Introduction
Over time, the administration of chemotherapy has
moved from an inpatient to an outpatient setting. Over 95% of
patients with solid tumors, including those with breast or
colorectal cancers, are treated in an outpatient setting. However,
only 30% of the patients with hematological malignancies are
treated in an outpatient setting, due to intensive myelotoxicity or
daily administration regimens. A regimen of cyclophosphamide,
doxorubicin, vincristine and prednisolone (CHOP) is considered to
be a standard treatment for non-Hodgkin's lymphoma (NHL). Although
CHOP therapy leads to intensive myelosuppression, an increasing
number of patients are being treated in an outpatient setting. It
is important that NHL patients receive the full chemotherapy
regimen, since the survival rate may be markedly decreased when
CHOP chemotherapy is delivered at <90% of the planned relative
dose intensity (RDI) (1,2). Therefore, prophylactic administration of
granulocyte-colony stimulating factor (G-CSF) is recommended for
NHL patients.
Patients receiving CHOP therapy often experience
febrile neutropenia (FN) due to myelotoxicity. The presence of FN
necessitates a reduction in the planned RDI, and also leads to
hospitalization with the administration of antibiotics (3). The prompt administration of antibiotics
is crucial, since infection can progress rapidly. Although varying
degrees of FN have been reported, the general risk factors for FN
are well known, and include an older age, advanced disease, poor
performance status (PS), comorbidities, baseline hemoglobin and
body surface area (4–7). The proper management of FN is essential
to maintain the RDI and to guarantee a positive outcome of the NHL
treatment. Therefore, the present study retrospectively examined
chemotherapy continuity and incidence of FN with CHOP therapy in an
outpatient setting.
Subjects and methods
Subjects
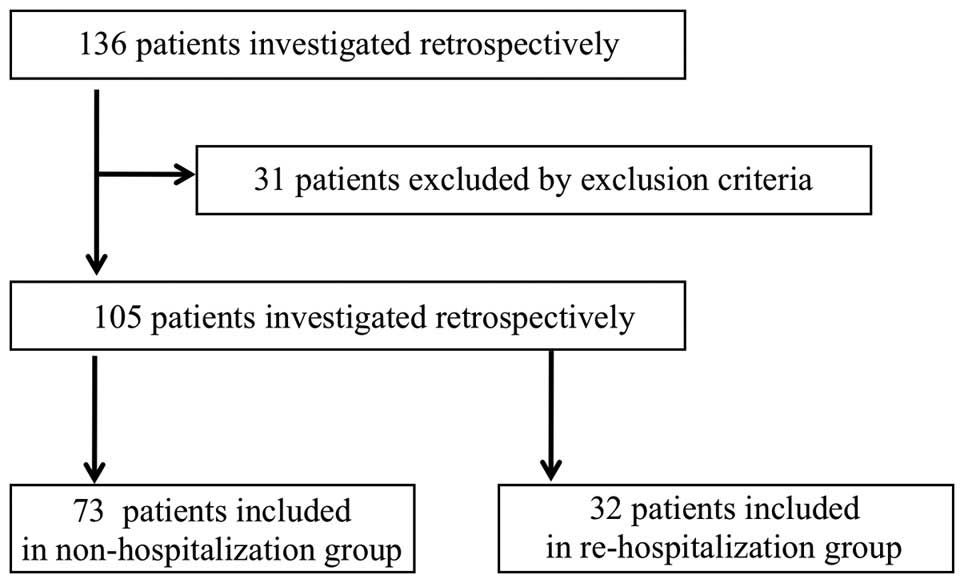
The subjects were 136 patients who received CHOP
chemotherapy at Ogaki Municipal Hospital (Ogaki-shi, Japan) between
January 2012 and December 2014. The first cycle of CHOP
chemotherapy was administered in an inpatient setting for all
patients, and the treatment was moved to an outpatient setting
based on a consideration of the patient's condition. A total of 31
patients were excluded since they were unable to be moved to an
outpatient treatment setting. Of the remaining 105 patients,32
(30.5%) were assigned to the re-hospitalization group, who required
hospitalization again during the chemotherapy treatment in an
outpatient setting, and 73 (69.5%) were assigned to the
non-hospitalization group, who did not require hospitalization
until after the patients had finished their outpatient chemotherapy
(Fig. 1). The CHOP regimen [750 mg/m2
cyclophosphamide, 50 mg/m2 doxorubicin and 1.4 mg/m2 vincristine,
all on day 1; and 100 mg/body or 60 mg/m2 (for patients >65
years) prednisolone daily for 5 days] was administered every 3
weeks. The present study was reviewed and approved by the Ethics
Committee at Ogaki Municipal Hospital.
