Introduction
The design of a tumor staging system depends on the
identification of individual predictors of survival in cancer
patients (1–15). The staging of hepatocellular carcinoma
(HCC) differs significantly from that of other malignancies, as the
underlying liver disease, apart from the biology of the tumor
itself, may significantly affect patient prognosis (1–15). Based
on the identification of relevant predictors for tumor burden and
liver functional reserve, several staging systems for HCC including
both aspects have been proposed in different parts of the world
(1–15). Of these prognostic systems for HCC,
the Japan Integrated Staging (JIS) system, the Barcelona Clinic
Liver Cancer (BCLC) classification system, the
tumor-node-metastasis (TNM) classification system, the Cancer of
the Liver Italian Program (CLIP) scoring system and the Chinese
University Prognostic Index (CUPI) scoring system are currently
used in daily clinical practice, with an ongoing debate between
Western and Eastern countries regarding their prognostic ability in
HCC (2,6,10–12,14).
Sorafenib (Nexavar; Bayer Healthcare
Pharmaceuticals, Montville, NJ, USA), a multikinase inhibitor that
blocks tumor growth and cell proliferation, was the first systemic
chemotherapeutic agent found to significantly improve the survival
of patients with advanced HCC in the Sorafenib HCC Assessment
Randomised Protocol (SHARP) trial and in the Asian Pacific trial,
and it is currently approved for use as first-line systemic
chemotherapy in these patients (16,17). In
order to optimize the beneficial effects of sorafenib, combination
or sequential therapies comprising sorafenib and other HCC
therapies, such as transcatheter arterial chemoembolization (TACE),
were recently investigated (18).
However, to the best of our knowledge, predictive factors of
responders to sorafenib among HCC patients have not been well
established, and none of the prognostic staging systems for HCC
patients who underwent sorafenib therapy is yet universally adopted
or preferred (19,20). Thus, there is an urgent need for
determining the prognostic ability of staging systems in patients
with advanced HCC receiving sorafenib therapy.
The aim of the present study was to compare
prognostic ability among the five aforementioned well-known
prognostic systems (JIS, BCLC, TNM, CLIP and CUPI systems) for HCC
patients who received sorafenib therapy.
Patients and methods
Patients
A total of 143 HCC patients were treated with
sorafenib monotherapy at the Osaka Red Cross Hospital (Osaka,
Japan) between June, 2009 and 2014. Subjects participating in
clinical trials of novel molecular targeted agents or sequential or
combination therapies with TACE and sorafenib were excluded from
the present analysis. Sorafenib therapy was indicated in patients
with unresectable HCC determined by dynamic computed tomography
(CT): i) Eastern Cooperative Oncology Group performance status
(ECOG PS) of 0–2; ii) presence of extrahepatic metastases; iii) HCC
refractory to previous therapies, such as TACE; iv) unsuitability
for TACE due to anatomical reasons; or v) vascular invasion, such
as tumor thrombus in the portal vein (19,21).
The disease was staged for all analysed patients by
means of five staging systems, including the JIS, BCLC, TNM, CLIP
and CUPI systems (2,6,10,11,14). We
investigated the prognostic ability of each prognostic system.
Furthermore, we investigated prognostic factors associated with
overall survival (OS) using univariate and multivariate analyses.
The following data were used for the analyses: gender, age, tumor
burden, presence of portal vein invasion, presence of extrahepatic
metastases, Child-Pugh classification, ECOG PS, cause of liver
disease, aspartate aminotransferase, alanine aminotransferase,
alkaline phosphatase (ALP), platelet count, tumor markers and
initial dose of sorafenib [recommended (800 mg/day) or reduced
dose].
Prior to sorafenib therapy for HCC, written informed
consent for HCC therapy was obtained from all the subjects. The
Ethics Committee of our department approved the study protocol. The
present study comprised a retrospective analysis of patients'
medical records in our database and all the treatments were
performed in an open-label manner.
Diagnosis of HCC and sorafenib
therapy
HCC was diagnosed based on the results of the
abdominal ultrasound and dynamic CT scan (hyperattenuation during
the arterial phase in the entire or part of the tumor, and
hypoattenuation in the portal venous phase) and/or magnetic
resonance imaging (MRI), mainly as recommended by the American
Association for the Study of Liver Diseases (22). Arterial and portal phase dynamic CT
images were obtained ~30 and 120 sec after injection of contrast
material. In our hospital, abdominal angiography combined with CT
(angio-CT) was routinely performed prior to therapy for HCC after
obtaining informed consent from the patients. This was performed
based on the fact that this technique was useful for detecting
small satellite nodules, as reported by Yamasaki et al
(23). Subsequently, HCC was
confirmed using CT during hepatic arteriography and during arterial
portography. Patients who presented with atypical liver tumors
underwent ultrasound-guided tumor biopsy. Vascular invasion was
determined using dynamic CT and/or angio-CT. During initial
evaluation for HCC, a chest X-ray was performed and, if abnormal,
it was followed by a chest CT scan. Bone scintigraphy, brain CT or
MRI was performed if there were any symptoms or clinical
indications.
