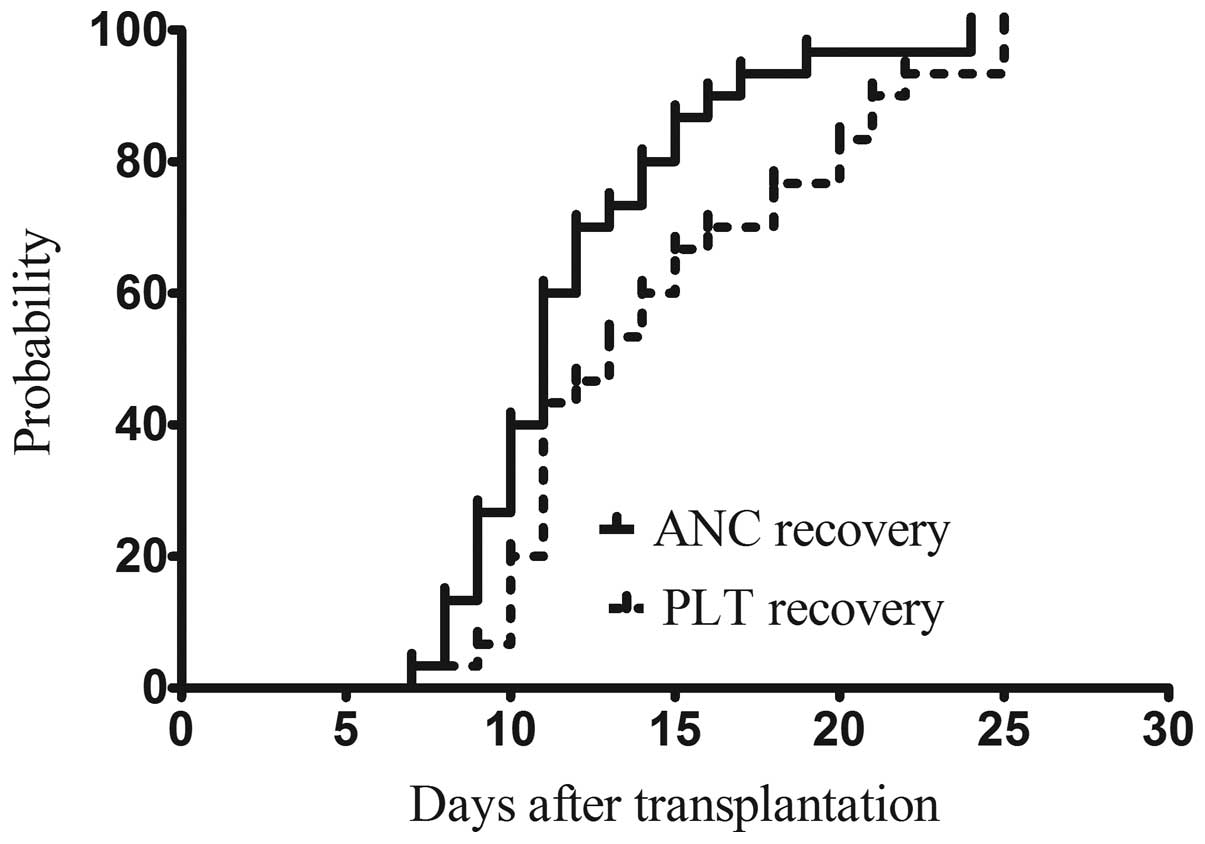
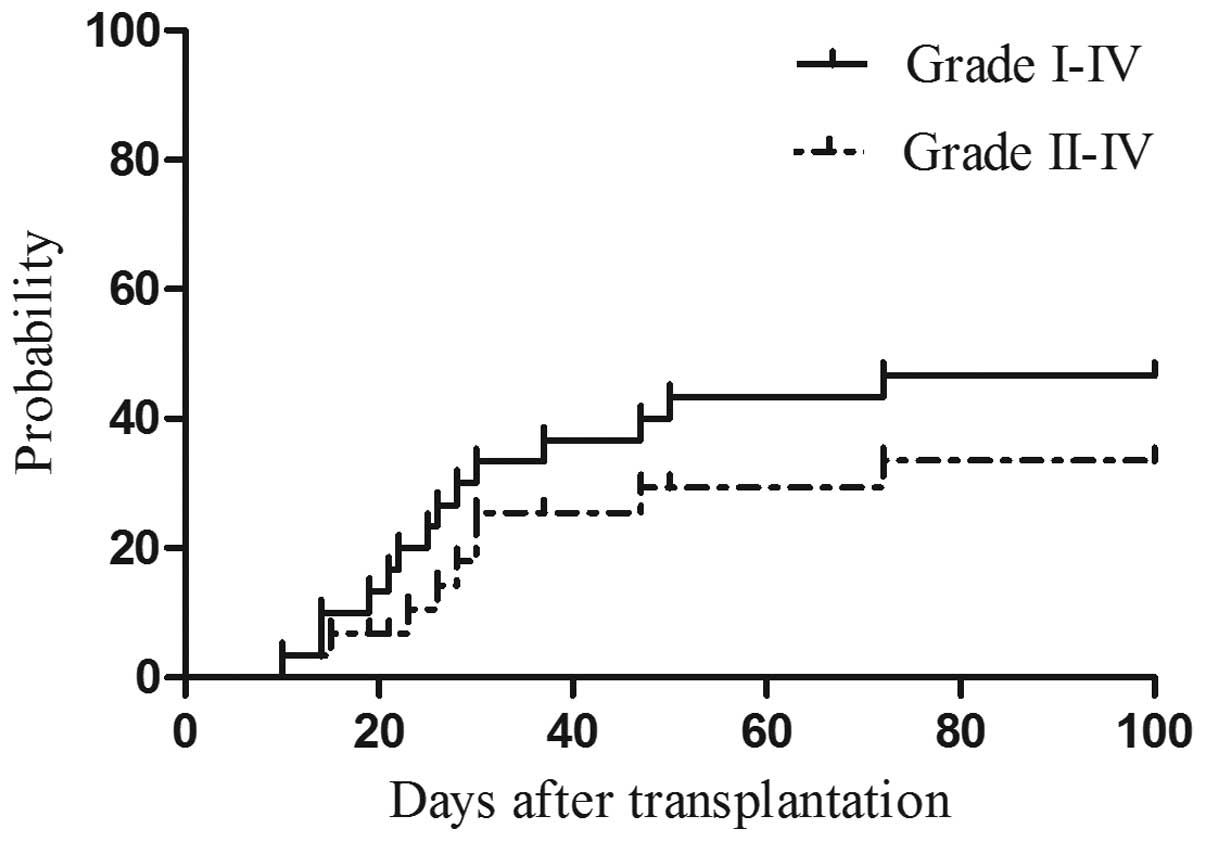
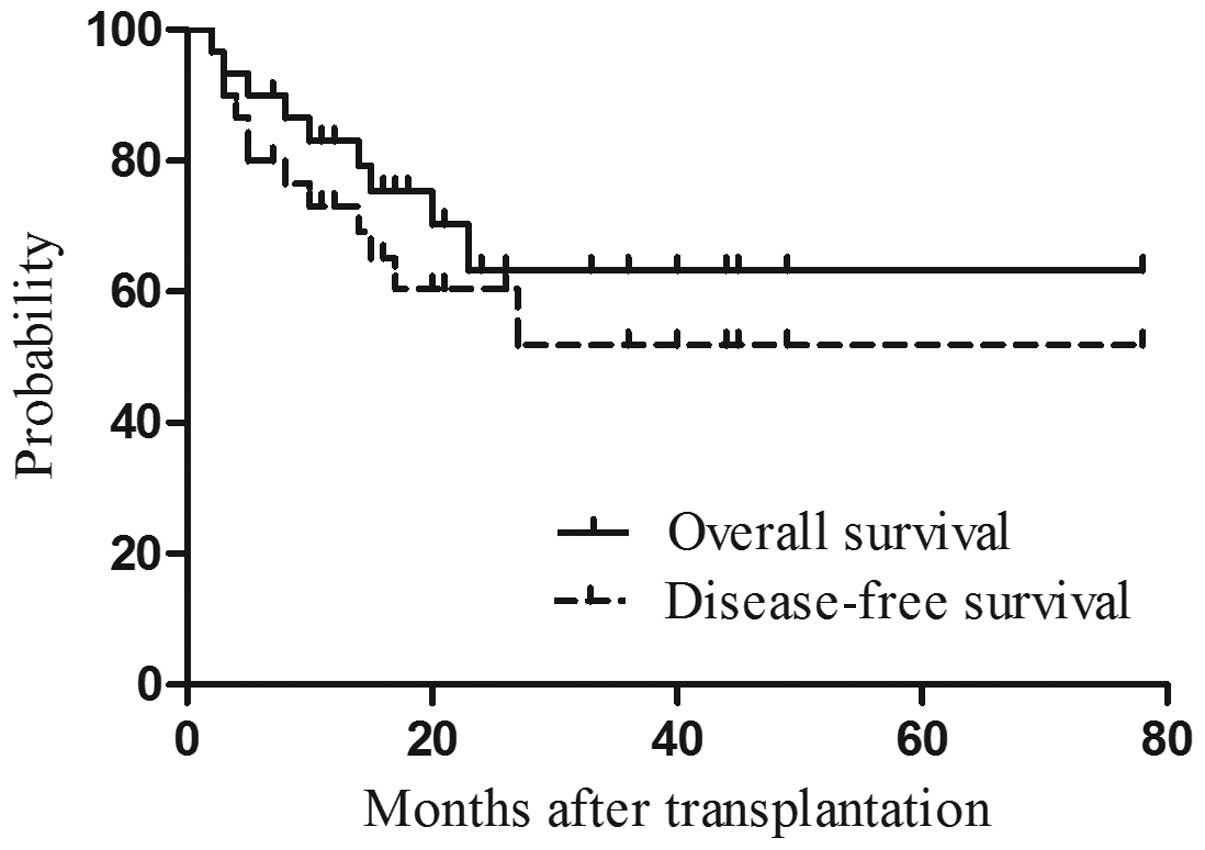
|
1
|
Kolb HJ, Schattenberg A, Goldman JM,
Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A,
Verdonck L, Niederwieser D, et al: Graft-versus-leukemia effect of
donor lymphocyte transfusions in marrow grafted patients. Blood.
86:2041–2050. 1995.PubMed/NCBI
|
|
2
|
Andersson BS, Kashyap A, Gian V, Wingard
JR, Fernandez H, Cagnoni PJ, Jones RB, Tarantolo S, Hu WW, Blume
KG, et al: Conditioning therapy with intravenous busulfan and
cyclophosphamide (IV BuCy2) for hematologic malignancies prior to
