Introduction
Serous epithelial ovarian cancer (EOC) accounts for
~75% of EOC subtypes (1,2). The majority of patients with EOC have
no identifiable risk factors or precursor lesions, and only few
effective screening tools for early diagnosis are currently
available (3).
EOC commonly originates from the ovarian surface
epithelium (OSE) and/or ovarian inclusion cysts (4,5).
However, an American pathology group recently proposed a novel
hypothesis, that high-grade serous ovarian cancer (HGSC) (6), the most common histological subtype of
EOC, develops from the Fallopian tubes. Moreover, previous studies
reported that salpingectomy may be associated with a reduced risk
of ovarian cancer, particularly serous EOC (4,6,7). However, this hypothesis does not yet
have an adequate scientific basis. We herein report a case of HGSC
that developed 3 years after bilateral salpingectomy (BS), which
contradicts the hypothesis of a tubal origin for HGSC.
Case report
A 51-year-old postmenopausal woman presented to the
Department of Obstetrics and Gynecology of Shimane University
Hospital (Izumo, Japan) on July, 2014 with low abdominal
distention. The patient had a history of uterine myoma and had
undergone total abdominal hysterectomy, left oophorectomy and BS 3
years prior. The patient was referred to our hospital from her
primary care provider for suspected ovarian cancer. Marked ascites
and a palpable mass were detected in the right adnexal region. The
serum carbohydrate antigen-125 levels were elevated to 604 ng/ml
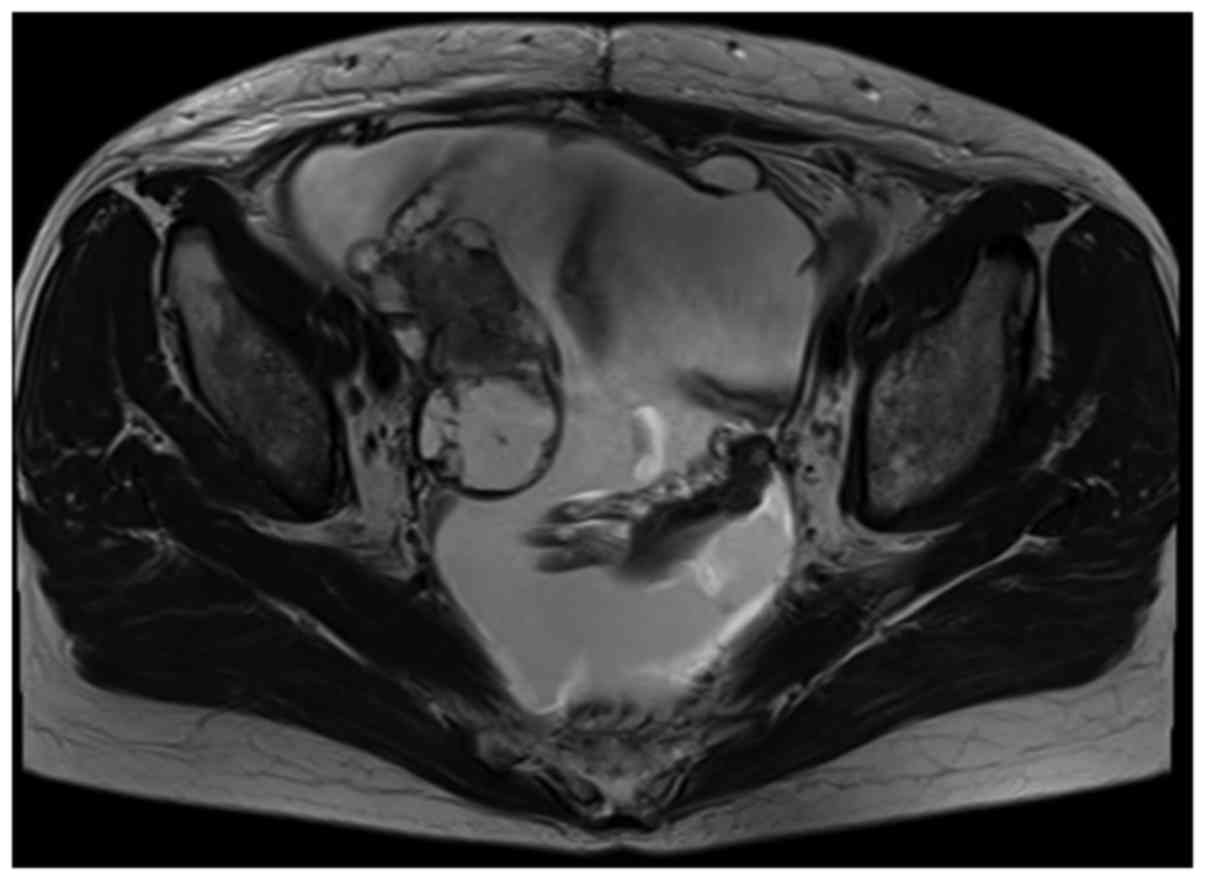
(normal, <35 U/ml). Magnetic resonance imaging revealed ascites
and a mass ~8 cm in diameter in the right adnexal region (Fig. 1). Positron emission
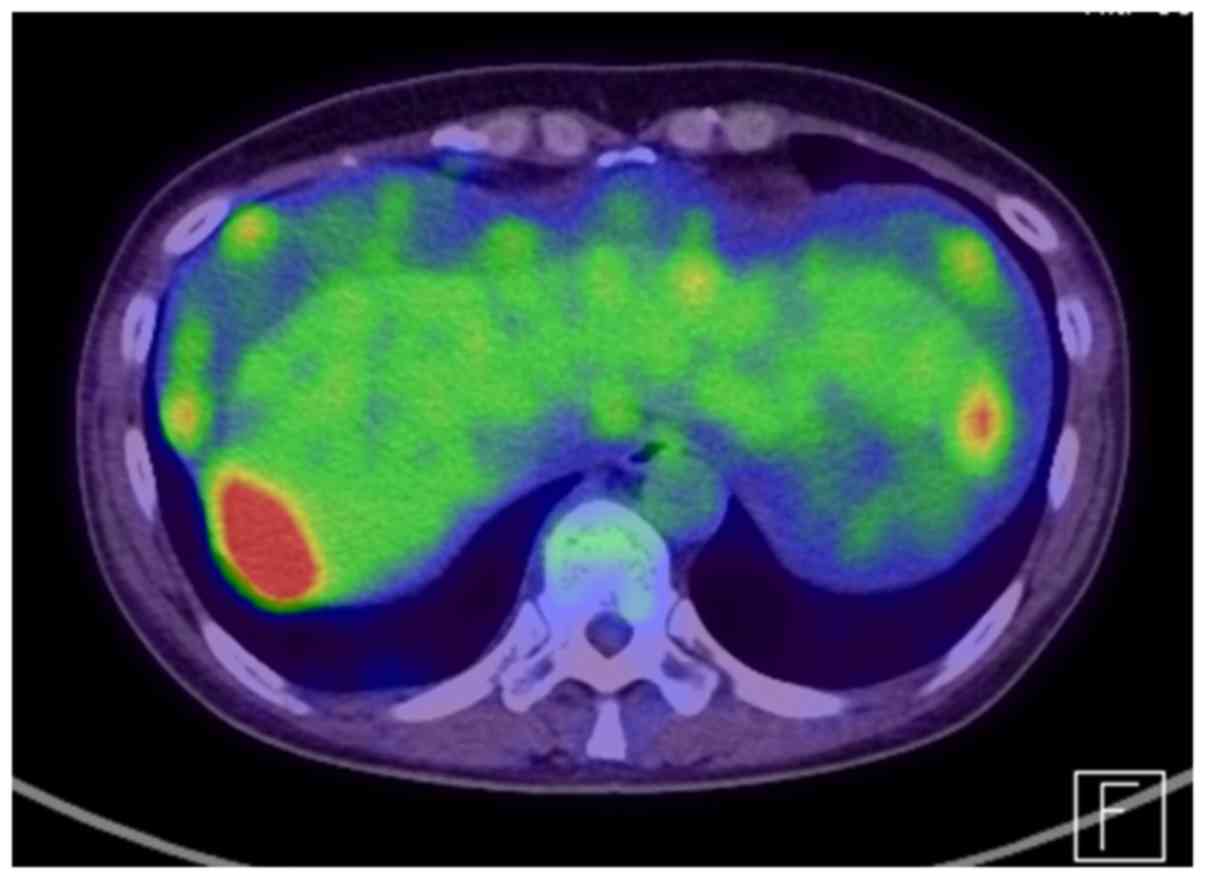
tomography-computed tomography revealed peritoneal dissemination,
omental cake and para-aortic lymph node metastasis (Fig. 2).
