Introduction
Allogeneic hematopoietic stem cell transplantation
(allo-HSCT) is considered to be a potential effective treatment
strategy for patients with hematological malignancies, particularly
acute leukemia. Allo-HSCT has been shown to cure leukemia via a
graft vs. leukemia (GVL) effect mediated by immunological cells
(1). Currently, high-dose busulfan
plus cyclophosphamide (BuCy) has been widely used as a
myeloablative conditioning regimen for HSCT. Although BuCy is
generally well-tolerated, high exposure to cyclophosphamide
metabolites after HSCT may cause serious adverse events and an
increase in non-relapse mortality, particularly in elderly
patients. By reducing the toxicity, reduced-intensity conditioning
(RIC) allows the extension of allo-HSCT to a significantly wider
patient population; however, RIC is associated with an increased
risk of relapse following HSCT (2,3).
Fludarabine may inhibit lymphocyte proliferation and
promote lymphocyte apoptosis by affecting DNA replication and
repair, and is considered as the conventional therapy for chronic
lymphocytic leukemia (4). As an
immunosuppressive purine analogue, fludarabine is used in
chemotherapy regimens for acute leukemia, replacing
cyclophosphamide in myeloablative and non-myeloablative
conditioning regimens (5–8). In addition, busulfan plus fludarabine
(BuFlu) exert a synergistic effect, impairing alkylator-induced DNA
damage repair. Previous studies demonstrated that fludarabine, when
used in a RIC regimen, allows adequate engraftment of allogeneic
hematopoietic cells to bring about immunosuppression (9,10). Several
clinical trials have also demonstrated that, as a myeloablative
conditioning regimen, BuFlu is associated with fewer
regimen-related toxicities (RRTs), a lower incidence of non-relapse
mortality, and higher disease-free survival (DFS) rates compared
with BuCy for allo-HSCT (5,11,12).
Fludarabine appears to be well-tolerated by patients undergoing
allo-HSCT and is a feasible conditioning regimen alternative to
cyclophosphamide.
Our study retrospectively analyzed the efficacy of
fludarabine 30 mg/m2 [intravenous (i.v.) injection
daily, 4 doses] and busulfan 3.2 mg/kg (i.v. daily, 4 consecutive
days) as a myeloablative conditioning regimen, considering RRT,
engraftment, hematological relapse/disease progression, acute and
chronic graft vs. host disease (GVHD), overall survival (OS) and
DFS, in 30 patients undergoing allo-HSCT for acute myeloid leukemia
(AML) and acute lymphoblastic leukemia (ALL).
Patients and methods
Patient population
Between January, 2008 and January, 2013, a total of
30 patients with AML or ALL were enrolled in this follow-up study
at the Department of Hematology and Hematopoietic Stem-cell
Transplantation Center of the Second Affiliated Hospital of Xi'an
Jiaotong University (Xi'an, China). The patient characteristics,
status at HSCT and donor source are outlined in Table I. All the patients had a Karnofsky
performance score of ≥70; normal cardiac, hepatic, and renal
function; and no uncontrolled bleeding or severe infection.
High-risk disease status was defined as patients who were beyond
the first remission, had sustained non-remission, had multiple
relapses, or were chemoresistant. All the study participants
provided written informed consent for the analysis of transplant
outcome data.
 | Table I.Patient characteristics (n=30). |
Table I.
Patient characteristics (n=30).
| Characteristics | Number (%) |
|---|
| Age, years [median
(range)] | 30 (13–59) |
| Gender |
|
| Male | 14 (47.0) |
|
Female | 16 (53.0) |
| Acute myeloid
leukemia (n=15) |
|
| Complete
remission 1 | 8 (54.0) |
| Complete
remission 2 | 5 (33.0) |
|
Non-remission | 2 (13.0) |
| Acute lymphoblastic
leukemia (n=15) |
|
| Complete
remission 1 | 9 (60.0) |
| Complete
remission 2 | 5 (33.0) |
|
Non-remission | 1 (7.0) |
| Donor gender |
|
|
Matched | 19 (63.0) |
|
Mismatch | 11 (37.0) |
| Disease risk |
|
|
Standarda | 17 (57.0) |
|
Highb | 13 (43.0) |
| Donor type |
|
|
Sibling | 18 (60.0) |
|
Unrelated | 12 (40.0) |
| Stem cell source |
|
| Bone
marrow | 4 (13.0) |
|
Peripheral blood | 26 (87.0) |
| Mononuclear cells,
×108/kg | 8.71
(0.87–15.97) |
| [median
(range)] |
|
| CD34+
cells, ×106/kg | 3.62 (0.66–12.9) |
| [median
(range)] |
|
| GVHD prophylaxis |
|
|
CSA+MTX | 18 (60.0) |
|
CSA+MTX+MMF | 12 (40.0) |
| Acute GVHD |
|
| 0 | 16 (53.3) |
| I | 5 (16.7) |
| II | 5 (16.7) |
|
III–IV | 4 (13.3) |
| Chronic GVHD | 6 (20.0) |
|
Limited | 4
(13.3) |
|
Extensive | 2 (6.7) |
Human leukocyte antigen (HLA) typing
and engraftment
HLA genotyping was performed in the same manner as
in all centers: The presence of class I antigens was tested using
standard serological techniques; and class II alleles were resolved
with low-resolution molecular typing using polymerase chain
reaction (PCR) amplification with sequence-specific oligonucleotide
primers for hybridization of amplified DNA, followed by
high-resolution typing in all patients and donors. Donor-recipient
pairs were considered fully matched in cases of HLA-A, HLA-B and
HLA-DRB1 compatibility.
