Introduction
The prognosis of pancreatic cancer patients with
peritoneal metastases remains very poor, as peritoneal metastases
are one of the major life-threatening factors in such patients
(1,2). S-1, an oral 5-fluorouracil (5-FU)
derivative and paclitaxel (PTX) have been proven to be effective in
the treatment of metastatic pancreatic cancer (3,4).
Combination chemotherapy consisting of intravenous (IV) and
intraperitoneal (IP) PTX with S-1 was well-tolerated and achieved
promising results against peritoneal metastases from gastric and
pancreatic cancer (5,6). However, to the best of our knowledge, a
conversion of an unresectable pancreatic cancer with peritoneal
metastases to a resectable one by this combination chemotherapy has
not yet been reported.
We herein present a case of clinical complete
response to IV and IP PTX with S-1 for peritoneal metastases from
pancreatic cancer. This combination chemotherapy was able to
convert an unresectable pancreatic cancer with peritoneal
metastases into resectable disease.
Case report
The patient was a 65-year-old Japanese woman,
presenting with intermittent constipation, abdominal distention and
back pain. The patient was a non-drinker and a non-smoker.
Laboratory examinations revealed increased levels of pancreatic
enzymes in the blood and urine. The patient was initially diagnosed
with acute pancreatitis and was repeatedly hospitalized; she was
referred to Tonan Hospital (Sapporo, Japan), as her symptoms had
continued for 5 months in 2013. Computed tomography and magnetic
resonance cholangiopancreatography revealed dilation of the tail of
the main pancreatic duct, which was consistent with
