Introduction
Primary vulvar cancer is a rare disease, with an
incidence of 2–3 per 100,000 women. Traditionally, primary vulvar
cancer affects elderly women (median age, 65–70 years) and the vast
majority of tumors are squamous cell carcinomas (90%) (1). Primary vulvar adenocarcinomas are rare
and their histogenesis has not been fully elucidated; they mainly
include extramammary Paget's disease, sweat gland carcinomas and
breast-like adenocarcinomas of the vulva (2,3). Primary
vulvar mucinous adenocarcinoma, in particular, is an extremely rare
entity, with only one case reported to date (4). The aim of this report was to describe
another case of mucinous adenocarcinoma arising from the vulva and
discuss its clinical management.
Case report
A Chinese woman, aged 43 years, gravida 3, para 2,
presented with a 1-month history of a pruritic, painless vulvar
mass. On gynecological examination, a 1.5-cm soft, non-tender gray
mass was identified adjacent to the hymen on the perineal body.
Bioptic excision of this mass was performed at another hospital and
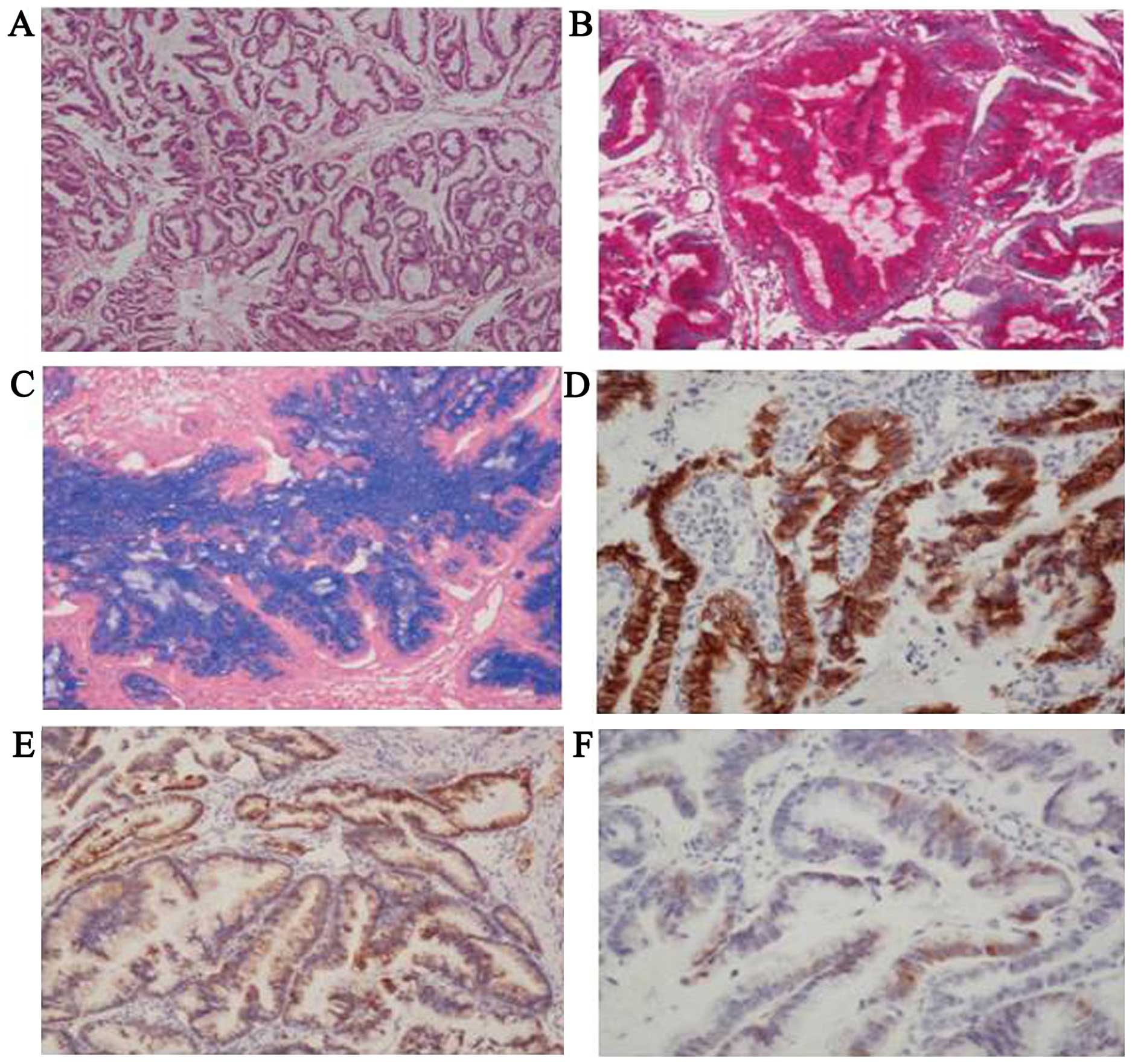
the pathological examination revealed an invasive, moderately
differentiated mucinous adenocarcinoma (Fig. 1A). On immunohistochemical analysis,
the tumor cells were diffusely positive for periodic acid-Schiff
(PAS), alcian blue (AB), cytokeratin (CK) 7 and cellular adhesion
molecule (CAM) 5.2, and focally positive for p16 (Fig. 1B–F). The tumor cells were negative for
estrogen receptor (ER), progesterone receptor (PR) and gross cystic
disease fluid protein (GCDFP) 15, thereby excluding mammary gland
and endometrial carcinoma. The tumor was also negative for
carbohydrate antigen (CA) 125, excluding ovarian cancer. The tumor
stained negatively for CK20, villin and CA19-9, excluding
adenocarcinoma of the gastrointestinal tract. The tumor stained
negatively for transcription intermediary factor (TTF) 1 and napsin
A, excluding lung adenocarcinoma. Therefore, the diagnosis of
primary mucinous adenocarcinoma of the vulva was confirmed. An
extensive workup, including chest X-ray, abdominal and pelvic
ultrasound, cervical Pap smear and systemic positron emission
