Introduction
Cervical cancer is the fourth most common malignancy
among women worldwide (1), with a
5-year recurrence rate of 28% according to the International
Federation of Gynecology and Obstetrics (FIGO) (2). Poor prognostic factors for cervical
cancer include stage, tumor size, histology and lymph node (LN)
metastasis (3,4). However, these parameters are not
sufficient to accurately predict prognosis.
Systemic inflammatory response (SIR) is an important
prognostic factor for survival in various types of cancer (5,6).
Neutrophils, platelets, lymphocytes and albumin play a prominent
role in cancer-related inflammation. Recent evidence has indicated
that relative differences in neutrophil-to-lymphocyte ratio (NLR),
platelet-to-lymphocyte ratio (PLR) and prognostic nutritional index
(PNI) (PNI=10 × albumin concentration +0.005 × total lymphocyte
count) affect SIR and, consequently, cancer survival (7–10).
Hypoalbuminemia is often observed in patients with advanced cancer
and is usually considered to be a marker of malnutrition and
cachexia. It has also been reported that albumin is involved in SIR
and survival in various types of cancer (11,12).
Although pretreatment NLR and PLR have been shown to predict
outcome in gynecological cancers, such as endometrial (13,14),
cervical (15,16) and ovarian cancer (17,18), PNI
has not been shown to be a predictive factor in patients with
cervical cancer. The aim of this study was to investigate the
correlation between pretreatment NLR, PLR, PNI and prognosis in
patients who had been treated with concurrent chemoradiotherapy
(CCRT) or radiotherapy (RT) for cervical cancer.
Patients and methods
Patients
The study population consisted of 131 patients with
primary cervical cancer who underwent CCRT or RT at the Department
of Obstetrics and Gynecology of Okayama University Hospital
(Okayama, Japan) between April, 2007 and March, 2013. The study
protocol was approved by the Institutional Review Board of Okayama
University Hospital. The patients' clinical data, including medical
history, physical examination and clinical staging, were reviewed.
Computed tomography (CT) and positron emission tomography-CT are
widely accepted modalities for assessing the extent of LN
metastasis; according to traditional criteria, LNs with short-axis
length of >10.0 mm are defined as metastatic (19). The baseline pretreatment
characteristics (stage, histology, LN metastasis, parametrial
involvement, vaginal invasion and maximum tumor size) of the
patients are listed in Table I.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Baseline
characteristics | All patients, no.
(%) |
|---|
| Age at diagnosis,
years [mean (range)] |
|
|
| Stage | 61.5 (25–88) |
| Ib1 | 7 | 5.8 |
| Ib2 | 10 | 8.3 |
| IIa1 | 7 | 5.8 |
| IIa2 | 4 | 3.3 |
| IIb | 51 | 42.1 |
| IIIa | 3 | 2.5 |
| IIIb | 33 | 27.3 |
| IVa | 6 | 4.9 |
| Histology |
|
|
| SCC | 104 | 85.9 |
| AD | 14 | 11.6 |
| ADSQ | 3 | 2.5 |
| Lymph node
metastasis |
|
|
|
Negative | 83 | 68.6 |
|
Positive | 38 | 31.4 |
| Parametrial
invasion |
|
|
|
Negative | 30 | 24.8 |
|
Positive | 91 | 75.2 |
| Vaginal invasion |
|
|
|
Negative | 63 | 52.1 |
|
Positive | 58 | 47.9 |
| Maximum tumor size,
cm |
|
|
| ≤4.0 | 41 | 33.9 |
|
>4.0 | 80 | 66.1 |
| Treatment |
|
|
| CCRT | 95 | 78.5 |
| RT | 36 | 21.5 |
| Chemotherapy
regimen (N=95) |
|
|
| Weekly
CDDP | 52 | 54.7 |
| Weekly
NED | 28 | 29.4 |
|
Ifosfamide + NED | 15 | 15.9 |
Laboratory analysis
All the patients had their white blood cell (WBC)
count and albumin levels recorded within 1 week prior to treatment.
Differential WBC counts and albumin levels were measured prior to
treatment with RT or CCRT; WBC, neutrophil, lymphocyte and platelet
counts were measured using automated blood cell counters (Bayer
HealthCare, Diagnostics Division, Tarrytown, NY, USA). The levels
of serum albumin were measured by latex nephelometry (LT Auto Wako,
Osaka, Japan). NLR was defined as the absolute neutrophil count
(µl) divided by the absolute lymphocyte count (µl), and PLR was
defined as the absolute platelet count (µl) divided by the
lymphocyte count (µl). The PNI was calculated as previously
described (20). Briefly, PNI was
defined as 10 × albumin concentration (g/dl)+0.005 × total
lymphocyte count (µl). During RT or CCRT, the WBC, neutrophil,
lymphocyte and platelet counts, albumin levels and weight were
measured weekly. Acute toxicities were evaluated and graded using
the Common Terminology Criteria for Adverse Events (CTCAE) version
4.0 (21). Seven toxicities,
including leukopenia, neutropenia, lymphocytopenia,
thrombocytopenia, weight loss, diarrhea and hyponatremia, were
recorded based on CTCAE v.4.0.