post-inflammatory changes. Tumor markers, including
carcinoembryonic antigen and carbohydrate antigen 19–9, were
normal. Endoscopic ultrasonography revealed poorly marginated
low-density lesion in the pancreatic body and fine-needle
aspiration was subsequently performed. Histological examination
revealed minute epithelial masses composed of cells with irregular
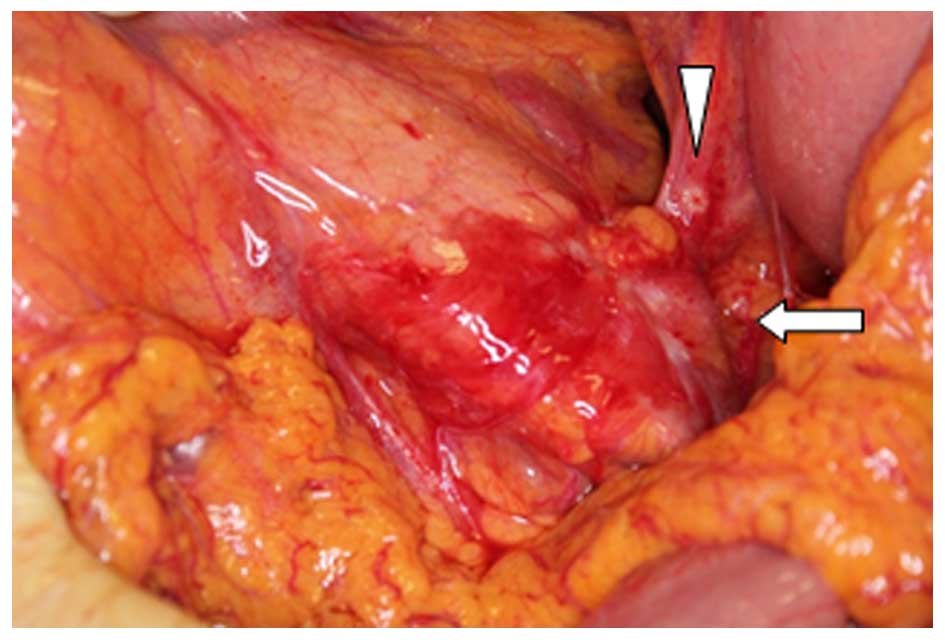
nuclei. The patient underwent open abdominal surgery due to
suspected pancreatic body cancer in June, 2013. However, as two
nodules in the bursa omentalis were found during the surgery and
were diagnosed as tubular adenocarcinoma, pancreatectomy was
abandoned (Fig. 1). Combination
therapy was initiated, consisting of IV and IP PTX with S-1 (Taiho
Pharmaceutical Co., Ltd., Tokyo, Japan), as a single-center
clinical trial in August, 2013. A peritoneal access device was
implanted in the right lower abdominal wall, and an indwelling
catheter was placed in the pelvic cavity. The chemotherapy regimen
was as follows: 2-week administration of S-1 (80 mg per day)
followed by a 1-week rest, with IV PTX 50 mg/m2 and IP
PTX 20 mg/m2 on days 1 and 8. The primary lesion did not
change in size and peritoneal lavage cytology remained negative
during seven cycles of chemotherapy. An exploratory laparoscopy was
performed and peritoneal white nodules were identified, which were
found to be negative for malignancy on frozen section biopsy in
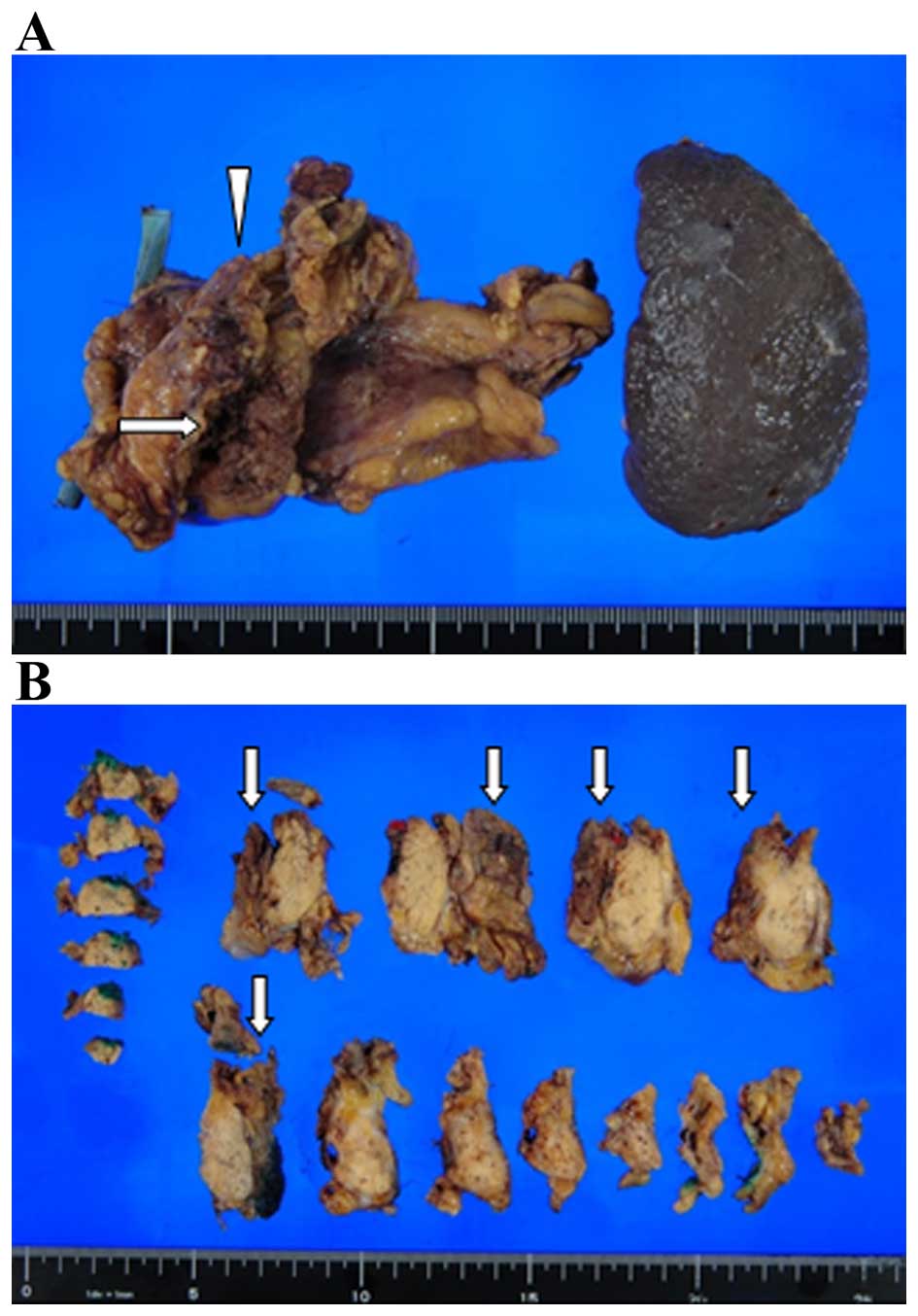
January, 2014. Subsequently, distal pancreatectomy was performed,
combined with resection of the transverse mesocolon and stomach
wall (Fig. 2). The postoperative
course was uneventful.