Engraftment was defined as an absolute neutrophil
count of >0.5×109/l on the first 3 consecutive days
and a platelet count of >20×109/l after at least 3
days without the need for transfusion. Chimeric status was
evaluated in nuclear cells of peripheral blood T cells and
polymorphonuclear neutrophils on days +20, +30 and +60 by using the
short tandem repeat PCR (STR-PCR) method.
Conditioning regimens, GVHD
prophylaxis and supportive care
The myeloablative conditioning regimen consisted of
fludarabine 30 mg/m2 infused over 30 min daily for 4
doses (days −5 to −2), followed by intravenous busulfan 3.2 mg/kg
of actual or adjusted ideal body weight over 4 h daily on 4
consecutive days (days −5 to −2). Acute GVHD (aGVHD) and chronic
GVHD (cGVHD) were diagnosed and classified according to previously
described clinical criteria (13–15).
Depending on the donor type, transplant recipients received
traditional cyclosporin A (CSA) and short-course methotrexate (MTX)
(sibling donor) or CSA, MTX and mycophenolate mofetil (unrelated
donor) for GVHD prophylaxis. The serum CSA concentration was
maintained between 100 and 300 ng/ml, and the dose was tapered off
by 5% every week from day +60 to day +90. Supportive care comprised
prophylactic transfusion of platelets if platelet counts decreased
to <20×109/l, or prophylactic transfusion of red
blood cells if hemoglobin levels decreased to <80 g/l.
Antimicrobial therapy and other
medications per-protocol
The patients underwent HSCT treatment in rooms with
a positive-pressure filtered flow. The patients were monitored for
cytomegalovirus (CMV) DNA with quantitative PCR once a week from
the first day of conditioning (day −6) until day +100, and then
twice a month until the discontinuation of GVHD prophylaxis.
Patients positive for CMV DNA were treated with ganciclovir and/or
foscarnet until two consecutive negative test results were
obtained. Neutropenic fever was managed according to the Infectious
Diseases Society of America Fever and Neutropenia guidelines
(16). Co-trimoxazole was initiated
after engraftment to prevent pneumocystis carinii pneumonia.
Prostaglandin E1 and Danshen injections were used for
veno-occlusive disease (VOD) prophylaxis, beginning with the
initiation of conditioning.
Statistical analysis
The day of stem cell infusion was defined as day 0.
Descriptive statistics were used to describe the baseline
characteristics of disease status at conditioning. Categorical
variables are summarized as frequency counts and percentages, and
continuous variables are summarized as median and range. DFS was
defined as the time between transplantation and the earliest
occurrence of relapse or death due to any cause. Cumulative
incidence or survival was plotted according to the Kaplan-Meier
method and the log-rank test was used to analyze differences
between groups. Basic statistical data were obtained using the SPSS
software package, version 17.0 (SPSS Inc., Chicago, IL, USA). A
cut-off value of 0.05 indicating statistically significant
differences was adopted for all statistical analyses.