The response to sorafenib was assessed every 4–8
weeks after the initiation of sorafenib therapy, using the modified
Response Evaluation Criteria in Solid Tumors (mRECIST) and/or tumor
markers (24). Sorafenib therapy was
continued until disease progression, unacceptable drug-related
toxicity, or the patient's wish to discontinue treatment. After
discontinuation of sorafenib therapy for any reason, any additional
therapies, such as TACE or systemic chemotherapy, were allowed
based on the status of each patient (19,21).
As regards the initial dose of sorafenib, for
patients without risk factors, we introduced the recommended
initial dose of 400 mg twice a day (800 mg/day) of sorafenib
(16,17,19,21,25,26).
Considering previous studies on dose reduction of sorafenib, the
initial dose was reduced based on clinical factors such as age,
body weight, ECOG PS and liver functional reserve (19,21,27).
During sorafenib therapy, each attending physician decided to
reduce the daily dose of sorafenib according to the grades of
adverse events or ECOG PS. Sorafenib-related toxicities, including
hand-foot skin reaction (HFSR), rash, diarrhea, fever,
hypertension, fatigue, liver injury, gastrointestinal bleeding and
lung injury were evaluated using the Common Terminology Criteria
for Adverse Events (CTCAE) version 3.0 (http://ctep.cancer.gov).
Statistical analysis
In this study, OS was the only endpoint. Data were
analysed using univariate and multivariate methods. To analyse the
significance of prognostic predictors, continuous variables were
divided by the median values for all cases (n=143) and treated as
dichotomous covariates. The cumulative OS rate was calculated by
the Kaplan-Meier method and tested by the log-rank test. A Cox
proportional hazards model via a stepwise forward method was used
for multivariate analyses of factors with a P-value of <0.05 in
the univariate analysis. These statistical methods were used to
estimate the interval from each date of initiation of sorafenib
therapy for HCC until the date of death or last follow-up.
The performance of a prognostic system has been
demonstrated to be related to homogeneity (small differences in
survival among subjects in the same stage within each system),
monotonicity of gradients (the survival of subjects in more
advanced stages is shorter compared with the survival of subjects
in earlier stages within the same system) and discriminatory
ability (greater differences in survival among subjects in
different stages within each system) (28). The prognostic performance of each
scoring system was statistically evaluated by homogeneity within
classification groups, monotonicity of the gradients and
discriminatory ability in the association between stage and
survival rate. Homogeneity was determined by the likelihood ratio
(LR) χ2 test based on a Cox proportional hazards
regression model (28). Monotonicity
of gradients was evaluated by the linear trend χ2 test
using a Cox regression model (28).
To evaluate the discriminatory ability for predicting survival, we
assessed the accuracy of prediction of death at 6 months and 1 year
for each scoring system. This score was assessed by calculating the
area under the receiver operating characteristic curve for each
score, which is equivalent to the concordance index (c-index)
(29). To perform this test, subjects
censored prior to 6 months or 1 year were excluded from the
analysis. The c-index ranges between 0.0 and 1.0; a c-index of 0.5
indicates that the model is no better than chance at making a
prediction of membership in a group, whereas a value of 1.0
indicates that the model perfectly identifies those within a group
and those not. Models are typically considered reasonable when the
c-index is >0.70 (30). In
conclusion, the higher values of the LR χ2 test, linear
trend χ2 test and c-index indicate that the prognostic
system is more informative.
Data were analysed using SPSS software version 21
(SPSS Inc., Chicago, IL, USA) for Microsoft Windows and are
expressed as median value (range). A P-value of <0.05 was
considered to indicate statistically significant differences.
Results
Patient demographic
characteristics
The baseline demographic characteristics of the
analysed patients (n=143) are listed in Table I. The patients included 114 men and 29
women, with a median age of 71 years (range, 45–89 years). A total
of 102 patients were classed as Child-Pugh A and 41 as Child-Pugh
B. In terms of ECOG PS, 119, 19 and 5 subjects had a PS score of 0,
1 and 2, respectively. A total of 31 patients (21.7%) had portal
vein invasion and 63 (44.1%) had extrahepatic metastases. The
proportion of viral hepatitis (hepatitis B, C or B+C)-related HCC
was 77.6% (111/143). In the present analysis, des-γ-carboxy
prothrombin (DCP) data were missing from 3 subjects.
 | Table I.Baseline characteristics (n=143). |
Table I.