allogeneic stem cell transplantation: A phase II study. Biol Blood
Marrow Transplant. 8:145–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill S and Porter DL: Reduced-intensity
hematopoietic stem cell transplants for malignancies: Harnessing
the graft-versus-tumor effect. Annu Rev Med. 64:101–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keating MJ, O'Brien S, Lerner S, Koller C,
Beran M, Robertson LE, Freireich EJ, Estey E and Kantarjian H:
Long-term follow-up of patients with chronic lymphocytic leukemia
(CLL) receiving fludarabine regimens as initial therapy. Blood.
92:1165–1171. 1998.PubMed/NCBI
|
|
5
|
Iravani M, Evazi MR, Mousavi SA, Shamshiri
AR, Tavakoli M, Ashouri A, Samiee S, Chahardovali B, Alimoghaddam
K, Ghaffari SH and Ghavamzadeh A: Fludarabine and busulfan as a
myeloablative conditioning regimen for allogeneic stem cell
transplantation in high- and standard-risk leukemic patients. Bone
Marrow Transplant. 40:105–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pidala J, Kim J, Anasetti C,
Kharfan-Dabaja MA, Nishihori T, Field T, Perkins J, Perez L and
Fernandez HF: Pharmacokinetic targeting of intravenous busulfan
reduces conditioning regimen related toxicity following allogeneic
hematopoietic cell transplantation for acute myelogenous leukemia.