The patient underwent primary debulking surgery
(right oophorectomy, omentectomy and resection of disseminated
nodules). Pathological examination revealed stage IV (International
Federation of Gynecology and Obstetrics 1988 guidelines) grade 2
ovarian serous adenocarcinoma, with right pleural metastasis, liver
surface metastasis and peritoneal dissemination. A large number of
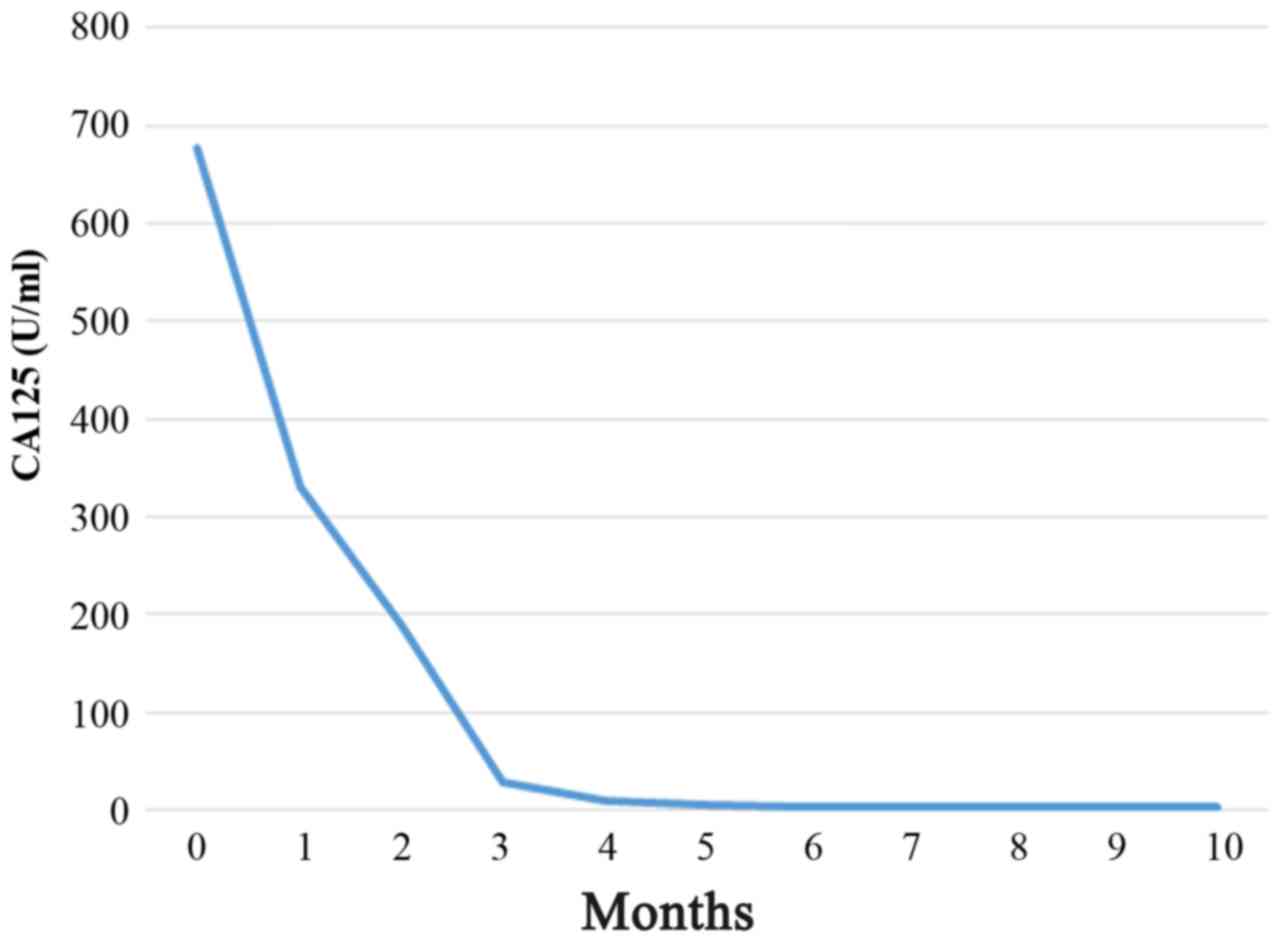
residual tumors were sized >2 cm. As adjuvant therapy, the
patient received combination chemotherapy with paclitaxel (175
mg/m2), carboplatin (area under the curve = 5) and
bevacizumab (15 mg/kg) (Fig. 3)
[Specifically, the patient received paclitaxel (Taxol)-carboplatin
(Carbo) plus bevacizumab (TC+BEV) therapy triweekly, for six
cycles; BEV monotherapy as a maintenance therapy, triweekly for 16
cycles; at the progression stage of the disease, 16 months after
adjuvant TC therapy+BEV, with the growth of intra-peritoneal
implantations, the patient received TC+BEV triweekly, for six
cycles), and subsequently BEV monotherapy (triweekly; now, the
patient receives 1 cycle, and she has stable disease].