Baseline characteristics (n=143).
|
Characteristics | No. or median value
(range) |
|---|
| Age (years) | 71 (45–89) |
| Gender,
male/female | 114/29 |
| Causes of liver
disease |
|
| Hepatitis
B/C/non-B, non-C/B+C | 22/85/32/4 |
| Child-Pugh class,
A/B | 102/41 |
| ECOG PS 0/1/2 | 119/19/5 |
| Tumor burden,
<50/≥50% | 129/14 |
| Portal vein
invasion, present/absent |
31/112 |
| Extrahepatic
metastases, present/absent | 63/80 |
| AST (IU/l) | 52 (17–791) |
| ALT (IU/l) | 34 (7–380) |
| ALP (IU/l) | 405 (162–4535) |
| Total bilirubin
(mg/dl) | 0.8 (0.3–2.5) |
| Serum albumin
(g/dl) | 3.4 (1.7–4.8) |
| Prothrombin time
(%) | 79 (48–116) |
| Platelet count
(×104/mm3) | 11.5
(3.4–29.5) |
| AFP (ng/ml) | 139.1
(1.8–688,400) |
| DCP
(mAU/ml)a | 1,341
(10–421,210) |
| Initial dose of
sorafenib (mg/day), 800/400/200 | 35/106/2 |
As regards previous therapies for HCC, the majority
of our cohort (90.9%, 130/143) underwent ≥1 sessions of TACE for
HCC prior to sorafenib therapy. Percutaneous ablative therapies,
such as radiofrequency ablation or percutaneous ethanol injection,
were performed in 73 (51.0%) and surgical resection in 33 (23.1%)
patients.
Overall survival and causes of death
for all cases
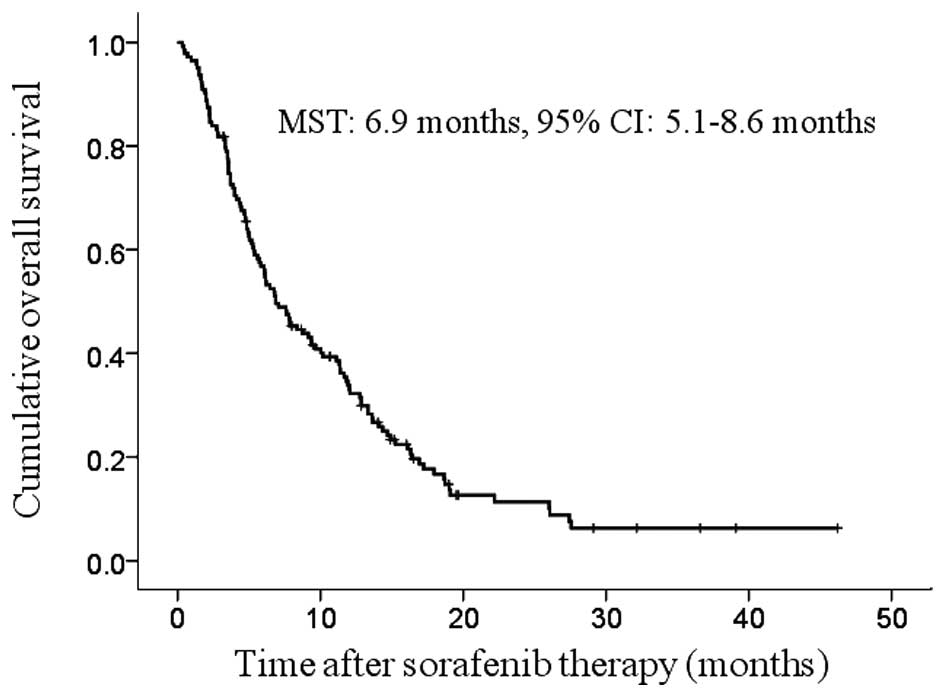
The median follow-up period was 6.8 months (range,
0.3–46.2 months) and the median survival time (MST) was 6.9 months
(95% CI: 5.1–8.6 months) (Fig. 1).
During the follow-up period, there were 121 (84.6%) deaths. The
causes of death were HCC progression in 97 patients, liver failure
in 4, sorafenib-related serious adverse events (SAE) in 1 and
miscellaneous causes in 19 patients.
Best treatment response, dose
adjustment or discontinuation, sorafenib-related adverse events and
therapy after sorafenib discontinuation
During sorafenib therapy, the best treatment
responses according to the mRECIST were as follows: complete
response in 2, partial response in 10, stable disease in 44,
progressive disease in 51 and not evaluated in 36 patients
(24).
In patients treated with the standard initial dose
of sorafenib (800 mg/day, n=35), dose reduction was performed in 15
patients during sorafenib therapy. In patients treated with a
reduced initial dose of sorafenib (400 or 200 mg/day, n=108), dose
escalation of sorafenib was performed in 25 and dose reduction in
22 patients during sorafenib therapy. Overall, the treatment
discontinuation rate was 93.7% (134/143).
In terms of sorafenib-related grade ≥3 SAEs
according to the CTCAE 3.0, rash was observed in 4 patients, HFSR
in 8, diarrhea in 7, gastrointestinal bleeding in 4, liver injury
in 33, general fatigue in 7, fever in 6 and lung injury in 3
patients.