Characteristics of the subjects
The characteristics of the subjects treated with
CHOP therapy in an outpatient setting were investigated. Gender,
age, histology, clinical stage, PS [Eastern Cooperative Oncology
Group (ECOG) (8)], B symptoms,
chemotherapy treatment times, doses and RDI, the total cycles,
planned treatment completion, duration of treatment with G-CSF,
nadir [day, white blood cell (WBC) and neutrophil count] and the
presence of FN were compared between the re-hospitalization and
non-hospitalization groups.
Incidence of FN in an outpatient
setting
Incidences of FN were compared between the
re-hospitalization and non-hospitalization groups in an outpatient
setting.
Timing of FN occurrence
The timing of FN during the CHOP treatment cycle was
examined.
Investigation of the factors affecting
re-hospitalization
The factors affecting re-hospitalization during
treatment in an outpatient setting were examined.
Causes of re-hospitalization
The factors affecting re-hospitalization during
outpatient treatment were investigated.
Statistical analysis
The data were analyzed using JMP software (version
5.0.1J; SAS Institute Japan Ltd., Tokyo, Japan). The Mann-Whitney U
test was used for comparison of the backgrounds of the subjects
between the groups. The recorded P-values were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference. The areas under the receiver-operator characteristic
(ROC) curves were calculated to estimate the accuracy and cut-off
values for the continuous variables obtained by univariate logistic
regression analysis. Subsequently, the data were analyzed using
multivariate logistic regression analysis.
Results
Characteristics of the subjects
Table I summarizes the
background characteristics of the subjects. The numbers of patients
in the non-hospitalization and re-hospitalization groups were 73
and 32, respectively, of which 71 and 24 completed the planned
treatment, respectively (therefore, 2 and 8, respectively, did not
complete the treatment). In addition, the duration of the G-CSF
treatment was 5.3±1.22 and 6.1±1.46 days, respectively. The numbers
of patients who experienced FN were 14 and 19, respectively.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
|
|
Non-hospitalization |
Re-hospitalization | P-value |
|---|
| No. of patients | 73 | 32 |
|
| Gender |
|
| 0.11 |
| Male | 31 | 19 |
|
|
Female | 42 | 13 |
|
| Age, median
(years) | 66.0 | 73.0 | 0.17 |
|
Range | (37–93) | (18–87) |
|
| Histology |
|
|
|
| Diffuse
large B-cell lymphoma | 38 | 17 |
|
|
Follicular lymphoma | 12 | 6 |
|
| T-cell
lymphoma | 3 | 1 |
|
|
Mucosa-associated lymphoid
tissue lymphoma | 10 | 3 |
|
| Mantle
cell lymphoma | 4 | 2 |
|
|
Others | 6 | 3 |
|
| Clinical stage (Ann
Arbor staging)a |
|
| 0.41 |
| I | 16 | 3 |
|
| II | 13 | 6 |
|
| III | 13 | 8 |
|
| IV | 31 | 15 |
|
| Performance status
(ECOG) |
|
| 0.11 |
| 0 | 62 | 22 |
|
| 1 | 5 | 6 |
|
| 2 | 6 | 3 |
|
| 3 | 0 | 1 |
|
| 4 | 0 | 0 |
|
| B symptoms |
|
| 0.32 |
|
Present | 9 | 2 |
|
|
Absent | 64 | 30 |
|
| Chemotherapy |
|
|
|
| Treatment
times, media (range) | 6 (3–8) | 6 (2–8) | 0.49 |
| Treatment
dose (%), median (range) | 100 (70–100) | 100 (80–100) | 0.71 |
| RDI,
median (range) | 0.93 (0.62–1.00) | 0.9 (0.61–1.00) | 0.22 |
| Total cycles of
CHOP |
|
| 0.49 |
| 2 | 0 | 1 |
|
| 3 | 14 | 1 |
|
| 4 | 1 | 1 |
|
| 5 | 0 | 4 |
|
| 6 | 27 | 10 |
|
| 7 | 0 | 1 |
|
| 8 | 31 | 14 |
|
| Planned
treatment |
|
| <0.01 |
|
Completion | 71 | 24 |
|
|
Unreached | 2 | 8 |
|
| G-CSF |
|
|
|
| Duration
of treatment (days), mean ± S.D. | 5.3±1.22 | 6.1±1.46 | <0.01 |
| Duration
of treatment (days), median (range) | 5 (3–8) | 5 (4–14) | 0.01 |
| Nadir |
|
|
|
| Day,
median (range) | 11 (6–16) | 11 (7–16) | 0.07 |
| WBC
(per µl), median (range) | 1230
(260–8180) | 1395 (50–6080) |
0.86 |
|
Neutrophil (per µl), median
(range) | 357 (15–7443) | 378 (6–4013) |
0.44 |
| FN |
|
| <0.01 |
|
Present | 14 | 19 |
|
|
Absent | 59 | 13 |
|
Incidence of FN in an outpatient
setting
Overall, 31.4% (33/105) of patients receiving CHOP
therapy experienced FN at a certain point during the present study.