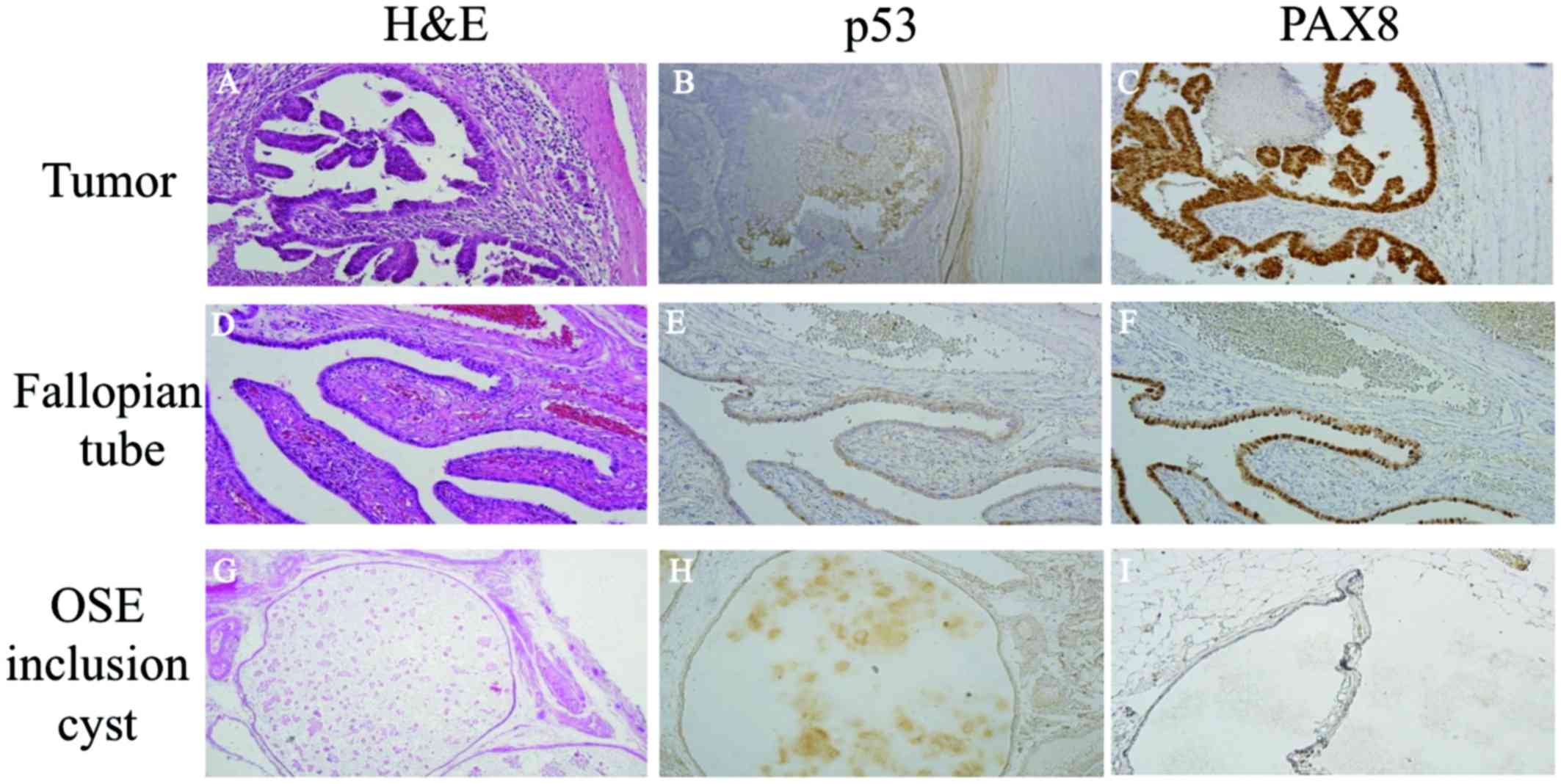
Immunohistochemical examination revealed that p53
was not overexpressed in the tumors or in the inclusion cyst of the
OSE. However, paired box gene 8 (PAX8), a marker of Fallopian tube
epithelium, was strongly positive in the bilateral Fallopian tubes
and the tumor cells, while it was not expressed in the inclusion
cyst of the OSE. The previously resected left ovary was examined,
and although there was no evidence of serous tubal intraepithelial
carcinoma (STIC) or any other cancer, PAX8-positive cells were
detected (Fig. 4).