As regards HCC therapy after sorafenib
discontinuation, ≥1 sessions of TACE were performed in 29 patients,
while chemotherapeutic agents other than sorafenib were
administered in 21 patients based on liver function or PS.
Univariate and multivariate analyses
of factors contributing to OS
On the univariate analysis of factors affecting OS,
gender (P=0.002), tumor burden (P=0.007), extrahepatic metastases
(P=0.001), Child-Pugh classification (P=0.007) and DCP >1,341
mAU/ml (P=0.018) were found to be significant factors associated
with OS (Table II). The multivariate
analysis involving five factors with P<0.05 in the univariate
analysis demonstrated that gender (P=0.003), tumor burden
(P=0.002), extrahepatic metastases (P<0.001) and Child-Pugh
classification (P=0.001) were significant independent predictors
associated with OS. Of note, gender was a significant predictor
that was not included in different staging systems. The hazard
ratios (HRs), 95% confidence intervals (CIs) and P-values for these
factors are listed in Table II.
 | Table II.Univariate and multivariate analyses
of factors contributing to overall survival (n=143). |
Table II.
Univariate and multivariate analyses
of factors contributing to overall survival (n=143).
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | n | Univariate
analysis | Hazard ratio (95%
CI) |
P-valuea |
|---|
| Gender, male vs.
female | 114/29 | 0.002 | 2.231
(1.320–3.770) | 0.003 |
| Age, >71 vs.
<71 years |
75/68 | 0.742 |
|
|
| Tumor burden,
<50 vs. >50% | 129/14 | 0.007 | 0.381
(0.207–0.702) | 0.002 |
| Portal vein
invasion, yes vs. no |
31/112 | 0.985 |
|
|
| Extrahepatic
metastases, yes vs. no |
63/80 | 0.001 | 2.273
(1.546–3.333) | <0.001 |
| Child-Pugh class, A
vs. B | 102/41 | 0.007 | 0.508
(0.335–0.771) | 0.001 |
| ECOG PS, 0 vs.
>1 | 119/24 | 0.278 |
|
|
| Cause of liver
disease, virus-related vs. NBNC | 111/32 | 0.844 |
|
|
| AST, >52 vs.
<52 IU/l |
72/71 | 0.234 |
|
|
| ALT, >34 vs.
<34 IU/l |
75/68 | 0.476 |
|
|
| ALP, >405 vs.
<405 IU/l |
72/71 | 0.221 |
|
|
| Platelet count,
>11.5 vs. <11.5 ×104/mm3 |
72/71 | 0.492 |
|
|
| AFP, >139.1 vs.
<139.1 ng/ml |
72/71 | 0.959 |
|
|
| DCP, >1,341 vs.
<1,341 mAU/mlb |
70/70 | 0.018 |
|
|
| Initial dose of
sorafenib, 800 mg/day vs. reduced dose |
35/108 | 0.665 |
|
|
Comparison of five prognostic systems
for all cases (n=143) using the LR χ2 test, linear trend
χ2 test and c-index
Kaplan-Meier curves of OS were constructed for the
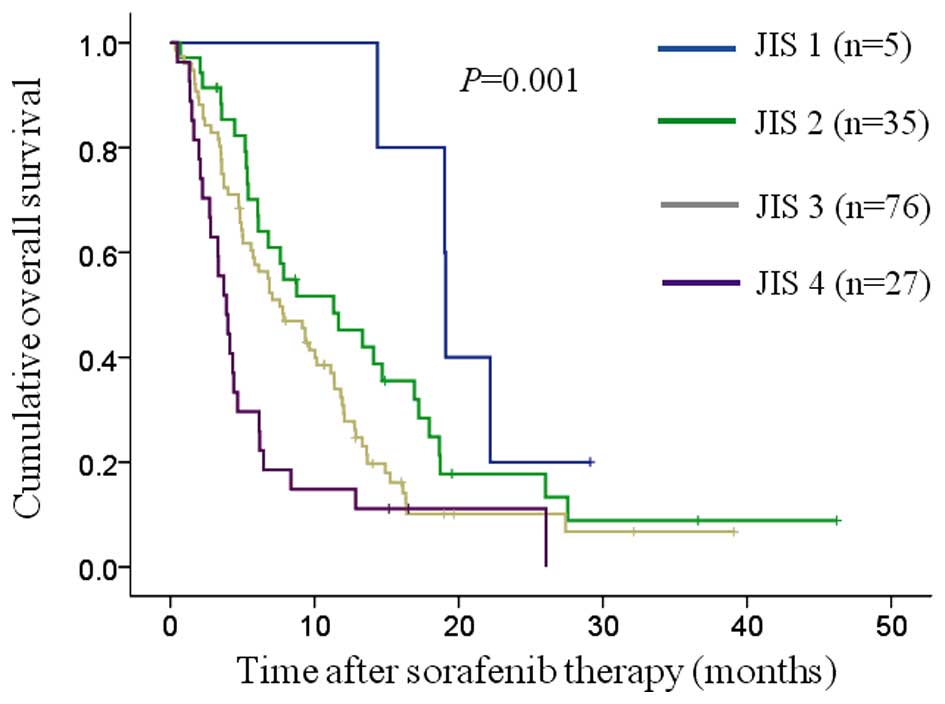
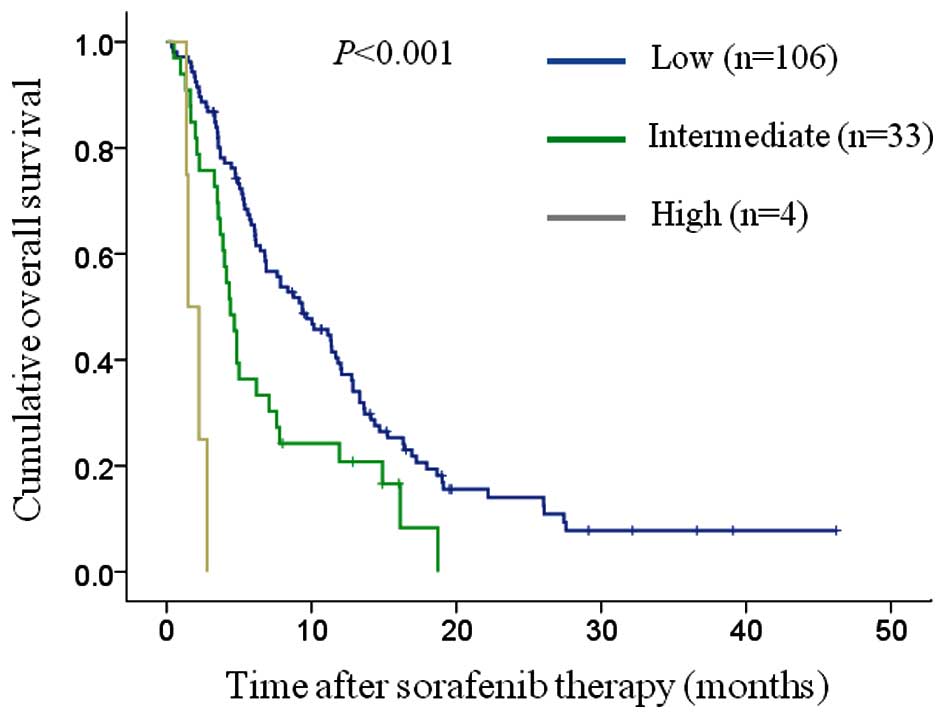
JIS, BCLC, TNM, CLIP and CUPI systems (Figs. 2–6). The
number and median OS of patients with each score are presented in