Treatment
The patients were treated with a combination of
external irradiation and intracavitary brachytherapy (ICBT) with
curative intent. RT was delivered at 2.0 Gy per fraction once
daily, 5 days per week, over 5 weeks. The median dose to the whole
pelvis was 50.0 Gy and ICBT as the high dose rate was 24 Gy/4
times. For CCRT, the patients were treated with either cisplatin
(CDDP; 40 mg/m2 infusion weekly for six cycles),
nedaplatin (NED; 30 mg/m2 infusion weekly for eight
cycles), or ifosfamide plus NED (IN) [ifosfamide (1
g/m2) infusion on days 1–5 and NED (80 mg/m2)
infusion on day 1 of a 3-week cycle, for three cycles], as
previously described (22,23). A total of 52 patients treated with
CDDP, 28 treated with NED and 15 treated with IN chemotherapy were
evaluated. The remaining 36 patients did not receive concurrent
chemotherapy due to the presence of comorbidities or advanced age
(≥75 years). CCRT was interrupted for up to 1 week in patients with
WBC counts <2,000/µl, neutrophil counts <1,000/µl, or
platelet counts <75,000/µl. If these side effects persisted for
>1 week, no additional chemotherapy was administered. RT was
suspended indefinitely in patients who exhibited a WBC count
<1,000/µl, neutrophil count <500/µl, or platelet count
<25,000/µl. Since the prognosis of patients with cervical cancer
is associated with their hemoglobin (Hb) level during RT or CCRT
(24), our treatment policy is to
administer red blood cell transfusions prior to and during CCRT if
the Hb level is <10.0 g/dl, until it exceeds 10 g/dl. Patients
underwent follow-up examinations approximately every 1–2 months for
the first 6 months, every 3 months for the next 2 years, and every
6 months thereafter.
Statistical analysis
Statistical analyses were performed using the
χ2 test and the Mann-Whitney U test for comparisons with
the controls and the one-factor analysis of variance, followed by
Fisher's protected least-significant difference test for all
pairwise comparisons. The survival curves were calculated by the
Kaplan-Meier method; differences in the recurrence or survival
curves were examined using the log-rank test. The analyses were
performed using the SPSS software, version 20.0 (IBM SPSS, Armonk,
NY, USA). P<0.05 was considered to indicate statistically
significant differences.
Results
Patient characteristics
The clinicopathological characteristics, including
patient age, tumor stage, histology, LN metastasis, parametrial
involvement, vaginal invasion, maximum tumor size and therapy, are
listed in Table I. The mean values
in the CCRT group (n=95) were as follows: NLR=3.17 (range,
0.89–9.52); PLR=188.29 (range, 40.61–466.49); and PNI=49.83 (range,
39.15–62.80). The mean values in the RT group (n=36) were as
follows: NLR=3.05 (range, 1.15–8.05); PLR=183.40 (range,
74.88–533.22); and PNI=47.41 (range, 30.34–56.10).
The distribution of these three values according to
the patients' clinical characteristics is shown in Table II. In the CCRT group, PLR was found
to be significantly associated with LN metastasis (P<0.001) and
vaginal invasion (P=0.005), whereas PNI was significantly
associated with stage (P=0.004), LN metastasis (P=0.031),
parametrial involvement (P=0.011) and vaginal invasion (P=0.027).
In the RT group, NLR was significantly associated with stage
(P=0.041), histology (P=0.012), maximum tumor size (P=0.013),
parametrial involvement (P<0.001) and vaginal invasion
(P=0.044); PLR was associated with FIGO stage (P=0.030), histology
(P=0.008), maximum tumor size (P=0.005) and parametrial involvement
(P=0.016); and PNI was associated with histology (P=0.018), LN
metastasis (P=0.010), maximum tumor size (P=0.001) and parametrial
involvement (P<0.001; Mann-Whitney U test, P<0.05).
 | Table II.Associations of NLR, PLR and PNI with
clinical factors in cervical cancer. |
Table II.
Associations of NLR, PLR and PNI with
clinical factors in cervical cancer.