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Discussion
HGSC is the most common histological subtype of EOC
and it was previously hypothesized that the risk reduction observed
among women who undergo BS mainly reflects a lower incidence of
HGSC (4). Previous findings support
the theory of ovarian cancer originating in the Fallopian tubes
(4,6,7).
However, data from observational studies are generally limited by
small sample size, hospital-based study populations, insufficient
control regarding the effects of oophorectomy, or the influence of
time from a bilateral salpingectomy to the formation of ovarian
cancer (4).
Studies emerging from The Cancer Genome Atlas
Research Network have shed some light on the genetics of ovarian
cancer (4,8,9). HGSC is
commonly driven by p53 and breast cancer, early onset (BRCA) gene
mutations (4). Morphological studies
of the Fallopian tubes in BRCA mutation carriers have also been
identified the STIC region (10).
Therefore, if HGSC develops from the Fallopian tubes, it is likely
that the STIC region and/or p53 mutations are present. The
association between STIC and the p53 signature has not yet been
investigated in HGSC.
To date, it has been hypothesized that STIC lesions
are shed through the Fallopian tubes and incorporated into ovarian
surface inclusion cysts, where they subsequently transform into
HGSC, and are associated with an activating p53 mutation. In the
present case, STIC or p53 overexpression were not detected, which
may overturn the theory that ovarian cancer, particularly HGSC,
originates in the Fallopian tubes. Based on these findings, we
consider that other mechanisms may be associated with HGSC
development in patients without p53 mutations. In the present case,
we hypothesize that HGSC may have originated from an inclusion cyst
of OSE or an inclusion cyst of the tubal epithelium, without STIC
reaching the surface of the ovary, as previously reported (11).
However, PAX8, a marker of Fallopian tube
epithelium, was strongly expressed in the bilateral Fallopian tubes
and in the tumor cells. We are currently investigating the
frequency of PAX8-positive cells in ovarian cancer in an attempt to
elucidate its role in ovarian carcinogenesis.
In conclusion, we herein report a case of HGSC that
developed 3 years after BS, contradicting the hypothesis of a tubal
origin. There was also no region of STIC or p53 expression detected
in the present case. Based on these findings, we consider that
other mechanisms must be responsible for carcinogenesis in HGSC in
patients without p53 mutations.
Acknowledgements
We would like to thank our colleagues at Shimane
University Hospital (Izumo, Japan).
References
|
1
|
Kim J, Coffey DM, Creighton CJ, Yu Z,
Hawkins SM and Matzuk MM: High-grade serous ovarian cancer arises
from fallopian tube in a mouse model. Proc Natl Acad Sci USA.
109:3921–3926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crum CP, Drapkin R, Kindelberger D,
Medeiros F, Miron A and Lee Y: Lessons from BRCA: The tubal fimbria
emerges as an origin for pelvic serous cancer. Clin Med Res.
5:35–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nusbaum R and Isaacs C: Management updates
for women with a BRCA1 or BRCA2 mutation. Mol Diagn Ther.
11:133–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Falconer H, Yin L, Grönberg H and Altman
D: Ovarian cancer risk after salpingectomy: A nationwide
population-based study. J Natl Cancer Inst. 107:2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scully RE: Classification of human ovarian
tumors. Environ Health Perspect. 73:15–25. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lessard-Anderson CR, Handloqten KS,
Molitor RJ, Dowdy SC, Cliby WA, Weaver AL, Sauver JS and
Bakkum-Gamez JN: Effect of tubal sterilization technique on risk of
serous epithelial ovarian and primary peritoneal carcinoma. Gynecol
Oncol. 135:423–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Madsen C, Baandrup L, Dehlendorff C and
Kjaer SK: Tubal ligation and salpingectomy and the risk of
epithelial ovarian cancer and borderline ovarian tumors: A
nationwide case-control study. Acta Obstet Gynecol Scand. 94:86–94.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verhaak RG, Tamayo P, Yang JY, Hubbard D,
Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH,
et al: Cancer Genome Atlas Research Network: Prognostically
relevant gene signatures of high-grade serous ovarian carcinoma. J
Clin Invest. 123:517–525. 2013.PubMed/NCBI
|
|
10
|
Kindelberger DW, Lee Y, Miron A, Hirsch
MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW,
Birch C, et al: Intraepithelial carcinoma of the fimbria and pelvic
serous carcinoma: Evidence for a causal relationship. Am J Surg
Pathol. 31:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banet N and Kurman RJ: Two types of
ovarian cortical inclusion cysts: Proposed origin and possible role
in ovarian serous carcinogenesis. Int J Gynecol Pathol. 34:3–8.
2015. View Article : Google Scholar : PubMed/NCBI
|