Friesenbichler J, Trajanoski S, Stojakovic T,
Eberhard K, Leithner A and Pichler M: The lymphocyte/monocyte ratio
predicts poor clinical outcome and improves the predictive accuracy
in patients with soft tissue sarcomas. Int J Cancer. 135:362–370.
2014. View Article : Google Scholar : PubMed/NCBI
|