Among these, a total of 18.1% (19/105) of the patients experienced
FN in an outpatient setting, and 9.5% (10/105) experienced it for
the first time in an outpatient setting. Additionally, 8.6% (9/105)
of the patients experienced more than two episodes of FN during all
the chemotherapy cycles.
Timing of FN occurrence
FN occurred in 21.0% (22/105) of the patients during
cycle 1, 2.9% (3/105) in cycle 2, 4.8% (5/104) in cycle 3, 5.6%
(5/89) in cycle 4, 6.9% (6/87) in cycle 5, 2.4% (2/83) in cycle 6
and 0% (0/46) of the patients in cycles 7 and 8.
Investigation of the factors affecting
re-hospitalization
A total of 14 factors affecting re-hospitalization
from an outpatient setting were analyzed using univariate logistic
regression analysis. The independent variables of the dosage data
were analyzed as continuous variables, and the results are shown in
Table II. The average duration of
G-CSF treatment [odds ratio (OR), 25.73; 95% confidence interval
(CI), 2.68–308.29; P<0.01], incidence of FN (OR, 4.77; 95% CI,
1.95–12.08; P<0.01), and patients who could not complete the
planned treatment (OR, 11.83; 95% CI, 2.74–82.09; P<0.01)
demonstrated significant differences between the
non-hospitalization and re-hospitalization groups. The area under
the ROC curve of the average duration of G-CSF treatment was 0.67,
and the cut-off value was 5.5 days. Table III shows the results of the
multivariate analysis based on the factors affecting
re-hospitalization, with P<0.25 by univariate logistic
regression analysis. This analysis revealed that the incidence of
FN (OR, 4.61; 95% CI, 1.68–13.19; P<0.01) and unreached planned
treatment (OR, 11.81; 95% CI, 2.07–99.74; P<0.01) were
independent factors that significantly contributed to
re-hospitalization.
 | Table II.Univariate analysis of factors
affecting re-hospitalization from an outpatient setting (n=103) |
Table II.
Univariate analysis of factors
affecting re-hospitalization from an outpatient setting (n=103)
| Factor | OR | 95% CI | P value | AUC | Cut-off |
|---|
| Duration of G-CSF
treatment (days) | 25.73 | 2.68–308.29 | <0.01 | 0.67 | 5.5 |
| Gender
(female) |
0.51 | 0.21–1.16 |
0.11 |
|
|
| Age |
7.21 | 0.47–144.71 |
0.17 |
|
|
| PS≥2 |
0.62 | 0.16–2.61 |
0.49 |
|
|
| Chemotherapy
treatment time |
1.63 | 0.41–7.34 |
0.51 |
|
|
| Chemotherapy
RDI |
0.36 | 0.06–1.89 |
0.22 |
|
|
| Chemotherapy
treatment dose (%) |
1.43 | 0.26–11.26 |
0.71 |
|
|
| Day of nadir
(days) |
0.13 | 0.01–1.19 |
0.08 |
|
|
| White blood cell
count of nadir (per µl) |
0.79 | 0.03–10.97 |
0.86 |
|
|
| Neutrophil count of
nadir (per µl) |
0.32 | 0.01–4.75 |
0.47 |
|
|
| Incidence of
FN |
4.77 | 1.95–12.08 | <0.01 |
|
|
| Clinical stage ≥3
(Ann Arbor) |
0.59 | 0.23–1.43 |
0.25 |
|
|
| B symptoms
present |
2.11 | 0.51–14.39 |
0.35 |
|
|
| Unreached planned
treatment | 11.83 | 2.74–82.09 | <0.01 |
|
|
 | Table III.Multivariate analysis of factors
affecting re-hospitalization from an outpatient setting
(n=103). |
Table III.