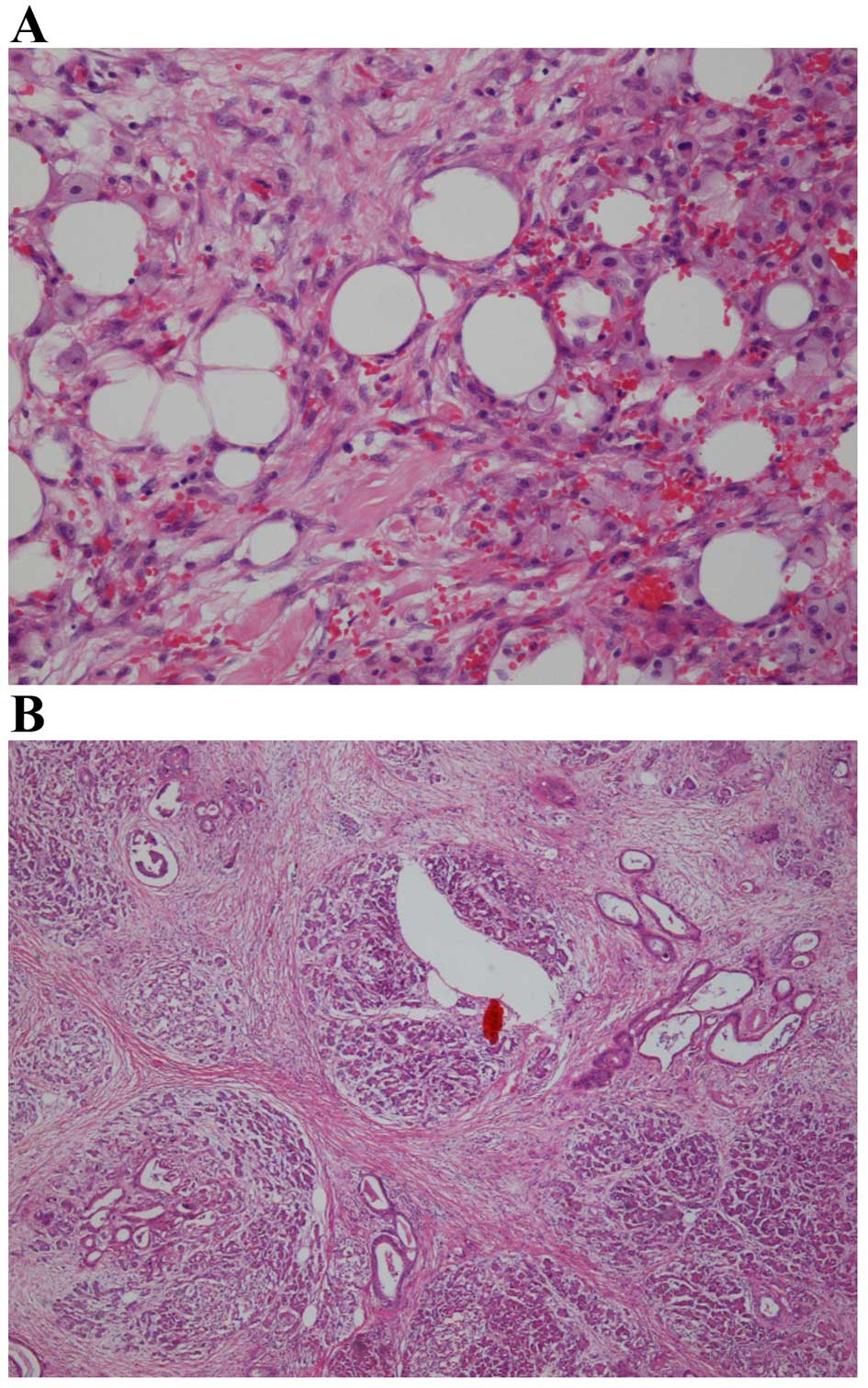
The pancreatic tumor was for the most part replaced
by extensive fibrosis (Fig. 3A).