Results
Engraftment
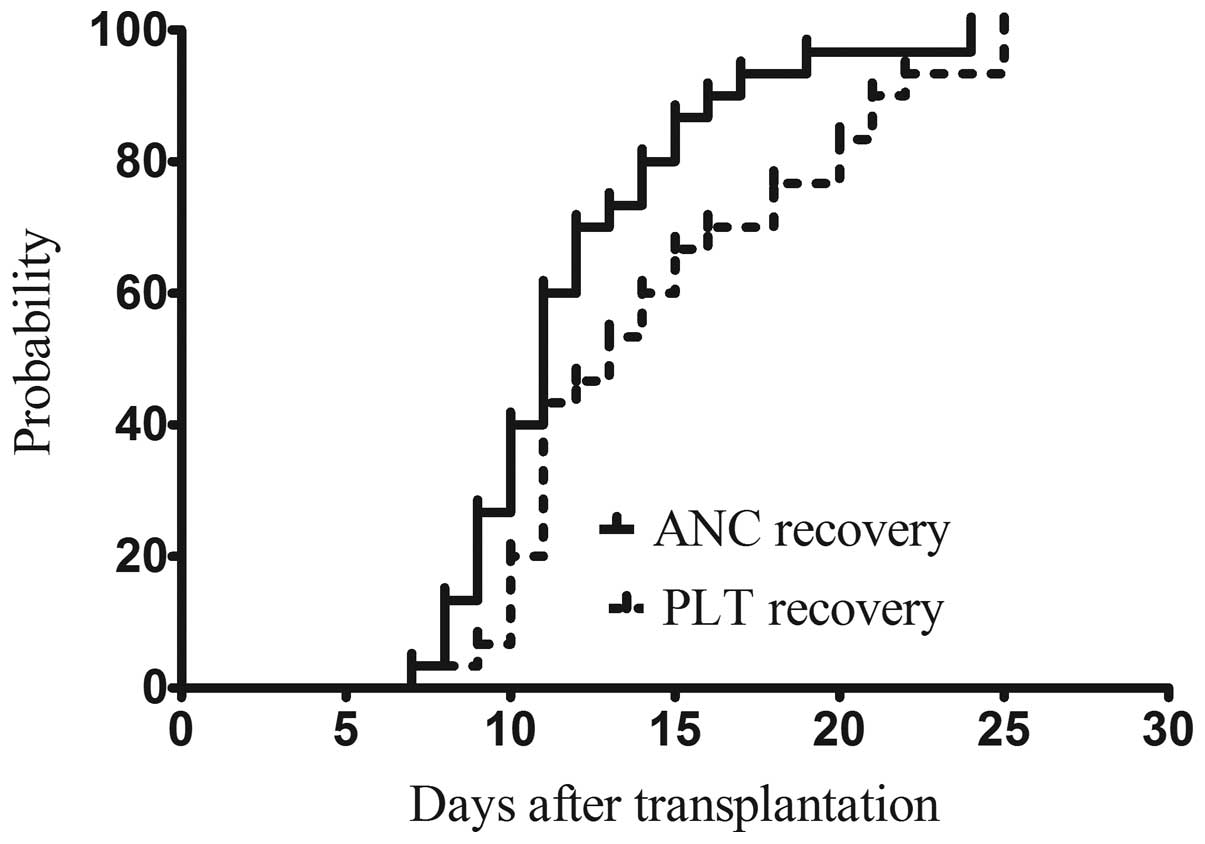
As shown in Fig. 1,
patients achieved absolute neutrophil count (ANC) recovery and
received platelet engraftment at 11 days (range, 7–17 days) and 13
days (range, 7–25 days), respectively. The median time to ANC
recovery or platelet engraftment was not statistically different
between the sibling and unrelated donor groups. Complete donor
chimerism was achieved in all patients, with neutrophil count
recovery being confirmed by STR-DNA detection on days +20, +30 and
+60.
Regimen-related toxicity
Of the 30 patients, 1 experienced hemorrhagic
cystitis (HC), which was resolved by the discontinuation of drugs
that may cause or aggravate HC, and the initiation of diuretic,
hemostatic and anti-infection treatment. CMV viremia was noted in
24 patients, of whom 2 developed CMV-associated interstitial
pneumonia. All 24 patients received antiviral treatment with
ganciclovir and foscarnet. A total of 26 patients developed
mucositis, which resolved with symptomatic therapy without any
serious or permanent sequelae. There was one case of cardiac
toxicity with tachycardia and no reported cases of VOD or
regimen-related death. Grade II, III and IV toxicities were
observed in 14 (46.7%), 8 (26.7%) and 1 (3.3%) patient,
respectively.