Multivariate analysis of factors
affecting re-hospitalization from an outpatient setting
(n=103).
| Factor | OR | 95% CI | P-value |
|---|
| Duration of G-CSF
treatment (≥5.5 days) |
0.21 | 0.17–1.42 |
0.20 |
| Female |
0.43 | 0.15–1.17 |
0.11 |
| Age |
0.48 |
0.01–17.76 |
0.67 |
| Chemotherapy
RDI |
0.51 | 0.06–4.01 |
0.51 |
| Day of nadir
(days) |
0.33 | 0.02–4.97 |
0.43 |
| Incidence of
FN |
4.61 |
1.68–13.19 | <0.01 |
| Unreached planned
treatment | 11.81 |
2.07–99.74 | <0.01 |
Causes of re-hospitalization
The cause of hospitalization with respect to the 32
re-hospitalization patients was FN in 59.4% (19/32) of the
patients, delayed WBC recovery in 12.5% (4/32), Pneumocystis
carinii pneumonia in 9.4% (3/32), pneumonia in 9.4% (3/32), a
decline in PS in 6.3% (2/32), and heart failure in 3.1% (1/32) of
the patients.
Discussion
The chemotherapy regimens used for patients with NHL
are often dose-intensive, with high rates of associated
neutropenic-related morbidity and occasional mortality (3). In the present study, grade 4 neutropenia
occurred in 70.5% (74/105) of the patients, with grade 3 occurring
in 20.0% (21/105) of the patients. Over 90% of the patients
experienced serious hematological toxicity. The myelotoxic regimen
often results in FN, which is the most serious hematological
toxicity (10,11). Hospitalization and prompt
administration of antibiotics are necessary for patients with FN,
since the infection can progress rapidly. Additionally, FN
increases medical costs and may lead to delays in the treatment
schedule and reductions in chemotherapy delivery. In the present
study, 43.4% (59/136) of all patients receiving CHOP therapy
experienced FN at a certain point during the study. The FN
occurrence rate of the patients who could be treated in an
outpatient setting was 31.4% (33/105), which is notably high.
Furthermore, 22.8% (31/136) of the patients could not be treated in
an outpatient setting, and 83.9% (26/31) of these experienced FN.
Almost all these patients experienced long- term hospitalization
and unreached treatment. The CHOP regimen is regarded as an
intermediate risk for FN, with an occurrence rate of 10–20%
recorded in the National Comprehensive Cancer Network and the
European Organization for Research and Treatment of Cancer
guidelines (4). However, several
studies have previously reported an FN occurrence rate of 28–58%
among NHL patients (5,12–14),
suggesting that the CHOP regimen itself poses a high risk for FN in
a real clinical situation.
Initially, CHOP chemotherapy was administered in an
inpatient setting to gauge the possible adverse events. The FN
occurrence rate for patients who received the CHOP regimen with
primary prophylaxis of G-CSF was 17–22% (13,15,16). G-CSF
primary prophylaxis was administered in 90.6% of the patients in
the present study. The FN occurrence rate of patients in cycle 1
was 21.0% (22/105). In agreement with a report by Mayordomo et
al (17), 53.7% of all FN events
(22/41) occurred during cycle 1. Lyman et al (18) reported that a lack of primary
prophylaxis with G-CSF in cycle 1 was associated with an increased
risk of FN (18). However, although
primary prophylaxis of G-CSF for almost all patients was
administered in the present study, the FN occurrence rate in cycle
1 was the highest of all the treatment cycles. This result suggests
that it is necessary to educate patients about FN prior to the
first chemotherapy cycle.