Furthermore, fibrosis and xanthogranulomatous reaction with
extensive fibrosis were observed in the pancreatic capsule and
transverse mesocolon, indicating that almost all peritoneal
metastases were eliminated after the chemotherapy; only one nodule
in the gastropancreatic fold was microscopically diagnosed as
residual metastasis (Fig. 3B). The
histopathological diagnosis was ductal carcinoma, ypT3, ypN1, ypM1
(PER), G2, L0, V1, R0, ypStage IV according to the 7th edition of
the American Joint Committee on Cancer/International Union Against
Cancer TNM classification (https://cancerstaging.org/references-tools/quickreferences/Documents/PancreasSmall.pdf).
The effect of the preoperative chemotherapy was classified as grade
II using the Evans classification (7).
The patient continued to receive the same
chemotherapy in the adjuvant setting for 8 months after the
surgery, with minor hair and appetite loss. However, peritoneal
carcinoma appeared again in the Douglas pouch in September, 2014.
Local recurrence or other metastases were not detected at the time
of recurrence. The patient succumbed to the disease 6 months later,
in February, 2015 (Table I).
 | Table I.Timeline of interventions and
outcomes. |
Table I.
Timeline of interventions and
outcomes.
| Month, year | Event |
|---|
| July, 2012 | Onset of intermittent
constipation, abdominal distention and back pain. |
| January, 2013 | Diagnosis of
pancreatic body cancer causing recurrent acute pancreatitis. |
| July, 2013 | Exploratory open
abdominal surgery revealing two peritoneal metastases. |
|
| Implantation of a
peritoneal access device. |
| August, 2013 | Seven cycles of
intraperitoneal and intravenous paclitaxel with S-1. |
| January, 2014 | Exploratory
laparoscopy revealing malignancy-negative peritoneal white
nodules. |
|
| Distal pancreatectomy
as radical resection. |
| February, 2014 | Chemotherapy resumed
as adjuvant therapy. |
| September, 2014 | Peritoneal recurrence
in Douglas pouch. |
| February, 2015 | Death from
disease. |
Discussion
The course of this patient draws attention to two
important clinical issues as follows: The first point is that the
2-way chemotherapy combining IV and IP PTX with S-1 was able to
achieve clinical complete response against peritoneal metastases
from pancreatic cancer. The ability of IP PTX to maintain high
concentrations in the peritoneal cavity may have contributed to the
strong antitumor effect against the peritoneal metastases, and the
tumor responded well to the drug combination with S-1. IP
administration of PTX was aimed at acting directly on the
peritoneal metastases at a high concentration. When PTX is
administered IP, it is absorbed slowly due to its high molecular
weight and lipophilic properties, and IP PTX itself is considered
to be associated with a relatively mild toxicity (8). IP administration of PTX has been used
together with systemic chemotherapy for peritoneal metastases from
ovarian cancer, with a significant survival benefit (9). Additionally, S-1 is one of the oral
5-FU agents that were found to be non-inferior to gemcitabine in
terms of overall survival in East-Asian patients with metastatic
pancreatic cancer (3). Yamaguchi
et al reported that IV and IP PTX with S-1 was well
tolerated and highly effective against peritoneal metastases from
gastric cancer (5), and the MPACT
trial demonstrated that PTX was effective in metastatic pancreatic
cancer (4). In fact, IV and IP PTX
with S-1 recently exhibited promising results in
gemcitabine-refractory pancreatic cancer (6). On pathological examination in our case,
almost all peritoneal metastases appeared to have been eradicated
by chemotherapy.
The second important point is that this combination
chemotherapy converted unresectable pancreatic cancer with
peritoneal metastases into resectable disease. Conversion therapy,
which is defined as therapy achieving transformation of
unresectable to resectable disease, has been established in colon
cancer management (10). However,
the viability of conversion therapy for other types of cancer has
not been sufficiently investigated. If chemotherapy manages to
control peritoneal metastases over a long time period, primary
tumor resection may prolong patient overall survival. Conversion
therapy for gastric cancer has been considered beneficial for
patients receiving tolerable and satisfactory chemotherapy, as
improved new regimens including systemic and IP chemotherapy may
achieve long-term control of the metastatic disease in certain
cases (11). This may be also
applicable to pancreatic cancer. It was recently reported that 3
patients with pancreatic cancer with macroscopic peritoneal
metastases underwent R0 resection of the primary tumor following
treatment with IV and IP PTX with S-1 (6). Those 3 cases, as well as our patient,
had residual cancer cells in the primary lesion after chemotherapy.