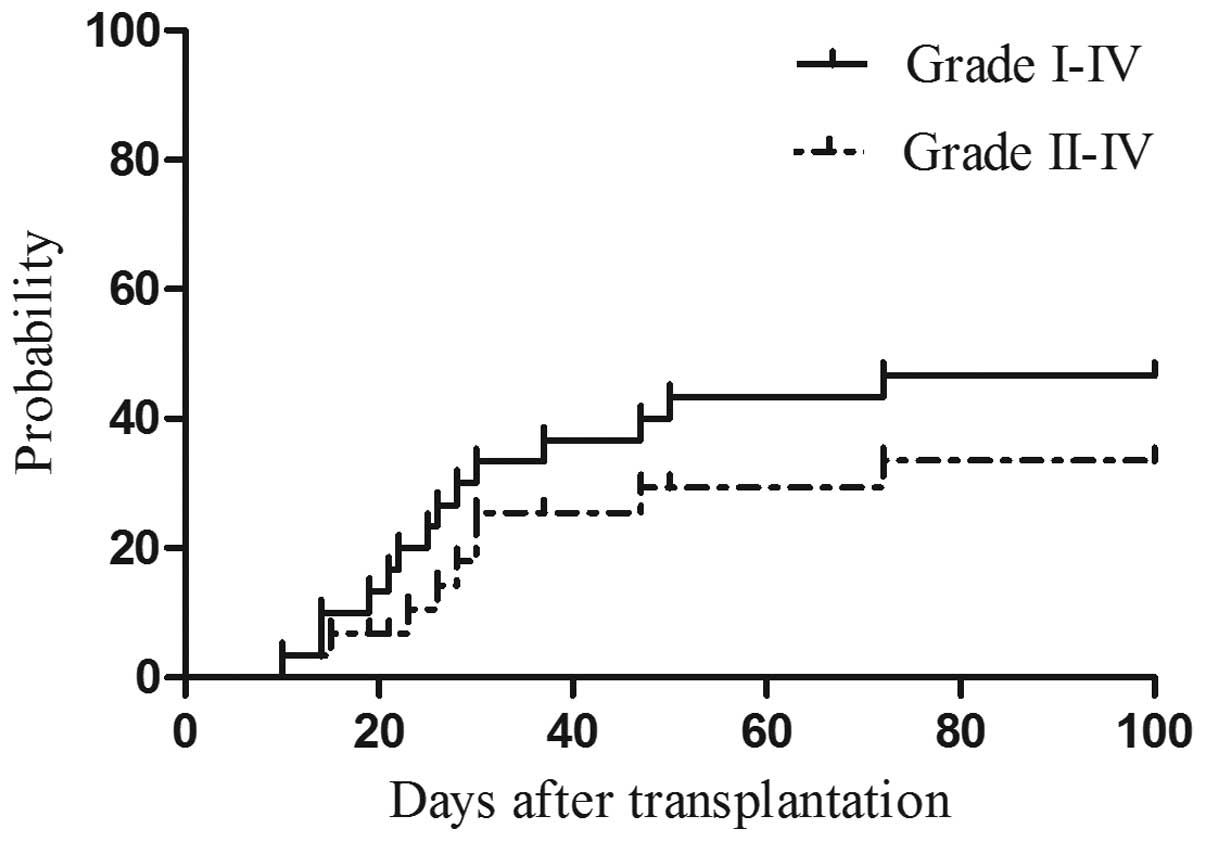
GVHD
As shown in Table I
and Fig. 2, 14 patients (46.7%)
experienced aGVHD. Of those, 9 (13.3%) had grade II–IV aGVHD, 4 of
whom succumbed due to severe rejection. cGVHD was observed in 6
patients (20%), including 2 (6.7%) with extensive cGVHD. The
incidence of aGVHD did not differ significantly between the AML and
ALL groups or between the sibling and unrelated donor groups.