| A, CCRT |
|---|
|
|---|
| Variables | N | NLR | P-value | PLR | P-value | PNI | P-value |
|---|
| Stage |
|
| 0.216 |
|
0.158 |
|
0.004a |
|
I–II | 67 | 3.05±1.46 |
| 179.29±70.98 |
| 50.65±4.50 |
|
|
III–IV | 28 | 3.49±1.81 |
|
209.81±102.60 |
| 47.86±3.73 |
|
| Histology |
|
| 0.089 |
|
0.208 |
| 0.471 |
|
SCC | 86 | 3.23±1.63 |
| 191.73±83.88 |
| 49.67±4.40 |
|
|
Non-SCC | 9 | 2.67±0.77 |
| 155.37±57.34 |
| 48.54±5.02 |
|
| LNM |
|
| 0.056 |
|
<0.001a |
|
0.031a |
|
Negative | 57 | 2.92±1.43 |
| 162.82±62.51 |
| 50.63±4.25 |
|
|
Positive | 38 | 3.55±1.73 |
| 226.48±93.58 |
| 48.63±4.54 |
|
| MTS, cm |
|
| 0.154 |
|
0.233 |
| 0.089 |
|
≤4.0 | 24 | 2.78±1.42 |
| 170.95±72.65 |
| 51.17±4.48 |
|
|
>4.0 | 71 | 3.31±1.61 |
| 194.15±84.86 |
| 49.38±4.39 |
|
| PI |
|
| 0.506 |
|
0.155 |
|
0.011a |
|
Negative | 20 | 3.00±1.19 |
| 169.06±60.55 |
| 52.04±3.69 |
|
|
Positive | 75 | 3.22±1.67 |
| 193.41±86.69 |
| 49.24±4.48 |
|
| VI |
|
| 0.229 |
|
0.005a |
|
0.027a |
|
Negative | 48 | 2.98±1.34 |
| 165.19±68.46 |
| 50.82±4.28 |
|
|
Positive | 47 | 3.37±1.77 |
| 211.88±88.84 |
| 48.81±4.44 |
|
|
| B, RT |
|
| Variables | N | NLR | P-value | PLR | P-value | PNI | P-value |
|
| Stage |
|
|
0.041a |
|
0.030a |
|
0.018a |
|
I–II | 21 | 2.58±1.55 |
| 151.11±53.27 |
| 49.38±5.61 |
|
|
III–IV | 15 | 3.70±1.59 |
|
228.61±119.62 |
| 44.65±5.76 |
|
| Histology |
|
|
0.012a |
|
0.008a |
|
0.109 |
|
SCC | 28 | 3.31±1.74 |
|
198.00±100.48 |
| 46.77±6.55 |
|
|
Non-SCC | 8 | 2.16±0.79 |
| 132.29±38.12 |
| 49.65±3.42 |
|
| LNM |
|
|
0.114 |
|
0.078 |
|
0.010a |
|
Negative | 26 | 2.78±1.66 |
| 158.49±54.16 |
| 48.97±5.36 |
|
|
Positive | 10 | 3.75±1.46 |
|
248.16±140.72 |
| 43.37±6.20 |
|
| MTS, cm |
|
|
0.013a |
|
0.005a |
|
0.001a |
|
≤4.0 | 17 | 2.35±0.84 |
| 139.37±36.57 |
| 50.62±4.67 |
|
|
>4.0 | 19 | 3.68±1.94 |
|
222.79±112.00 |
| 44.54±5.81 |
|
| PI |
|
|
<0.001a |
|
0.016a |
|
<0.001a |
|
Negative | 10 | 1.96±0.72 |
| 140.77±36.30 |
| 51.83±3.26 |
|
|
Positive | 26 | 3.47±1.71 |
|
199.79±104.42 |
| 45.71±6.08 |
|
| VI |
|
|
0.044a |
| 0.076 |
|
0.249 |
|
Negative | 15 | 2.40±1.70 |
| 153.01±62.18 |
| 48.81±6.18 |
|
|
Positive | 21 | 3.51±1.48 |
|
205.11±107.64 |
| 46.42±5.94 |
|
Overall, the CCRT group had a median
progression-free survival (PFS) of 41.82 months and an overall
survival (OS) of 49.70 months; at the last follow-up, 59 patients
in the CCRT group remained alive with no evidence of disease, 28
had succumbed to the disease and 8 were alive with disease. In the
RT group, the median PFS was 24.72 months and the OS was 29.56
months; at the last follow-up, 22 patients remained alive with no
evidence of disease, 13 had succumbed to the disease and 1 was
alive with disease.
Receiver operating characteristic curve analyses
were used to determine the optimal cut-off values of NLR, PLR and
PNI to predict recurrence (PFS) and survival (OS). The analyses
identified NLR≥2.78 [area under the curve (AUC)=0.635, sensitivity:
63.9%, specificity: 49.2%], PLR≥172.50 (AUC=0.597, sensitivity:
58.3%, specificity: 55.9%) and PNI≤48.55 (AUC=0.720, sensitivity:
72.9%, specificity: 58.3%) as the most accurate cut-off values for
predicting recurrence (PFS) in the CCRT group. The analyses
identified NLR≥2.85 (AUC=0.679, sensitivity: 66.7%, specificity:
66.7%), PLR≥128.00 (AUC=0.829, sensitivity: 80.0%, specificity:
52.4%) and PNI≤45.80 (AUC=0. 876, sensitivity: 85.7%, specificity:
60.0%) as the most accurate cut-off values for predicting
recurrence (PFS) in the RT group.
The analyses identified NLR≥2.78 (AUC=0.616,
sensitivity: 60.7%, specificity: 47.8%), PLR≥171.00 (AUC=0.609,
sensitivity: 60.7%, specificity: 50.7%), and PNI≤48.55 (AUC=0.736,
sensitivity: 73.1%, specificity: 64.3%) as the most accurate
cut-off values for predicting survival (OS) in the CCRT group. The
analyses identified NLR≥2.85 (AUC=0.724, sensitivity: 71.4%,
specificity: 68.2%), PLR≥130.00 (AUC=0.792, sensitivity: 78.6%,
specificity: 50.0%) and PNI≤45.80 (AUC=0.851, sensitivity: 86.4%,
specificity: 57.1%), as the most accurate cut-off values for
predicting survival (OS) in the RT group (Fig. 1).