J Hematol Oncol. 3:362010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie
D, Zhang Y, Huang F, Zhou H, Fan Z, et al: Busulfan plus
fludarabine as a myeloablative conditioning regimen compared with
busulfan plus cyclophosphamide for acute myeloid leukemia in first
complete remission undergoing allogeneic hematopoietic stem cell
transplantation: A prospective and multicenter study. J Hematol
Oncol. 6:152013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chunduri S, Dobogai LC, Peace D,
Saunthararajah Y, Quigley J, Chen YH, Mahmud N, Hurter E, Beri R
and Rondelli D: Fludarabine/i.v. BU conditioning regimen:
Myeloablative, reduced intensity or both? Bone Marrow Transplant.
41:935–940. 2008.
|
|
9
|
Laport GG, Sandmaier BM, Storer BE, Scott
BL, Stuart MJ, Lange T, Maris MB, Agura ED, Chauncey TR, Wong RM,
et al: Reduced-intensity conditioning followed by allogeneic
hematopoietic cell transplantation for adult patients with
myelodysplastic syndrome and myeloproliferative disorders. Biol
Blood Marrow Transplant. 14:246–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Joo YD, Kim H, Ryoo HM, Kim MK,
Lee GW, Lee JH, Lee WS, Park JH, Bae SH, et al: Randomized trial of
myeloablative conditioning regimens: Busulfan plus cyclophosphamide
versus busulfan plus fludarabine. J Clin Oncol. 31:701–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson BS, de Lima M, Thall PF, Wang X,
Couriel D, Korbling M, Roberson S, Giralt S, Pierre B, Russell JA,
et al: Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu)
compares favorably with i.v. busulfan and cyclophosphamide (i.v.
BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood
Marrow Transplant. 14:672–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimoni A, Hardan I, Shem-Tov N, Yeshurun
M, Yerushalmi R, Avigdor A, Ben-Bassat I and Nagler A: Allogeneic
hematopoietic stem-cell transplantation in AML and MDS using
myeloablative versus reduced-intensity conditioning: The role of
dose intensity. Leukemia. 20:322–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK,
Lee KB, Sohn SK, Kim JG, Kim DH, Seol M, et al: Prognostic factors
identifiable at the time of onset of acute graft-versus-host
disease after allogeneic hematopoietic cell transplantation.
Haematologica. 90:939–948. 2005.PubMed/NCBI
|
|
14
|
Filipovich AH, Weisdorf D, Pavletic S,
Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D,
Couriel D, et al: National institutes of health consensus
development project on criteria for clinical trials in chronic
graft-versus-host disease: I. Diagnosis and staging working group
report. Biol Blood Marrow Transplant. 11:945–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jagasia MH, Greinix HT, Arora M, Williams
KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS,
et al: National Institutes of Health consensus development project
on criteria for clinical trials in chronic graft-versus-host
disease: I. The 2014 Diagnosis and Staging Working Group report.
Biol Blood Marrow Transplant. 21:389–401.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hughes WT, Armstrong D, Bodey GP, Bow EJ,
Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL and
Young LS: 2002 Guidelines for the use of antimicrobial agents in
neutropenic patients with cancer. Clin Infect Dis. 34:730–751.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDonald GB, Slattery JT, Bouvier ME, Ren
S, Batchelder AL, Kalhorn TF, Schoch HG, Anasetti C and Gooley T:
Cyclophosphamide metabolism, liver toxicity, and mortality
following hematopoietic stem cell transplantation. Blood.