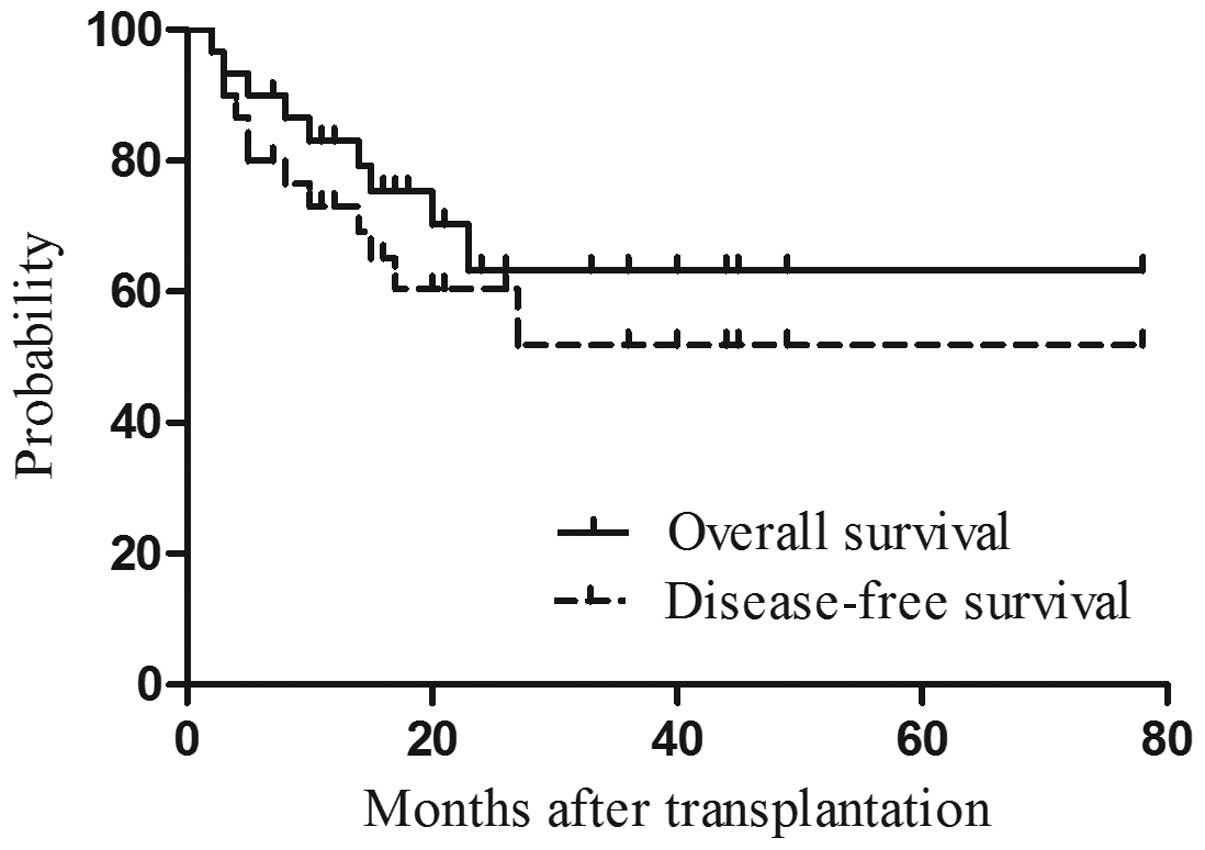
Survival data
With a median follow-up period of 25 months (range,
2–78 months) for surviving patients, 20 of the 30 patients remained
alive. A total of 4 patients succumbed to disease relapse, whereas
6 deaths were due to non-relapse causes: aGVHD (n=4), uninduced
epileptic seizures (n=1), and multifactorial respiratory failure
following severe pulmonary infection (n=1) (Table II). The OS and DFS rates were 66.7
and 53%, respectively, at the end of follow-up (Fig. 3). Three (10%) and one (3.3%) relapses
occurred in ALL and AML patients, respectively. The median
time-to-relapse was 79 days (range, 65–155 days) after
transplantation. The 3 patients with ALL who relapsed were
chemoresistant, and the patient with AML who relapsed 155 days
after transplantation was in non-remission. After receiving a full
chimerism sibling donor lymphocyte infusion, the patient achieved a
transient complete remission but relapsed twice and succumbed 1
year later. The probability of OS at 2 years of AML vs. ALL
patients was 73.3 vs. 60%, respectively, which was not
statistically significant (P=0.70). Furthermore, the cumulative
incidence rate of 2-year OS did not differ significantly between
high-risk and standard-risk patients (53.8 and 76.5%, respectively;
P=0.76).
 | Table II.Number of deaths according to primary
disease. |
Table II.
Number of deaths according to primary
disease.
| Causes of death | ALL, n
(%)a | AML, n
(%)a | HR, n
(%)a | SR, n
(%)a |
|---|
| Relapse-related
mortality | 3 (30.0) | 1 (10.0) | 4 (40.0) | 0 (0.0) |
| Non-relapse-related
mortality | 3 (30.0) | 3 (30.0) | 2 (20.0) | 4 (40.0) |
| GVHD | 2 (20.0) | 2 (20.0) | 1 (10.0) | 3 (30.0) |
| Infection | 1 (10.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) |
| Otherb | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (10.0) |
| Total | 6 (60.0) | 4 (40.0) | 6 (60.0) | 4 (40.0) |
Discussion
BuCy is a traditionally used myeloablative
conditioning regimen for HLA-matched allo-HSCT. However, the high
RRT due to the additive effect of the two alkylators is a major
concern; in particular, cyclophosphamide metabolism is associated
with sinusoidal obstruction syndrome, hemorrhagic cystitis and
bilirubin level elevation, in addition to increased non-relapse
mortality (17). Although a
non-myeloablative transplant is associated with lower conditioning
regimen-related mortality, it has a higher rate of leukemia relapse
compared with a classical myeloablative transplant regimen.