tomography-computed tomography (PET-CT) did not reveal any other
local genital or systemic cancer. In particular, colonoscopy and
gastroscopy examination were both negative, excluding
adenocarcinoma of the gastrointestinal tract (5). The carcinoembryonic antigen (CEA) and
CA-125 levels were not increased. A diagnosis of stage I primary
mucinous adenocarcinoma of the vulva was made, according to the
classification of the International Federation of Gynecology and
Obstetrics. The patient's general health was good and her routine
laboratory examinations were considered satisfactory for her
age.
A local resection of the vulvar neoplasm without
lymph node dissection was recommended. The postoperative
pathological examination revealed an invasive, moderately
differentiated mucinous adenocarcinoma, with clear resection
margins of the surgical specimen. With the patient's informed
consent, she then underwent systemic venous chemotherapy. The
combined chemotherapy consisted of paclitaxel (150
mg/m2) and carboplatin (220 mg/m2) for a
total of four cycles (6). The patient
underwent a regular clinical, vulvoscopic and colonoscopic
follow-up and she remains alive and cancer-free at 24 months after
treatment.
Discussion
Mucinous adenocarcinoma is a rare variant of sweat
gland carcinoma, first described by Mendoza and Helwig in 1971
(7). A total of 76 cases of mucinous
adenocarcinoma have been reported in the with three cases on the
vulva (Table I). Two of them were
primary neuroendocrine differentiated mucinous adenocarcinomas of
the vulva (8,9), and only one was a true primary vulvar
mucinous adenocarcinoma, which was also the case in our patient.
The case report of primary vulvar mucinous adenocarcinoma in the
literature was that of an 80-year-old, nulliparous, Caucasian
woman, who presented with a 2-month history of a 2-cm,
asymptomatic, reddish-blue, well-defined cystic mass on the
upper-medial aspect of the right labium majus. The patient
underwent radical vulvectomy, with bilateral inguinofemoral lymph
node dissection (4).
 | Table I.Cases of primary mucinous
adenocarcinomas of the vulva in the literature. |
Table I.