Table III. The P-values between
adjacent groups in each system are also shown in Table III. The overall significance in all
prognostic systems was P<0.05. The differences between adjacent
groups reached statistical significance: In the JIS system, between
JIS 3 and 4 (P=0.013); in the BCLC classification system, between
BCLC B and C (P=0.017); in the TNM classification system, between
stages III and IV (P=0.007); and in the CUPI scoring system,
between the low-and intermediate-risk groups (P=0.005) and between
the high- and intermediate-risk groups (P=0.001).
 | Table III.Patient survival according to
different staging systems. |
Table III.
Patient survival according to
different staging systems.
| Staging system | MST (months) | 95% CI | P-value
(overall) | P-value in each
adjacent group |
|---|
| JIS system |
|
|
0.001 |
|
| 1
(n=5) | 19.1 | 19.0–19.2 |
| 1 vs. 2, 0.132 |
| 2
(n=35) | 11.3 |
5.2–17.5 |
| 2 vs. 3, 0.088 |
| 3
(n=76) |
7.6 |
5.2–10.0 |
| 3 vs. 4, 0.013 |
| 4
(n=27) |
3.9 |
2.8–5.0 |
|
|
| BCLC classification
system |
|
|
0.045 |
|
| A
(early stage, n=1) | NT | NT |
|
|
| B
(intermediate stage, n=49) | 11.4 |
7.6–15.1 |
| B vs. C, 0.017 |
| C
(advanced stage, n=93) |
6.1 |
4.5–7.7 |
|
|
| TNM classification
system |
|
|
0.007 |
|
| Stage
II (n=7) | 19.0 |
7.1–31.0 |
| II vs. III,
0.336 |
| Stage
III (n=45) | 11.4 |
6.5–16.3 |
| III vs. IV,
0.007 |
| Stage
IV (n=91) |
5.7 |
4.5–7.0 |
|
|
| CLIP scoring
system |
|
|
0.038 |
|
| 1
(n=54) | 11.8 |
7.7–15.9 |
| 1 vs. 2, 0.315 |
| 2
(n=48) |
6.1 |
3.3–8.9 |
| 2 vs. 3, 0.117 |
| 3
(n=31) |
4.1 |
1.2–7.1 |
| 3 vs. 4, 0.895 |
| 4
(n=10) |
4.3 |
2.8–5.9 |
|
|
| CUPI scoring
system |
|
| <0.001 |
|
|
Low-risk group (L)
(n=106) |
9.4 |
6.3–12.4 |
| L vs. I, 0.005 |
|
Intermediate-risk group (I)
(n=33) |
4.4 |
3.6–5.2 |
| I vs. H, 0.001 |
|
High-risk group (H) (n=4) |
1.5 |
0.7–2.3 |
|
|
Using the LR χ2 test, the CUPI
classification system had the highest value (35.804, P<0.001)
among the five prognostic systems, followed by the JIS system
(17.469, P=0.001), indicating small differences in survival among
subjects in the same stages of these two groups (Table IV). Using the linear trend
χ2 test, the CUPI scoring system had the highest value
(17.523), followed by the JIS system (15.819), indicating that
these two prognostic systems gave an accurate prediction of patient
survival (monotonicity of the prognostic system) (Table IV). Using the c-index, the JIS system
had the highest value at 6 months (0.659) and 1 year (0.674),
suggesting that the JIS system had the highest discriminative
ability among the five prognostic systems (Table V).