The BuFlu regimen has been confirmed to be safe and
effective for allo-HSCT in patients with hematological
malignancies. Slavin et al (18) first reported the efficacy of
fludarabine (180 mg/m2) in an RIC regimen, with an OS
rate of 85% and a DFS rate of 81% after a median follow-up of 8
months for HLA-matched peripheral blood stem cell transplant. The
replacement of cyclophosphamide with fludarabine appeared to
decrease toxicity, while exhibiting efficacy comparable to that of
BuCy. However, the graft failure rate was higher in patients
treated with the RIC regimen (18,19). Other
studies have demonstrated that intense conditioning may decrease
the incidence of graft failure (20,21). Or
et al (22) reported a 54%
rate of aGVHD with negligible toxicity after a median follow-up of
42 months with the same conditioning regimen as that used by Slavin
et al (18). Bornhauser et
al (23) reported that an
ablative dose of BuFlu resulted in 100% engraftment and 7% RRT in
42 patients with high-risk chronic myeloid leukemia or
myelodysplastic syndrome, with estimated OS and DFS rates of 42.4
and 34.9%, respectively, at a median follow-up of 18 months. The MD
Anderson Cancer Center reported that a regimen of fludarabine 40
mg/m2 and i.v. busulfan 130 mg/m2 once daily
for 4 days was well-tolerated and effective (11). In that study, patients who received a
transplant in first complete remission had 3-year OS and event-free
survival rates of 78 and 74%, respectively. The overall incidence
of grade II–IV aGVHD was 15.8% and that of extensive cGVHD was
34.1%. Thus, i.v. BuFlu was found to be a well-tolerated and
efficacious myeloablative conditioning regimen with reduced
toxicity. Russell et al (24)
reported that high-dose fludarabine 250 mg/m2 and
busulfan 12.8 mg/kg plus thymoglobulin was also a well-tolerated
and effective regimen, with a particularly low incidence of grade
III–IV aGVHD (3%) and cGVHD (38%) at 2 years.
In our study, we analyzed the clinical data of
Chinese Han patients with acute leukemia who underwent HLA-matched
allo-HSCT with the BuFlu conditioning regimen. As in most other
studies, we did not observe graft failure. Neutrophil and platelet
engraftment occurred on days +11 and +13 for patients who received
sibling donor transplants, and on days +13 and +14 for patients who
received unrelated donor transplants, respectively. There were no
significant differences with the BuCy conditioning regimen
regarding the time to hematopoietic reconstitution. Grade III–IV
RRT was observed in 30% of the patients. The most common and
serious RRTs were mucositis (86.7%) and HC (3.3%), respectively.
CMV infection was common (80%). Mucositis and HC were resolved with
drug adjustment and supportive treatment. Our results were similar
to those of Iravani et al (5).
Grade III–IV aGVHD was detected in 13.3% of the patients, without
significant differences in the incidence of aGVHD according to
donor type. The rate of cGVHD in all patients (20%) was lower
compared with that observed with BuCy (25,26). A
probable reason for this is the strong immune inhibitory effect and
alkylator-induced DNA damage repair with fludarabine.
Several studies have reported an association of the
BuFlu conditioning regimen with a decreased relapse rate following
allo-HSCT (27,28). However, other studies have reported
greater relapse or progression in the BuFlu regimen arm compared
with that in the BuCy arm (10). In
our study, the rate of overall relapse was 30%. The majority of the
relapsed patients had a high risk or advanced status prior to the
transplant, indicating that the rate of relapse is also associated
with the pre-transplant disease status and post-transplant
adjustment of immune inhibitors.
In conclusion, our study indicated that BuFlu was an
acceptable regimen, due to its low rates of RRT, GVHD and morbidity
in the Chinese population. BuFlu may replace BuCy, with the aim to
decrease regimen-related side effects, without compromising the
efficacy. However, further comparative studies on BuFlu and
standard regimens with subgroup analyses according to standard- vs.
high-risk leukemia are required.
Acknowledgements
The present study was supported by the Science and
Technology Foundation of Shaanxi Province, China (grant no.
2014K11020107).
References
|
1
|
Kolb HJ, Schattenberg A, Goldman JM,
Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A,
Verdonck L, Niederwieser D, et al: Graft-versus-leukemia effect of
donor lymphocyte transfusions in marrow grafted patients. Blood.