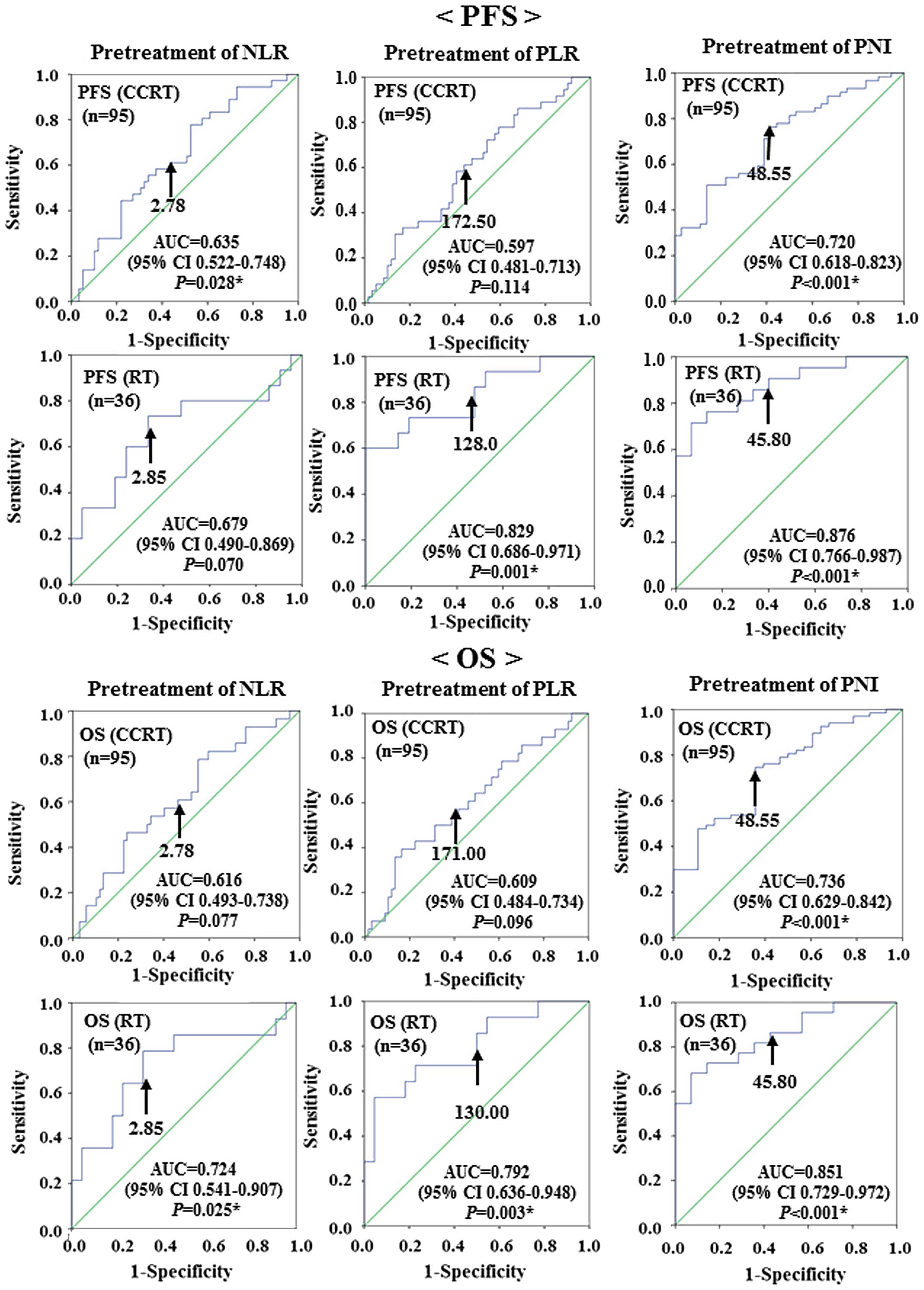 | Figure 1.Receiver operating characteristic
curve analysis determining the optimal cut-off values for NLR, PLR
and PNI to predict recurrence (PFS) and survival (OS) in patients
with cervical cancer treated with CCRT (n=95) or with RT (n=36).