Primary tumor resection after chemotherapy may be preferable if
peritoneal metastases are controlled well over a long time period.
Our patient received the combination chemotherapy for 5 months
before primary tumor resection. Distal pancreatectomy following
chemotherapy was not complicated. Thus, combination therapy
consisting of IV and IP PTX with S-1 was effective in controlling
peritoneal metastases from pancreatic cancer and convert the
disease status to resectable.
Longer duration of chemotherapy (>8 months prior
to surgery) may be preferable as conversion therapy. Unfortunately,
the disease recurred at the Douglas pouch 8 months after surgery,
which was likely due to drug resistance of the malignant cells. In
a phase II study, all 8 pancreatic cancer patients with peritoneal
metastases received the combination chemotherapy for >8 months
prior to surgery (6). Additionally,
a Japanese multicenter retrospective study demonstrated that
preoperative treatment for >8 months was favorable in terms of
overall survival for initially unresectable pancreatic cancer
(12).
In conclusion, the 2-way chemotherapy consisting of
IV and IP PTX with S-1 converted unresectable pancreatic cancer
with peritoneal metastases into resectable disease and this
combination chemotherapy achieved clinical complete response
against the peritoneal metastases with good tolerance. IP PTX has
the ability to maintain high concentrations for prolonged periods
of time, and this combination chemotherapy has the potential to
become a conversion therapy for pancreatic cancer with peritoneal
metastases. However, further investigation is required to evaluate
the substantivity of this treatment and to determine whether the
pancreatic tumor should be resected after a satisfactory response
of the peritoneal metastases to chemotherapy.
Acknowledgements
The authors would like to thank the patient for her
kind cooperation, including permission to publish the details of
this case, and Mr. David Hochman for reviewing the language of our
article. Taiho Pharmaceutical Co., Ltd. provided lecture fees to Dr
Kitayama and a gratitude scholarship to Professor Hirano. Ono
Pharmaceutical Co., Ltd. provided lecture fees to Dr Tsuji. The
present study was approved by the Institutional Review Board of
Tonan Hospital.
Glossary
Abbreviations
Abbreviations:
|
PTX
|
paclitaxel
|
|
IV
|
intravenous
|
|
IP
|
intraperitoneal
|
References
|
1
|
DeWitt J, Yu M, Al-Haddad MA, Sherman S,
McHenry L and Leblanc JK: Survival in patients with pancreatic
cancer after the diagnosis of malignant ascites or liver metastases
by EUS-FNA. Gastrointest Endosc. 71:260–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morizane C, Okusaka T, Morita S, Tanaka K,
Ueno H, Kondo S, Ikeda M, Nakachi K and Mitsunaga S: Construction
and validation of a prognostic index for patients with metastatic
pancreatic adenocarcinoma. Pancreas. 40:415–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi H, Kitayama J, Ishigami H, Emoto
S, Yamashita H and Watanabe T: A Phase 2 trial of Intravenous and
intraperitoneal paclitaxel combined with S-1 for treatment of
gastric cancer with macroscopic peritoneal metastasis. Cancer.
119:3354–3358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satoi S, Fujii T, Yanagimoto H, Motoi F,
Kurata M, Takahara N, Yamada S, Yamamoto T, Mizuma M, Honda G, et
al: Multicenter Phase II study of intravenous and intraperitoneal
paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients
with peritoneal metastasis. Ann Surg. Mar 11–2016.(Epub ahead of
print). View Article : Google Scholar
|
|
7
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gelderblom H, Verweij J, van Zomeren DM,
Buijs D, Ouwens L, Nooter K, Stoter G and Sparreboom A: Influence
of Cremophor El on the bioavailability of intraperitoneal
paclitaxel. Clin Cancer Res. 8:1237–1241. 2002.PubMed/NCBI
|
|
9
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Gynecologic Oncology Group: Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Power DG and Kemeny NE: Chemotherapy for
the conversion of unresectable colorectal cancer liver metastases
to resection. Crit Rev Oncol Hematol. 79:251–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida K, Yamaguchi K, Okumura N,
Tanahashi T and Kodera Y: Is conversion therapy possible in stage
IV gastric cancer: The proposal of new biological categories of
classification. Gastric Cancer. 19:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese society of
Hepato-Biliary-Pancreatic surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|