86:2041–2050. 1995.PubMed/NCBI
|
|
2
|
Andersson BS, Kashyap A, Gian V, Wingard
JR, Fernandez H, Cagnoni PJ, Jones RB, Tarantolo S, Hu WW, Blume
KG, et al: Conditioning therapy with intravenous busulfan and
cyclophosphamide (IV BuCy2) for hematologic malignancies prior to
allogeneic stem cell transplantation: A phase II study. Biol Blood
Marrow Transplant. 8:145–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill S and Porter DL: Reduced-intensity
hematopoietic stem cell transplants for malignancies: Harnessing
the graft-versus-tumor effect. Annu Rev Med. 64:101–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keating MJ, O'Brien S, Lerner S, Koller C,
Beran M, Robertson LE, Freireich EJ, Estey E and Kantarjian H:
Long-term follow-up of patients with chronic lymphocytic leukemia
(CLL) receiving fludarabine regimens as initial therapy. Blood.
92:1165–1171. 1998.PubMed/NCBI
|
|
5
|
Iravani M, Evazi MR, Mousavi SA, Shamshiri
AR, Tavakoli M, Ashouri A, Samiee S, Chahardovali B, Alimoghaddam
K, Ghaffari SH and Ghavamzadeh A: Fludarabine and busulfan as a
myeloablative conditioning regimen for allogeneic stem cell
transplantation in high- and standard-risk leukemic patients. Bone
Marrow Transplant. 40:105–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pidala J, Kim J, Anasetti C,
Kharfan-Dabaja MA, Nishihori T, Field T, Perkins J, Perez L and
Fernandez HF: Pharmacokinetic targeting of intravenous busulfan
reduces conditioning regimen related toxicity following allogeneic
hematopoietic cell transplantation for acute myelogenous leukemia.
J Hematol Oncol. 3:362010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie
D, Zhang Y, Huang F, Zhou H, Fan Z, et al: Busulfan plus
fludarabine as a myeloablative conditioning regimen compared with
busulfan plus cyclophosphamide for acute myeloid leukemia in first
complete remission undergoing allogeneic hematopoietic stem cell
transplantation: A prospective and multicenter study. J Hematol
Oncol. 6:152013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chunduri S, Dobogai LC, Peace D,
Saunthararajah Y, Quigley J, Chen YH, Mahmud N, Hurter E, Beri R
and Rondelli D: Fludarabine/i.v. BU conditioning regimen:
Myeloablative, reduced intensity or both? Bone Marrow Transplant.
41:935–940. 2008.
|
|
9
|
Laport GG, Sandmaier BM, Storer BE, Scott
BL, Stuart MJ, Lange T, Maris MB, Agura ED, Chauncey TR, Wong RM,
et al: Reduced-intensity conditioning followed by allogeneic
hematopoietic cell transplantation for adult patients with
myelodysplastic syndrome and myeloproliferative disorders. Biol
Blood Marrow Transplant. 14:246–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Joo YD, Kim H, Ryoo HM, Kim MK,
Lee GW, Lee JH, Lee WS, Park JH, Bae SH, et al: Randomized trial of
myeloablative conditioning regimens: Busulfan plus cyclophosphamide
versus busulfan plus fludarabine. J Clin Oncol. 31:701–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson BS, de Lima M, Thall PF, Wang X,
Couriel D, Korbling M, Roberson S, Giralt S, Pierre B, Russell JA,
et al: Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu)
compares favorably with i.v. busulfan and cyclophosphamide (i.v.
BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood
Marrow Transplant. 14:672–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimoni A, Hardan I, Shem-Tov N, Yeshurun
M, Yerushalmi R, Avigdor A, Ben-Bassat I and Nagler A: Allogeneic
hematopoietic stem-cell transplantation in AML and MDS using
myeloablative versus reduced-intensity conditioning: The role of
dose intensity. Leukemia. 20:322–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK,
Lee KB, Sohn SK, Kim JG, Kim DH, Seol M, et al: Prognostic factors
identifiable at the time of onset of acute graft-versus-host
disease after allogeneic hematopoietic cell transplantation.
Haematologica. 90:939–948. 2005.PubMed/NCBI
|
|
14
|
Filipovich AH, Weisdorf D, Pavletic S,
Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D,
Couriel D, et al: National institutes of health consensus
development project on criteria for clinical trials in chronic
graft-versus-host disease: I. Diagnosis and staging working group
report. Biol Blood Marrow Transplant. 11:945–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jagasia MH, Greinix HT, Arora M, Williams
KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS,
et al: National Institutes of Health consensus development project
on criteria for clinical trials in chronic graft-versus-host
disease: I. The 2014 Diagnosis and Staging Working Group report.