Pegfilgrastim (Peg-G) comprises the protein
filgrastim, to which a polyethylene glycol (Peg) molecule is bound
covalently to the N-terminal methionine residue (19,20). It
was first approved in the United States in 2002, and approved for
use in Japan in November 2014. The addition of the Peg molecule
increases the serum half-life of Peg-G, thus requiring fewer
injections compared with unmodified G-CSF. In the present study,
the duration of G-CSF treatment was longer in the
re-hospitalization group compared with the non-hospitalization
group (P<0.01). Since Peg-G was not used in the present study, a
daily administration of G-CSF was required, in spite of severe
neutropenia and the risk of infection in the outpatient setting.
Requiring only one subcutaneous injection per cycle, Peg-G may be
more convenient, and pose less of a risk, for the patient. It may
become the standard of care to maintain the patients' quality of
life, and to reduce the occurrence of FN.
Unplanned re-hospitalizations occurred in 30.5%
(32/105) of the patients, which was due to FN or pneumonia in ~80%
of the patients. Pettengell et al (15) reported a similar rate of
re-hospitalization. The incidence of FN is an independent factor
significantly contributing to the re-hospitalizations (OR, 4.61;
95% CI, 1.68–13.19; P<0.01). The FN rate in an outpatient
setting was 18.1% (19/105). In this outpatient setting, 9.5%
(10/105) of the patients experienced FN for the first time.
Therefore, there is a continual requirement to educate patients on
infection prevention.
In the present study, 9.5% (10/105) of the patients
were not able to complete their planned treatment. The numbers of
patients unable to complete the treatment were significantly
different between the re-hospitalization and non-hospitalization
groups (OR, 11.81; 95% CI, 2.07–99.74; P<0.01). The reasons why
patients were unable to complete treatment were severe infection
(5/10), a poor PS (4/10) and heart failure (1/10). Infection has
been identified as an important risk factor for the completion of
planned treatment. The mean age of the patients who were unable to
complete their planned treatment was 80 years (range 63–93 years),
which was significantly older compared with the age of the patients
who were able to complete their planned treatment (P<0.01). In
the present study, 20.6% (28/136) of all the patients who received
CHOP chemotherapy were >80 years, and only ~50% of them (13/28)
could be treated in an outpatient setting. From the first cycle,
76.9% (10/13) of the patients were treated with chemotherapy at an
80% reduced dose, and 38.5% (5/13) of patients were not able to
complete their planned treatment. Age was not identified as an
independent risk factor for re-hospitalization and the incidence of
FN in the present study. However, Lymam et al (5) and Salar et al (16) reported that an older age was an
independent risk factor for the occurrence of FN. Therefore,
elderly patients may require special attention concerning infection
prevention.
In conclusion, ~90% of the patients treated with
CHOP chemotherapy experienced a greater- than- grade-3 neutrophil
count decrease, and 31.4% (33/105) of the patients who were able to
be treated in an outpatient setting experienced FN in the present
study. Although primary prophylaxis with G-CSF was administered in
this study, the incidence of FN was still as high as 21.0% (22/105)
in cycle 1, which accounted for 53.7% of all FN events (22/41).
These results suggested that the education of patients is required
to prevent infections prior to the first chemotherapy cycle. In
addition, for patients who require long-term G-CSF and are at risk
of unplanned re-hospitalization, Peg-G treatment strategies should
be taken into consideration. Therefore, proper supportive therapy
and the management of infection are important to safely treat
patients undergoing the CHOP regimen in an outpatient setting.
References
|
1
|
Bosly A, Bron D, Van Hoof A, De Bock R,
Berneman Z, Ferrant A, Kaufman L, Dauwe M and Verhoef G:
Achievement of optimal average relative dose intensity and
correlation with survival in diffuse large B-cell lymphoma patients
treated with CHOP. Ann Hematol. 87:277–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pettengell R, Schwenkglenks M and Bosly A:
Association of reduced relative dose intensity and survival in
lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol.
87:429–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trillet-Lenoir V, Green J, Manegold C, Von
Pawel J, Gatzemeier U, Lebeau B, Depierre A, Johnson P, Decoster G,
Tomita D, et al: Recombinant granulocyte colony stimulating factor
reduces the infectious complications of cytotoxic chemotherapy. Eur
J Cancer. 29A:319–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, et al: 2010 update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
47:8–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lyman GH, Morrison VA, Dale DC, Crawford
J, Delgado DJ and Fridman M: OPPS Working Group and ANC Study
Group: Risk of febrile neutropenia among patients with
intermediate-grade non-Hodgkin's lymphoma receiving CHOP
chemotherapy. Leuk Lymphoma. 44:2069–2076. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pettengell R, Bosly A, Szucs TD, Jackisch
C, Leonard R, Paridaens R, Constenla M and Schwenkglenks M: Impact
of Neutropenia in Chemotherapy-European Study Group (INC-EU):
Multivariate analysis of febrile neutropenia occurrence in patients
with non-Hodgkin lymphoma: Data from the INC-EU prospective
observational European neutropenia study. Br J Haematol.