Cases of primary mucinous
adenocarcinomas of the vulva in the literature.
| First author
(Refs.) | Age (years) | Reproductive
history | Symptoms | History (months) | Signs | FIGO stage | Treatment | Histological
diagnosis | Outcome |
|---|
| Ghamande (4) | 80 | Nulliparous | Asymptomatic | 2 | 2-cm reddish-blue,
well-defined cystic mass of the right labium majus | – | Radical vulvectomy,
bilateral inguinofemoral lymph node dissection | Mucinous
adenocarcinoma | Alive 19 months after
surgery |
| Rahilly (8) | 69 | Parous | Vulval pruritus,
occasionally painful | 2 | 8.5×6-cm ulcerated
swelling of the right labium majus | – | Extended right
hemivulvectomy | Mucinous
adenocarcinoma with NE differentiation | Disease-free 35
months after surgery |
| Graf (9) | 75 | G4P3 | Genital bleeding | 9 | 4.5×4.0×3.5–cm tumor
of the labium majus | II | Radical vulvectomy,
bilateral inguinofemoral lymph node dissection, postoperative
radiotherapy | Mucinous
adenocarcinoma with NE differentiation | Alive 4 years after
surgery, but presented with bilateral breast cancer 16 months after
surgery |
| Present case | 43 | G3P2 | Vulval pruritus,
painless | 1 | 1.5–cm soft,
non-tender gray mass on the perineal body, adjacent to the
hymen | I | Local excision,
postoperative chemotherapy | Invasive, moderately
differentiated mucinous adenocarcinoma | Disease-free 24
months after surgery |
As regards the clinical management of primary vulvar
cancer, up to the 1990s, radical vulvectomy with bilateral
inguinofemoral lymphadenectomy was considered the standard therapy.
The aim of this intervention was to remove all tissue possibly
affected by vulvar cancer, including the bridge of skin between the
vulva and the inguinal area. However, this mutilating procedure was
associated with severe morbidity, due to the significant
psychosexual impairment (10). To
avoid overtreatment, increasing efforts to modify surgical
management were undertaken. The introduction of radical local
excision instead of complete vulvectomy was a major step towards
further reduction of surgical complications. No compromise in
oncological safety was observed in patients with early-stage
disease compared with the control group (1). Considering the significant morbidity of
radical lymphadenectomy and the fact that only 25–30% of the
patients present with lymph node metastases at first diagnosis,
sentinel node dissection is considered a favorable alternative for
patients with a clinically node-negative inguinal area (11). Patients with early-stage disease have
a fairly good prognosis with an individualized treatment plan and a
less radical surgical approach (12).
Local tumor resection rather than radical vulvectomy and the
implementation of the sentinel technique have decreased
therapy-associated morbidity and psychosocial impairment in these
patients, while maintaining oncological safety (11).
The clinical management in our case was different
from that of the first case of primary vulvar mucinous
adenocarcinoma. The reasons were as follows: First, the lesion was
adjacent to the hymen on the perineal body in our case. In general,
lymphatic metastasis is one of the main pathways of metastasis in
gynecological malignant tumors, and a poor prognostic factor
(13). As regards vulvar cancer lymph
node metastases from the superficial to the deep inguinal and
femoral lymph nodes (including the Cloquet's lymph node) and then
to the pelvic lymph nodes, the mean incidence is 22–39% and the
lesion size, infiltration depth and stage are closely associated.
The case report of vulvar mucinous adenocarcinoma in the
literature, the lesion was on the upper medial aspect of the right
labium majus, with lymphatic flow mainly to the inguinal lymph
nodes; in our case, the tumor was on the perineal body, with
lymphatic flow mainly to the pelvic lymph nodes (11). There was no pelvic lymph node
metastasis on the systemic PET-CT. Therefore, bilateral
inguinofemoral node dissection was not performed. Second, vulvar
mucinous adenocarcinoma is rare and its pathway of metastasis has
not been elucidated; thus, the main pathway of metastasis may not
be lymphogenic. Adjuvant chemotherapy was administered
postoperatively to prevent other metastatic pathways (6,14).
Finally, the patient's diagnosis in this case was stage IA primary
mucinous adenocarcinoma of the vulva and, for tumors with an
infiltration depth of <1 mm, a lymphadenectomy is not considered
necessary (15,16). In addition, our patient displayed no
signs of pelvic lymph node metastasis. As the lesion was close to
the anus and rectum, radical resection would have required removal
of those anatomical structures, which would have severely
compromised the quality of life of this patient. Therefore, a local
tumor resection was performed, followed by chemotherapy.