Biol Blood Marrow Transplant. 21:389–401.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hughes WT, Armstrong D, Bodey GP, Bow EJ,
Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL and
Young LS: 2002 Guidelines for the use of antimicrobial agents in
neutropenic patients with cancer. Clin Infect Dis. 34:730–751.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDonald GB, Slattery JT, Bouvier ME, Ren
S, Batchelder AL, Kalhorn TF, Schoch HG, Anasetti C and Gooley T:
Cyclophosphamide metabolism, liver toxicity, and mortality
following hematopoietic stem cell transplantation. Blood.
101:2043–2048. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slavin S, Nagler A, Naparstek E,
Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M,
Ackerstein A, Samuel S, et al: Nonmyeloablative stem cell
transplantation and cell therapy as an alternative to conventional
bone marrow transplantation with lethal cytoreduction for the
treatment of malignant and nonmalignant hematologic diseases.
Blood. 91:756–763. 1998.PubMed/NCBI
|
|
19
|
Giralt S, Thall PF, Khouri I, Wang X,
Braunschweig I, Ippolitti C, Claxton D, Donato M, Bruton J, Cohen
A, et al: Melphalan and purine analog-containing preparative
regimens: Reduced-intensity conditioning for patients with
hematologic malignancies undergoing allogeneic progenitor cell
transplantation. Blood. 97:631–637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Blanc K, Remberger M, Uzunel M,
Mattsson J, Barkholt L and Ringdén O: A comparison of
nonmyeloablative and reduced-intensity conditioning for allogeneic
stem-cell transplantation. Transplantation. 78:1014–1020. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niederwieser D, Maris M, Shizuru JA,
Petersdorf E, Hegenbart U, Sandmaier BM, Maloney DG, Storer B,
Lange T, Chauncey T, et al: Low-dose total body irradiation (TBI)
and fludarabine followed by hematopoietic cell transplantation
(HCT) from HLA-matched or mismatched unrelated donors and
postgrafting immunosuppression with cyclosporine and mycophenolate
mofetil (MMF) can induce durable complete chimerism and sustained
remissions in patients with hematological diseases. Blood.
101:1620–1629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Or R, Shapira MY, Resnick I, Amar A,
Ackerstein A, Samuel S, Aker M, Naparstek E, Nagler A and Slavin S:
Nonmyeloablative allogeneic stem cell transplantation for the
treatment of chronic myeloid leukemia in first chronic phase.
Blood. 101:441–445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bornhauser M, Storer B, Slattery JT,
Appelbaum FR, Deeg HJ, Hansen J, Martin PJ, McDonald GB, Nichols
WG, Radich J, et al: Conditioning with fludarabine and targeted
busulfan for transplantation of allogeneic hematopoietic stem
cells. Blood. 102:820–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russell JA, Tran HT, Quinlan D, Chaudhry
A, Duggan P, Brown C, Stewart D, Ruether JD, Morris D, Glick S, et
al: Once-daily intravenous busulfan given with fludarabine as
conditioning for allogeneic stem cell transplantation: Study of
pharmacokinetics and early clinical outcomes. Biol Blood Marrow
Transplant. 8:468–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chae YS, Sohn SK, Kim JG, Cho YY, Moon JH,
Shin HJ, Chung JS, Cho GJ, Yang DH, Lee JJ, et al: New
myeloablative conditioning regimen with fludarabine and busulfan
for allogeneic stem cell transplantation: Comparison with BuCy2.
Bone Marrow Transplant. 40:541–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bredeson CN, Zhang MJ, Agovi MA,
Bacigalupo A, Bahlis NJ, Ballen K, Brown C, Chaudhry MA, Horowitz
MM, Kurian S, et al: Outcomes following HSCT using fludarabine,
busulfan, and thymoglobulin: A matched comparison to allogeneic
transplants conditioned with busulfan and cyclophosphamide. Biol
Blood Marrow Transplant. 14:993–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo
DJ, Stone R, Ritz J, Antin JH and Soiffer RJ: Impact of
conditioning regimen intensity on outcome of allogeneic
hematopoietic cell transplantation for advanced acute myelogenous
leukemia and myelodysplastic syndrome. Biol Blood Marrow
Transplant. 12:1047–1055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martino R, Iacobelli S, Brand R, Jansen T,
van Biezen A, Finke J, Bacigalupo A, Beelen D, Reiffers J, Devergie
A, et al: Retrospective comparison of reduced-intensity
conditioning and conventional high-dose conditioning for allogeneic
hematopoietic stem cell transplantation using HLA-identical sibling
donors in myelodysplastic syndromes. Blood. 108:836–846. 2006.
View Article : Google Scholar : PubMed/NCBI
|