 | Table IV.Values of LR χ2 test and
linear trend χ2 test in each prognostic system. |
Table IV.
Values of LR χ2 test and
linear trend χ2 test in each prognostic system.
| Systems | LR χ2
test | P-value | Linear trend
χ2 test |
|---|
| JIS | 17.469 |
0.001 | 15.819 |
| BCLC |
6.138 |
0.013 | 6.162 |
| TNM |
9.470 |
0.002 | 9.505 |
| CLIP |
7.891 |
0.005 | 7.922 |
| CUPI | 35.804 | <0.001 | 17.523 |
 | Table V.Comparison of discriminative ability
using 6 months and 1-year concordance index (c-index) among five
prognostic systems. |
Table V.
Comparison of discriminative ability
using 6 months and 1-year concordance index (c-index) among five
prognostic systems.
|
| 6 months | 1 year |
|---|
|
|
|
|
|---|
| Systems | c-index | 95% CI | P-value | c-index | 95% CI | P-value |
|---|
| JIS | 0.659 | 0.568–0.749 | 0.001 | 0.674 | 0.573–0.775 | 0.001 |
| BCLC | 0.580 | 0.485–0.674 | 0.107 | 0.606 | 0.500–0.712 | 0.051 |
| TNM | 0.613 | 0.519–0.706 | 0.022 | 0.658 | 0.553–0.762 | 0.004 |
| CLIP | 0.627 | 0.534–0.720 | 0.010 | 0.628 | 0.528–0.728 | 0.018 |
| CUPI | 0.634 | 0.539–0.729 | 0.007 | 0.590 | 0.490–0.689 | 0.099 |
Discussion
In the present analysis, in terms of homogeneity and
monotonicity of gradients, the CUPI scoring system had the highest
values among the five prognostic systems, followed by the JIS
system. In terms of discriminative ability, the JIS system had the
highest c-index at the time points of 6 months and 1 year, while
the CUPI scoring system had the lowest c-index at the 1-year time
point. The ideal cancer staging system must provide maximal
discrimination of clinical outcomes among different stages of the
disease, while maintaining the variability of outcomes within each
stage to a minimum (31). In view of
our present results, the JIS system may be the most appropriate
among the five prognostic systems for HCC patients undergoing
sorafenib therapy. The JIS system was introduced in Japan, whereas
the CUPI scoring system was introduced in China (10,14). The
major difference in the HCC characteristics between these two Asian
countries is the main etiology of liver disease: In Japan it is
chronic hepatitis C virus infection, whereas in China it is chronic
hepatitis B virus infection (10,14).
However, these differences may not affect survival of patients with
advanced HCC who received sorafenib therapy.
In our results, the values of the LR χ2
test, linear trend χ2 test and 6-month c-index in the
BCLC classification were the lowest among the five systems.
Although the BCLC classification system is still widely used and is
the most comprehensive staging system available, previous studies
have demonstrated that the performance of the BCLC classification
system may be better in Caucasian HCC patients and earlier-stage
disease only (6–9,13,32). Our data were consistent with these
findings.
The TNM classification system was inferior to JIS
and CUPI in terms of homogeneity, monotonicity and discriminative
ability, although at 1 year the value of the c-index for TNM was
the second highest in the present study. JIS was based on TNM,
followed by the addition of liver function, whereas CUPI was based
on TNM, followed by the addition of liver function and symptom
evaluation in the risk stratification (10,11,14). In
advanced HCC patients, factors other than tumor-related factors may
be essential for risk stratification (2,10,11,14).