The optimal cut-off values for predicting recurrence (PFS) in the
CCRT group were as follows: NLR=2.78 (AUC=0.635; 95% CI:
0.522–0.748; P=0.028); PLR=172.50 (AUC=0.597; 95% CI: 0.481–0.713;
P=0.114); and PNI=48.55 (AUC=0.720; 95% CI: 0.618–0.823;
P<0.001). The optimal cut-off values for predicting recurrence
(PFS) in the RT group were as follows: NLR=2.85 (AUC=0.679; 95% CI:
0.490–0.869; P=0.070); PLR=128.00 (AUC=0.829; 95% CI: 0.686–0.971;
P=0.001); and PNI=45.80 (AUC=0.876; 95% CI: 0.766–0987;
P<0.001). The optimal cut-off values for predicting survival
(OS) in the CCRT group were as follows: NLR=2.78 (AUC=0.616; 95%
CI: 0.493–0.738; P=0.077); PLR=171.00 (AUC=0.609; 95% CI:
0.484–0.734; P=0.096); and PNI=48.55 (AUC=0.736; 95% CI:
0.629–0.842; P<0.001). The optimal cut-off values for predicting
survival (OS) in the RT group were as follows: NLR=2.85 (AUC=0.724;
95% CI: 0.541–0.907; P=0.025); PLR=130.00 (AUC=0.792; 95% CI:
0.636–0.948; P=0.003); and PNI=45.80 (AUC=0.851; 95% CI:
0.729–0.972; P<0.001). PFS, progression-free survival; OS,
overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR,
platelet-to-lymphocyte ratio; PNI, prognostic nutritional index;
CCRT, concurrent chemoradiotherapy; RT, radiotherapy; AUC, area
under the curve; CI, confidence interval. |
The correlations between clinical factors and
recurrence (PFS) or survival (OS) were assessed in univariate and
multivariate analyses (Tables III
and IV). In the univariate
analysis, LN metastasis (P=0.032), histology (P=0.006), maximum
tumor size (P=0.013), PNI (P=0.002) and extended radiation duration
(>6 weeks; P=0.036) were significantly associated with
recurrence (PFS) in the CCRT group. Moreover, histology (P=0.016),
maximum tumor size (P=0.045) and PNI (P=0.012) were independent
predictors of recurrence (PFS) in the CCRT group on multivariate
analysis. Univariate analysis suggested that LN metastasis
(P=0.013), histology (P=0.012), PNI (P=0.001) and extended
radiation duration (P=0.016) were significantly associated with OS
in the CCRT group. Moreover, histology (P=0.010) and PNI (P=0.003)
were independent predictors of OS in the CCRT group.
 | Table III.Prognostic factors for
progression-free and overall survival in cervical cancer patients
who underwent CCRT (n=95) by Cox's multivariate analysis. |
Table III.
Prognostic factors for
progression-free and overall survival in cervical cancer patients
who underwent CCRT (n=95) by Cox's multivariate analysis.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Factors | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value |
|---|
| Stage | 1.397 | (0.707–2.758) | 0.336 |
|
|
| 1.335 | (0.616–2.895) | 0.464 |
|
|
|
| Lymph node
metastasis | 2.049 | (1.064–3.947) | 0.032a | 1.485 | (0.764–2.888) | 0.244 | 2.612 | (1.222–5.585) | 0.013a | 2.019 | (0.936–4.354) | 0.073 |
| Histology | 0.313 | (0.137–0.717) | 0.006a | 0.356 | (0.153–0.824) | 0.016a | 0.315 | (0.127–0.779) | 0.012a | 0.300 | (0.120–0.751) | 0.010a |
| Maximum tumor
size | 4.510 |
(1.382–14.715) | 0.013a | 3.401 | (1.027–11.267) | 0.045a | 3.270 |
(0.987–10.835) | 0.053 |
|
|
|
| Parametrial
involvement | 1.803 | (0.701–4.638) | 0.221 |
|
|
| 1.757 | (0.609–5.065) | 0.297 |
|
|
|
| Vaginal
invasion | 0.803 | (0.416–1.550) | 0.513 |
|
|
| 1.275 | (0.606–2.680) | 0.522 |
|
|
|
| NLR | 1.423 | (0.728–2.783) | 0.303 |
|
|
| 1.536 | (0.717–3.289) | 0.269 |
|
|
|
| PLR | 1.522 | (0.784–2.954) | 0.215 |
|
|
| 1.634 | (0.753–3.543) | 0.214 |
|
|
|
| PNI | 2.796 | (1.438–5.437) | 0.002a | 2.380 | (1.212–4.676) | 0.012a | 3.754 | (1.726–8.161) | 0.001a | 3.273 | (1.481–7.237) | 0.003a |
| Leukopenia (grade
4) | 0.858 | (0.303–2.427) | 0.772 |
|
|
| 1.126 | (0.390–3.248) | 0.827 |
|
|
|
| Neutropenia (grade
4) | 0.047 |
(0.000–78.691) | 0.419 |
|
|
| 0.047 |
(0.000–219.951) | 0.478 |
|
|
|
| Lymphocytopenia
(grade 4) | 0.874 | (0.430–1.776) | 0.709 |
|
|
| 1.022 | (0.462–2.258) | 0.958 |
|
|
|
| Thrombocytopenia
(grade 4) | 1.853 | (0.445–7.720) | 0.397 |
|
|
| 2.102 | (0.498–8.861) | 0.312 |
|
|
|
| Weight loss (grade
≥2) | 1.072 | (0.446–2.577) | 0.876 |
|
|
| 1.211 | (0.460–3.187) | 0.699 |
|
|
|
| Diarrhea (grade
≥3) | 0.742 | (0.288–1.909) | 0.535 |
|
|
| 0.940 | (0.356–2.485) | 0.901 |
|
|
|
| Hyponatremia(grade
≥3) | 1.090 | (0.545–2.179) | 0.808 |
|
|
| 1.621 | (0.757–3.470) | 0.214 |
|
|
|
| ERD (>6
weeks) | 2.322 | (1.056–5.105) | 0.036a | 1.799 | (0.814–3.976) | 0.147 | 2.893 | (1.221–6.852) | 0.016a | 2.096 | (0.887–4.952) | 0.092 |
 | Table IV.Clinical factors affecting
progression-free and overall survival in cervical cancer patients
who underwent RT (n=36) by Cox's multivariate analysis. |
Table IV.