101:2043–2048. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slavin S, Nagler A, Naparstek E,
Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M,
Ackerstein A, Samuel S, et al: Nonmyeloablative stem cell
transplantation and cell therapy as an alternative to conventional
bone marrow transplantation with lethal cytoreduction for the
treatment of malignant and nonmalignant hematologic diseases.
Blood. 91:756–763. 1998.PubMed/NCBI
|
|
19
|
Giralt S, Thall PF, Khouri I, Wang X,
Braunschweig I, Ippolitti C, Claxton D, Donato M, Bruton J, Cohen
A, et al: Melphalan and purine analog-containing preparative
regimens: Reduced-intensity conditioning for patients with
hematologic malignancies undergoing allogeneic progenitor cell
transplantation. Blood. 97:631–637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Blanc K, Remberger M, Uzunel M,
Mattsson J, Barkholt L and Ringdén O: A comparison of
nonmyeloablative and reduced-intensity conditioning for allogeneic
stem-cell transplantation. Transplantation. 78:1014–1020. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niederwieser D, Maris M, Shizuru JA,
Petersdorf E, Hegenbart U, Sandmaier BM, Maloney DG, Storer B,
Lange T, Chauncey T, et al: Low-dose total body irradiation (TBI)
and fludarabine followed by hematopoietic cell transplantation
(HCT) from HLA-matched or mismatched unrelated donors and
postgrafting immunosuppression with cyclosporine and mycophenolate
mofetil (MMF) can induce durable complete chimerism and sustained
remissions in patients with hematological diseases. Blood.
101:1620–1629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Or R, Shapira MY, Resnick I, Amar A,
Ackerstein A, Samuel S, Aker M, Naparstek E, Nagler A and Slavin S:
Nonmyeloablative allogeneic stem cell transplantation for the
treatment of chronic myeloid leukemia in first chronic phase.
Blood. 101:441–445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bornhauser M, Storer B, Slattery JT,
Appelbaum FR, Deeg HJ, Hansen J, Martin PJ, McDonald GB, Nichols
WG, Radich J, et al: Conditioning with fludarabine and targeted
busulfan for transplantation of allogeneic hematopoietic stem
cells. Blood. 102:820–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russell JA, Tran HT, Quinlan D, Chaudhry
A, Duggan P, Brown C, Stewart D, Ruether JD, Morris D, Glick S, et
al: Once-daily intravenous busulfan given with fludarabine as
conditioning for allogeneic stem cell transplantation: Study of
pharmacokinetics and early clinical outcomes. Biol Blood Marrow
Transplant. 8:468–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chae YS, Sohn SK, Kim JG, Cho YY, Moon JH,
Shin HJ, Chung JS, Cho GJ, Yang DH, Lee JJ, et al: New
myeloablative conditioning regimen with fludarabine and busulfan
for allogeneic stem cell transplantation: Comparison with BuCy2.
Bone Marrow Transplant. 40:541–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bredeson CN, Zhang MJ, Agovi MA,
Bacigalupo A, Bahlis NJ, Ballen K, Brown C, Chaudhry MA, Horowitz
MM, Kurian S, et al: Outcomes following HSCT using fludarabine,
busulfan, and thymoglobulin: A matched comparison to allogeneic
transplants conditioned with busulfan and cyclophosphamide. Biol
Blood Marrow Transplant. 14:993–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo
DJ, Stone R, Ritz J, Antin JH and Soiffer RJ: Impact of
conditioning regimen intensity on outcome of allogeneic
hematopoietic cell transplantation for advanced acute myelogenous
leukemia and myelodysplastic syndrome. Biol Blood Marrow
Transplant. 12:1047–1055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martino R, Iacobelli S, Brand R, Jansen T,
van Biezen A, Finke J, Bacigalupo A, Beelen D, Reiffers J, Devergie
A, et al: Retrospective comparison of reduced-intensity
conditioning and conventional high-dose conditioning for allogeneic
hematopoietic stem cell transplantation using HLA-identical sibling
donors in myelodysplastic syndromes. Blood. 108:836–846. 2006.
View Article : Google Scholar : PubMed/NCBI
|