Similarly, the CLIP scoring system was inferior to JIS and CUPI in
our results. The CLIP scoring system uses portal vein invasion as a
marker of tumor extension (2).
However, in our analysis, patients with portal vein invasion had an
almost identical prognosis compared with those without portal vein
invasion in our univariate analysis (P=0.985). These observation
may be associated with our present results.
In our data, the MST was 6.9 months, which is
shorter compared with that in the SHARP trial (10.7 months). This
is probably due to the difference in the proportion of patients
with Child-Pugh class B between our cohort 28.7% (41/143) and the
SHARP study (5%) (16). Of note,
gender was a significant predictor associated with OS in the
multivariate analysis, along with other well-known predictors
(P=0.003). One possibility is genomic alterations, such as mutation
or amplification in female HCC patients (33). However, we did not investigate these
alterations in our cohorts; thus, further examination is required.
However, the initial dose of sorafenib was not a significant
predictor. The optimal dose of sorafenib for Japanese HCC patients
with a relatively lower body weight compared with Western
populations remains unclear (21) and
further investigation is required.
We acknowledge several limitations to the present
analysis. First, this was a single-center retrospective study
including only Japanese HCC patients. Second, the initial dose of
sorafenib varied among individual patients, leading to bias. Third,
various therapies were applied after discontinuation of sorafenib,
also potentially leading to bias regarding their OS. Therefore, our
results must be interpreted with caution. Fourth, since any staging
system is constructed from selected prognostic factors for a
certain stage of HCC in a specific population, the predictive
ability of the staging system may be considerably impaired if it is
applied to another patient population and the clinical outcome is
closely associated with patient characteristics. Thus, various
staging systems for HCC patients undergoing sorafenib therapy
should be compared in other independent populations (7,34,35). Finally, there were several values
missing from our study. However, our results demonstrated that the
JIS system exhibited a high prognostic ability for HCC patients
treated with sorafenib.
In conclusion, the JIS system may be a useful
prognostic tool for patients undergoing sorafenib therapy.
Acknowledgements
We would like to thank Mrs. Haruko Takada for the
data collection.
References
|
1
|
Huitzil-Melendez FD, Capanu M, O'Reilly
EM, Duffy A, Gansukh B, Saltz LL and Abou-Alfa GK: Advanced
hepatocellular carcinoma: Which staging systems best predict
prognosis? J Clin Oncol. 28:2889–2895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
No authors listed: A new prognostic system
for hepatocellular carcinoma: a retrospective study of 435
patients: the Cancer of the Liver Italian Program (CLIP)
investigators. Hepatology. 28:751–755. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al:
Asian Oncology Summit: Management of hepatocellular carcinoma in
Asia: Consensus statement from the Asian Oncology Summit 2009.
Lancet Oncol. 10:1111–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takanishi DM Jr, Severino R and Wong LL:
The Cancer of the Liver Italian Program (CLIP) score: Validation of
a new prognostic system for hepatocellular carcinoma. Hawaii Med J.
66:209–212. 2007.PubMed/NCBI
|
|
5
|
Levy I and Sherman M: Liver Cancer Study
Group of the University of Toronto. Staging of hepatocellular
carcinoma: Assessment of the CLIP, Okuda, and Child-Pugh staging
systems in a cohort of 257 patients in Toronto. Gut. 50:881–885.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marrero JA, Fontana RJ, Barrat A, Askari
F, Conjeevaram HS, Su GL and Lok AS: Prognosis of hepatocellular
carcinoma: Comparison of 7 staging systems in an American cohort.
Hepatology. 41:707–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cillo U, Vitale A, Grigoletto F, Farinati
F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D'Amico F, et
al: Prospective validation of the Barcelona Clinic Liver Cancer
staging system. J Hepatol. 44:723–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J, Sherman M, Llovet JM, Beaugrand
M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M
and Rodés J: EASL Panel of Experts on HCC; European Association for
the Study of the Liver: Clinical management of hepatocellular
carcinoma. Conclusions of the Barcelona-2000 EASL conference. J
Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M, Chung H and Osaki Y: Prognostic
staging system for hepatocellular carcinoma (CLIP score): Its value
and limitations, and a proposal for a new staging system, the Japan
Integrated Staging Score (JIS score). J Gastroenterol. 38:207–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
No authors listed: Liver Cancer Study
Group of Japan: The general rules for the clinical and pathological
study of primary liver cancer. Jpn J Surg. 19:98–129. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kudo M, Chung H, Haji S, Osaki Y, Oka H,
Seki T, Kasugai H, Sasaki Y and Matsunaga T: Validation of a new
prognostic staging system for hepatocellular carcinoma: The JIS
score compared with the CLIP score. Hepatology. 40:1396–1405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomaa AI, Hashim MS and Waked I: Comparing
staging systems for predicting prognosis and survival in patients
with hepatocellular carcinoma in Egypt. PLoS One. 9:e909292014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leung TW, Tang AM, Zee B, Lau WY, Lai PB,
Leung KL, Lau JT, Yu SC and Johnson PJ: Construction of the Chinese
University Prognostic Index for hepatocellular carcinoma and
comparison with the TNM staging system, the Okuda staging system,
and the Cancer of the Liver Italian Program staging system: A study
based on 926 patients. Cancer. 94:1760–1769. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henderson JM, Sherman M, Tavill A,
Abecassis M, Chejfec G and Gramlich T: AHPBA/AJCC consensus
conference on staging of hepatocellular carcinoma: Consensus
statement. HPB Oxf. 5:243–250. 2003. View Article : Google Scholar
|
|
16
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdel-Rahman O and Fouad M:
Sorafenib-based combination as a first line treatment for advanced
hepatocellular carcinoma: A systematic review of the literature.