Clinical factors affecting
progression-free and overall survival in cervical cancer patients
who underwent RT (n=36) by Cox's multivariate analysis.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Factors | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value |
|---|
| Stage | 3.846 | (1.308–11.310) | 0.014a | 0.603 | (0.114–3.197) | 0.552 | 4.263 | (1.331–13.652) | 0.015a | 0.757 | (0.129–4.444) | 0.758 |
| Lymph node
metastasis | 4.091 | (1.472–11.370) | 0.007a | 1.919 | (0.383–9.610) | 0.428 | 4.101 | (1.413–11.904) | 0.009a | 1.459 | (0.270–7.885) | 0.661 |
| Histology | 1.134 | (0.320–4.021) | 0.846 |
|
|
| 1.040 | (0.289–3.742) | 0.952 |
|
|
|
| Maximum tumor size
(>4.0 cm) | 18.773 |
(2.457–143.465) | 0.005a | 11.976 |
(1.263–1,013.546) | 0.030a | 15.656 |
(2.044–119.908) | 0.008a | 8.072 | (0.873–74.642) | 0.066 |
| Parametrial
involvement | 3.075 | (0.691–13.674) | 0.140 |
|
|
| 2.850 | (0.635–12.788) | 0.172 |
|
|
|
| Vaginal
invasion | 1.639 | (0.559–4.803) | 0.368 |
|
|
| 1.922 | (0.601–6.140) | 0.271 |
|
|
|
| NLR | 3.587 | (1.139–11.292) | 0.029a | 0.724 | (0.158–3.321) | 0.678 | 4.766 | (1.324–17.154) | 0.017a | 1.682 | (0.342–8.261) | 0.522 |
| PLR | 3.059 | (0.862–10.858) | 0.084 |
|
|
| 2.660 | (0.741–9.547) | 0.133 |
|
|
|
| PNI | 5.315 | (1.8741–5.074) | 0.002a | 3.127 | (0.944–10.356) | 0.062 | 4.219 | (1.456–12.228) | 0.008a | 1.783 | (0.551–5.764) | 0.334 |
| Lymphocytopenia
(grade 4) | 0.047 |
(0.000–16,748.526) | 0.639 |
|
|
| 0.047 |
(0.000–25,878.809) | 0.651 |
|
|
|
| Weight loss (grade
≥2) | 2.770 | (0.770–9.965) | 0.119 |
|
|
| 2.775 | (0.762–10.106) | 0.122 |
|
|
|
| Diarrhea (grade
≥3) | 1.828 | (0.238–14.027) | 0.562 |
|
|
| 1.940 | (0.251–14.981) | 0.525 |
|
|
|
| Hyponatremia (grade
≥3) | 2.467 | (0.875–6.959) | 0.088 |
|
|
| 2.635 | (0.913–7.605) | 0.073 |
|
|
|
| ERD (>6
weeks) | 10.637 | (3.071–36.843) |
<0.001a | 5.888 | (1.332–26.026) | 0.019a | 13.439 | (3.515–51.378) |
<0.001a | 4.853 | (1.044–22.558) | 0.044a |
In the RT group, univariate analysis suggested that
stage (P=0.014), LN metastasis (P=0.007), maximum tumor size
(P=0.005), NLR (P=0.029), PNI (P=0.002) and extended radiation
duration (P<0.001) were significantly associated with recurrence
(PFS). Moreover, maximum tumor size (P=0.030) and extended
radiation duration (P=0.019) were independent predictors of
recurrence (PFS) in the RT group. Univariate analysis suggested
that stage (P=0.015), LN metastasis (P=0.009), maximum tumor size
(P=0.008), NLR (P=0.017), PNI (P=0.008) and extended radiation
duration (P<0.001) were significantly associated with OS in the
RT group. Moreover, extended radiation duration (P<0.001) were
independent predictors of OS in the RT group.
Discussion
In cervical cancer, stage, tumor size, histological
type, presence of lymphovascular invasion and metastasis to the
regional LNs at the time of diagnosis are significant prognostic
factors (3,4). However, the prognostic value of SIR in
cervical cancer remains unknown. To the best of our knowledge, this
is the first study to evaluate whether NLR, PLR and PNI are
predictors of poor prognosis for patients with cervical cancer
treated with CCRT or RT.