144:677–685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith TJ, Khatcheressian J, Lyman GH, Ozer
H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J,
Cross SJ, et al: 2006 update of recommendations for the use of
white blood cell growth factors: An evidence-based clinical
practice guideline. J Clin Oncol. 24:3187–3205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Armitage JO: Staging non-Hodgkin lymphoma.
CA Cancer J Clin. 55:368–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aapro MS, Cameron DA, Pettengell R,
Bohlius J, Crawford J, Ellis M, Kearney N, Lyman GH, Tjan-Heijnen
VC, Walewski J, et al: The European Organisation for Research and
EORTC guidelines for the use of granulocyte-colony stimulating
factor to reduce the incidence of chemotherapy-induced febrile
neutropenia in adult patients with lymphomas and solid tumours. Eur
J Cancer. 42:2433–2453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith TJ, Khatcheressian J, Lyman GH, Ozer
H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J,
Cross SJ, et al: 2006 update of recommendations for the use of
white blood cell growth factors: An evidence-based, clinical
practice guideline. J Clin Oncol. 24:3187–3205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balducci L, Al-Halawani H, Charu V, Tam J,
Shahin S, Dreiling L and Ershler WB: Elderly cancer patients
receiving chemotherapy benefit from first-cycle pegfilgrastim.
Oncologist. 12:1416–1424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Case DC Jr, Desch CE, Kalman LA, Vongkovit
P, Mena RR, Fridman M and Allen B: Community-based trial of R-CHOP
and maintenance rituximab for intermediate- or high-grade
non-Hodgkin lymphoma with first-cycle filgrastim for older
patients. Clin Lymphoma Myeloma. 7:354–360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crawford J, Dale DC, Kuderer NM, Culakova
E, Poniewierski MS, Wolff D and Lyman GH: Risk and timing of
neutropenic events in adult cancer patients receiving chemotherapy:
The results of a prospective nationwide study of oncology practice.
J Natl Compr Canc Netw. 6:109–118. 2008.PubMed/NCBI
|
|
15
|
Pettengell R, Schwenkglenks M, Leonard R,
Bosly A, Paridaens R, Constenla M, Szucs TD and Jackisch C: Impact
of Neutropenia in Chemotherapy-European Study Group (INC-EU):
Neutropenia occurrence and predictors of reduced chemotherapy
delivery: Results from the INC-EU prospective observational
European neutropenia study. Support Care Cancer. 16:1299–1309.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salar A, Haioun C, Rossi FG, Duehrsen U,
Pettengell R, Johnsen HE, Jaeger U, Verhoef G, Schwenkglenks M,
Bacon P, et al: The need for improved neutropenia risk assessment
in DLBCL patients receiving R-CHOP-21: Findings from clinical
practice. Leuk Res. 36:548–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayordomo JI, López A, Viñolas N,
Castellanos J, Pernas S, Alonso Domingo J, Frau A, Layola M,
Antonio Gasquet J and Sánchez J: ENIA Study Group: Retrospective
cost analysis of management of febrile neutropenia in cancer
patients in Spain. Curr Med Res Opin. 25:2533–2542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lyman GH and Delgado DJ: Risk and timing
of hospitalization for febrile neutropenia in patients receiving
CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade
non-Hodgkin lymphoma. Cancer. 98:2402–2409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morstyn G, Foote MA, Walker T and Molineux
G: Filgrastim (r-metHuG-CSF) in the 21st century: SD/01. Acta
Haematol. 105:151–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lord BI, Woolford LB and Molineux G:
Kinetics of neutrophil production in normal and neutropenic animals
during the response to filgrastim (r-metHu G-CSF) or filgrastim
SD/01 (PEG-r-metHu G-CSF). Clin Cancer Res. 7:2085–2090.
2001.PubMed/NCBI
|