Crit Rev Oncol Hematol. 91:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takeda H, Nishikawa H, Osaki Y, Tsuchiya
K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y and Izumi N:
Japanese Red Cross Liver Study Group: Clinical features associated
with radiological response to sorafenib in unresectable
hepatocellular carcinoma: a large multicenter study in Japan. Liver
Int. 35:1581–1589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Llovet JM, Peña CE, Lathia CD, Shan M,
Meinhardt G and Bruix J: SHARP Investigators Study Group: Plasma
biomarkers as predictors of outcome in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 18:2290–2300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishikawa H, Osaki Y, Endo M, Takeda H,
Tsuchiya K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y, et al:
Japanese Red Cross Liver Study Group: Comparison of standard-dose
and half-dose sorafenib therapy on clinical outcome in patients
with unresectable hepatocellular carcinoma in field practice: A
propensity score matching analysis. Int J Oncol. 45:2295–2302.
2014.PubMed/NCBI
|
|
22
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamasaki T, Kurokawa F, Shirahashi H,
Kusano N, Hironaka K and Okita K: Percutaneous radiofrequency
ablation therapy with combined angiography and computed tomography
assistance for patients with hepatocellular carcinoma. Cancer.
91:1342–1348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JW, Amarapurkar D, Chao Y, Chen PJ,
Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, et al:
Consensus recommendations and review by an International Expert
Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver
Int. 33:327–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudo M: Targeted therapy for liver cancer:
Updated review in 2012. Curr Cancer Drug Targets. 12:1062–1072.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shingina A, Hashim AM, Haque M, Suen M,
Yoshida EM, Gill S, Donnellan F and Weiss AA: In a ‘real-world’,
clinic-based community setting, sorafenib dose of 400 mg/day is as
effective as standard dose of 800 mg/day in patients with advanced
hepatocellular carcinoma, with better tolerance and similar
survival. Can J Gastroenterol. 27:393–396. 2013.PubMed/NCBI
|
|
28
|
Ueno S, Tanabe G, Sako K, Hiwaki T,
Hokotate H, Fukukura Y, Baba Y, Imamura Y and Aikou T:
Discrimination value of the new western prognostic system (CLIP
score) for hepatocellular carcinoma in 662 Japanese patients.
Cancer of the Liver Italian Program. Hepatology. 34:529–534. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanley JA and McNeil BJ: The meaning and
use of the area under a receiver operating characteristic (ROC)
curve. Radiology. 143:29–36. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pencina MJ and D'Agostino RB: Overall C as
a measure of discrimination in survival analysis: Model specific
population value and confidence interval estimation. Stat Med.
23:2109–2123. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maetani S, Onodera H, Nishikawa T and Tobe
T: Systematic computer-aided search of optimal staging system for
colorectal cancer. J Clin Epidemiol. 44:285–291. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grieco A, Pompili M, Caminiti G, Miele L,
Covino M, Alfei B, Rapaccini GL and Gasbarrini G: Prognostic
factors for survival in patients with early-intermediate
hepatocellular carcinoma undergoing non-surgical therapy:
Comparison of Okuda, CLIP, and BCLC staging systems in a single
Italian centre. Gut. 54:411–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu ZZ, Wang D, Cong WM, Jiang H, Yu Y,
Wen BJ, Dong H, Zhang X, Liu SF, Wang AZ, et al: Sex-related
differences in DNA copy number alterations in hepatitis B
virus-associated hepatocellular carcinoma. Asian Pac J Cancer Prev.
13:225–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cillo U, Bassanello M, Vitale A,
Grigoletto FA, Burra P, Fagiuoli S, D'Amico F, Ciarleglio FA,
Boccagni P, Brolese A, et al: The critical issue of hepatocellular
carcinoma prognostic classification: Which is the best tool
available? J Hepatol. 40:124–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collette S, Bonnetain F, Paoletti X,
Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Bedenne L
and Barbare JC: Prognosis of advanced hepatocellular carcinoma:
Comparison of three staging systems in two French clinical trials.
Ann Oncol. 19:1117–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|