Neutrophils release inflammatory cytokines,
leukocyte chemotactic factors and other phagocytic mediators that
may damage cellular DNA, inhibit apoptosis and promote angiogenesis
(25–28). Platelets release potent mitogens or
adhesive glycoproteins, such as platelet-derived growth factor,
transforming growth factor-β and vascular endothelial growth factor
(29–31). The albumin levels decrease with
increased levels of pro-inflammatory cytokines, such as interleukin
(IL)-1, IL-6 and tumor necrosis factor, which modulate albumin
production (32). Lymphocytes, such
as CD3+ T cells and natural killer cells may affect
tumor growth and metastasis (33).
Recent evidence has shown that relative differences in neutrophil,
platelet and lymphocyte counts, albumin levels, NLR, PLR, and PNI,
are systemic indicators of prognosis. PNI is based on albumin and
absolute lymphocyte count, which are measured routinely in clinical
practice, and it is designed to assess nutritional and
immunological status, which may predict prognosis (34). Mizunuma et al reported that
NLR was a significant prognostic factor for PFS and OS in patients
with cervical cancer treated with CCRT or RT (15).
We investigated whether pretreatment
clinicopathological parameters were correlated with NLR, PLR and
PNI, which reflect SIR. In the CCRT group, PLR was significantly
associated with LN metastasis and vaginal invasion; and PNI was
significantly associated with stage, LN metastasis, parametrial
involvement and vaginal invasion. In the RT group, NLR was
significantly associated with stage, histology, maximum tumor size,
parametrial involvement and vaginal invasion; PLR was associated
with stage, histology, maximum tumor size and parametrial
involvement; and PNI was associated with stage, LN metastasis,
maximum tumor size and parametrial involvement.
The present study mainly aimed to evaluate the
correlation of certain parameters, such as NLR, PLR and PNI, with
recurrence and survival in cervical cancer patients who underwent
CCRT or RT. In the CCRT group, the PFS and OS of patients with
lower PNI were significantly worse compared with those of patients
with higher PNI. Multivariate analysis identified PNI as an
independent prognostic factor for both PFS and OS. In the RT group,
the PFS and OS of patients with higher NLR were significantly
shorter compared with those of patients with lower NLR; and the PFS
and OS of patients with lower PNI were significantly shorter
compared with those of patients with higher PNI. Furthermore, PNI
was found to be superior to NLR and PLR as a predictor of survival
in the CCRT group.
There were certain limitations to our study,
including the limited number of patients and the relatively short
duration of follow-up. Further prospective studies with more
patients and longer follow-up periods would provide more definitive
data to elucidate the significance of our findings.
In conclusion, our results demonstrated that the
determination of PNI may serve as a useful indicator of prognosis
in cervical cancer patients who undergo CCRT.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benedet JL, Odicino F, Maisonneuve P,
Beller U, Creasman WT, Heintz AP, Ngan HY, Sideri M and Pecorelli
S: Carcinoma of the cervix uteri. J Epidemiol Biostat. 6:7–43.
2001.PubMed/NCBI
|
|
3
|
Burghardt E, Pickel H, Haas J and Lahousen
M: Prognostic factors and operative treatment of stages IB to IIB
cervical cancer. Am J Obstet Gynecol. 156:988–996. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Bommel PF, Van Lindert AC, Kock HC,
Leers WH and Neijt JP: A review of prognostic factors in
early-stage carcinoma of the cervix (FIGO I B and II A) and
implications for treatment strategy. Eur J Obstet Gynecol Reprod
Biol. 26:69–84. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Acmaz G, Aksoy H, Unal D, Ozyurt S,
Cingillioglu B, Aksoy U and Muderris I: Are neutrophil/lymphocyte
and platelet/lymphocyte ratios associated with endometrial
precancerous and cancerous lesions in patients with abnormal
uterine bleeding? Asian Pac J Cancer Prev. 15:1689–1692. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Babu SN, Chetal G and Kumar S: Macrophage
migration inhibitory factor: A potential marker for cancer
diagnosis and therapy. Asian Pac J Cancer Prev. 13:1737–1744. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Absenger G, Szkandera J, Stotz M,
Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H,
Samonigg H and Gerger A: Preoperative neutrophil-to-lymphocyte
ratio predicts clinical outcome in patients with stage II and III
colon cancer. Anticancer Res. 33:4591–4594. 2013.PubMed/NCBI
|
|
8
|
Migita K, Takayama T, Saeki K, Matsumoto
S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N and
Nakajima Y: The prognostic nutritional index predicts long-term
outcomes of gastric cancer patients independent of tumor stage. Ann
Surg Oncol. 20:2647–2654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda M, Fujii T, Kodera Y, Nagai S,
Takeda S and Nakao A: Nutritional predictors of postoperative
outcome in pancreatic cancer. Br J Surg. 98:268–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang
J, Wang T, Zhu W and Liu P: Prognostic value of PLR in various
cancers: A meta-analysis. PLoS One. 9:e1011192014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fathalla MF: Factors in the causation and
incidence of ovarian cancer. Obstet Gynecol Surv. 27:751–768. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi T, Teruya M, Kishiki T, Endo D,
Takenaka Y, Tanaka H, Miki K, Kobayashi K and Morita K:
Inflammation-based prognostic score, prior to neoadjuvant
chemoradiotherapy, predicts postoperative outcome in patients with
esophageal squamous cell carcinoma. Surgery. 144:729–735. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haruma T, Nakamura K, Nishida T, Ogawa C,
Kusumoto T, Seki N and Hiramatsu Y: Pre-treatment neutrophil to
lymphocyte ratio is a predictor of prognosis in endometrial cancer.