In the case of vulvar mucinous adenocarcinoma in the
literature, the patient remained alive and well 19 months after the
surgery. In our case, during the 2 years of follow-up, the patient
remained clinically disease-free, without recurrence or
treatment-related complications. We consider local excision, with
or without chemotherapy, to be an effective therapeutic approach to
this type of tumor. However, further studies are required to
support our conclusions for early-stage vulvar mucinous
adenocarcinoma. Patients with early-stage disease have a fairly
good prognosis with an individualized treatment plan and a less
radical surgical approach. Therefore, we recommend local excision,
with or without chemotherapy, as an effective treatment for
early-stage vulvar mucinous adenocarcinoma.
Acknowledgements
The authors would like to thank to Mrs. Huiting Liu,
who revised the manuscript.
Glossary
Abbreviations
Abbreviations:
|
PAS
|
periodic acid-Schiff
|
|
AB
|
alcian-blue
|
|
CK
|
cytokeratin
|
|
CAM
|
cellular adhesion molecule
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
GCDFP
|
gross cystic disease fluid protein
|
|
TIF
|
transcription intermediary factor
|
|
CA
|
carbohydrate antigen
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
CEA
|
carcinoembryonic antigen
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Woelber L, Kock L, Gieseking F, Petersen
C, Trillsch F, Choschzick M, Jaenicke F and Mahner S: Clinical
management of primary vulvar cancer. Eur J Cancer. 47:2315–2321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kajal B, Talati H, Daya D and Alowami S:
Apocrine adenocarcinoma of the vulva. Rare Tumors. 5:e402013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bogani G, Uccella S, Cromi A, Casarin J,
Donadello N and Ghezzi F: Primary mammary-like ductal carcinoma of
the vulva: A case report and analysis of the literature. Am J
Dermatopathol. 35:685–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghamande SA, Kasznica J, Griffiths CT,
Finkler NJ and Hamid AM: Mucinous adenocarcinomas of the vulva.
Gynecol Oncol. 57:117–120. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cormio G, Carriero C, Loizzi V, Gissi F,
Leone L, Putignano G, Resta L and Selvaggi L: ‘Intestinal-type’
mucinous adenocarcinoma of the vulva: A report of two cases. Eur J
Gynaecol Oncol. 33:433–435. 2012.PubMed/NCBI
|
|
6
|
Geisler JP, Manahan KJ and Buller RE:
Neoadjuvant chemotherapy in vulvar cancer: Avoiding primary
exenteration. Gynecol Oncol. 100:53–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendoza S and Helwig EB: Mucinous
(adenocystic) carcinoma of the skin. Arch Dermatol. 103:68–78.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahilly MA, Beattie GJ and Lessells AM:
Mucinous eccrine carcinoma of the vulva with neuroendocrine
differentiation. Histopathology. 27:82–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Graf AH, Su HC, Tubbs RR, Hacker GW, Dietz
O and Staudach A: Primary neuroendocrine differentiated mucinous
adenocarcinoma of the vulva: case report and review of the
literature. Anticancer Res. 18:2041–2045. 1998.PubMed/NCBI
|
|
10
|
Magrina JF, Gonzalez-Bosquet J, Weaver AL,
Gaffey TA, Webb MJ, Podratz KC and Cornella JL: Primary squamous
cell cancer of the vulva: Radical versus modified radical vulvar
surgery. Gynecol Oncol. 71:116–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woelber L, Trillsch F, Kock L, Grimm D,
Petersen C, Choschzick M, Jaenicke F and Mahner S: Management of
patients with vulvar cancer: A perspective review according to
tumour stage. Ther Adv Med Oncol. 5:183–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dittmer C, Fischer D, Diedrich K and Thill
M: Diagnosis and treatment options of vulvar cancer: A review. Arch
Gynecol Obstet. 285:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woelber L, Mahner S, Voelker K, Eulenburg
CZ, Gieseking F, Choschzick M, Jaenicke F and Schwarz J:
Clinicopathological prognostic factors and patterns of recurrence
in vulvar cancer. Anticancer Res. 29:545–552. 2009.PubMed/NCBI
|
|
14
|
Domingues AP, Mota F, Durão M, Frutuoso C,
Amaral N and de Oliveira CF: Neoadjuvant chemotherapy in advanced
vulvar cancer. Int J Gynecol Cancer. 20:294–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stehman FB and Look KY: Carcinoma of the
vulva. Obstet Gynecol. 107:719–733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herr D, Juhasz-Boess I and Solomayer EF:
Therapy for primary vulvar carcinoma. Geburtshilfe Frauenheilkd.
74:271–275. 2014. View Article : Google Scholar : PubMed/NCBI
|