Anticancer Res. 35:337–343. 2015.PubMed/NCBI
|
|
14
|
Cummings M, Merone L, Keeble C, Burland L,
Grzelinski M, Sutton K, Begum N, Thacoor A, Green B, Sarveswaran J,
et al: Preoperative neutrophil: Lymphocyte and platelet: Lymphocyte
ratios predict endometrial cancer survival. Br J Cancer.
113:311–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizunuma M, Yokoyama Y, Futagami M, Aoki
M, Takai Y and Mizunuma H: The pretreatment
neutrophil-to-lymphocyte ratio predicts therapeutic response to
radiation therapy and concurrent chemoradiation therapy in uterine
cervical cancer. Int J Clin Oncol. 20:989–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kose M, Celik F, Kose SK, Arioz DT and
Yilmazer M: Could the platelet-to-lymphocyte ratio be a novel
marker for predicting invasiveness of cervical pathologies? Asian
Pac J Cancer Prev. 16:923–926. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim
YT and Lee K: Pre-treatment neutrophil to lymphocyte ratio is
elevated in epithelial ovarian cancer and predicts survival after
treatment. Cancer Immunol Immunother. 58:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SH, Kim SC, Choi BI and Han MC:
Uterine cervical carcinoma: Evaluation of pelvic lymph node
metastasis with MR imaging. Radiology. 190:807–811. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nozoe T, Ninomiya M, Maeda T, Matsukuma A,
Nakashima H and Ezaki T: Prognostic nutritional index: A tool to
predict the biological aggressiveness of gastric carcinoma. Surg
Today. 40:440–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
U.S. Department of Health and Human
Services; National Institutes of Health. National Cancer Institute,
. Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
2010.
|
|
22
|
Kodama J, Takemoto M, Seki N, Nakamura K,
Hongo A, Moriya S, Kanazawa S and Hiramatsu Y: Phase I study of
chemoradiation with nedaplatin and ifosfamide in patients with
advanced squamous cell carcinoma of uterine cervix. Int J Gynecol
Cancer. 18:1300–1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kodama J, Takemoto M, Seki N, Nakamura K,
Hongo A, Kanazawa S and Hiramatsu Y: Phase I study of weekly
nedaplatin and concurrent pelvic radiotherapy as adjuvant therapy
after radical surgery for cervical cancer. Int J Gynecol Cancer.
18:1037–1041. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Münstedt K, Johnson P, Bohlmann MK,
Zygmunt M, von Georgi R and Vahrson H: Adjuvant radiotherapy in
carcinomas of the uterine cervix: The prognostic value of
hemoglobin levels. Int J Gynecol Cancer. 15:285–291. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jackson JR, Seed MP, Kircher CH,
Willoughby DA and Winkler JD: The codependence of angiogenesis and
chronic inflammation. FASEB J. 11:457–465. 1997.PubMed/NCBI
|
|
28
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Assoian RK and Sporn MB: Type beta
transforming growth factor in human platelets: Release during
platelet degranulation and action on vascular smooth muscle cells.
J Cell Biol. 102:1217–1223. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dubernard V, Arbeille BB, Lemesle MB and
Legrand C: Evidence for an alpha-granular pool of cytoskeletal
protein alpha-actinin in human platelets that redistributes with
the adhesive glycoprotein thrombospondin-1 during the exocytotic
process. Arterioscler Tromb Vasc Biol. 17:2293–2305. 1997.
View Article : Google Scholar
|
|
31
|
Kaplan KL, Broekman MJ, Chernoff A,
Lesznik GR and Drillings M: Platelet alpha-granule proteins:
Studies on release and subcellular localization. Blood. 53:604–618.
1979.PubMed/NCBI
|
|
32
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin X, Li W, Lai J, Okazaki M, Sugimoto S,
Yamamoto S, Wang X, Gelman AE, Kreisel D and Krupnick AS: Five-year
update on the mouse model of orthotopic lug transplantation:
Scientific uses, tricks of the trade, and tips for success. J
Thorac Dis. 4:247–258. 2012.PubMed/NCBI
|
|
34
|
Yao ZH, Tian GY, Wan YY, Kang YM, Guo HS,
Liu QH and Lin DJ: Prognostic nutritional index predicts outcomes
of malignant pleural mesothelioma. J Cancer Res Clin Oncol.
139:2117–2123. 2013. View Article : Google Scholar : PubMed/NCBI
|















