Introduction
With the continued aging and increase in the life
expectancy of the population worldwide, the age of patients with
cancer is also expected to increase. Indeed, over 50% of solid
cancers are diagnosed in patients aged >65 years, and the cancer
incidence at ages >65 years is ~10 times higher compared with
that in the younger population (1).
Accordingly, the use of chemotherapeutic agents in elderly patients
with cancer may also increase. However, there is not sufficient
evidence of the tolerability and effectiveness of chemotherapeutic
drugs among elderly patients with cancer. Moreover, treatment
guidelines for elderly patients with cancer have not yet been
established; this may be because these patients are often excluded
from clinical trials, and also because the number of the clinical
reports on such patients is insufficient. Even among the relatively
few trials that include elderly patients, the majority are subset
analyses of clinical trials. Therefore, determining the benefits of
chemotherapy and doses of chemotherapeutic agents for elderly
patients with cancer is often difficult in clinical practice.
The therapeutic problem for elderly patients
originates from the fact that treatment response is affected by
aging-related physiological changes that differ from those in
younger patients; this is due to decreased drug absorption, liver
metabolic capacity and renal excretory function being more common
among elderly individuals. Additionally, several elderly patients
with cancer have multiple chronic diseases, which may result in the
use of a wide variety of drugs with consequent deleterious drug
interactions and adverse events.
Endometrial cancer is the most common gynecological
malignancy, with 52,630 new cases and 8,590 deaths in the United
States reported in 2014 (2).
Approximately 50% of patients with endometrial cancer are older
than 65 years (3), with a further
increase anticipated in the future. Thus, determining the optimal
treatment strategy for endometrial cancer in elderly patients will
become increasingly more important. The majority of patients with
endometrial cancer present with early-stage disease and may be
cured with primary therapy; however, women with metastatic cancer
or relapse have poor prognosis and require chemotherapy.
The Gynecologic Oncology Group 122 randomized study
(GOG122 trial) compared the efficacy of radiotherapy to that of
doxorubicin and cisplatin (AP therapy) for advanced endometrial
cancer; the results showed a favorable prognosis for patients who
underwent AP therapy (4).
Furthermore, the results of the GOG177 trial that paclitaxel,
doxorubicin and cisplatin (TAP therapy) was more effective compared
with AP therapy, although the toxicity of TAP therapy was severe
(5). paclitaxel and carboplatin (TC
therapy) exerted a therapeutic effect similar to that of TAP
therapy, but with less toxicity (6).
Moreover, a previous phase 2 trial found no differences in the
effectiveness of docetaxel and cisplatin (DP), docetaxel and
carboplatin (DC), and TC therapies for advanced endometrial cancer
(7). As described above, the
combination of an anthracycline-based drug, a platinum-containing
drug and a taxane agent is used in endometrial cancer chemotherapy,
with DC therapy being one of the most important treatment options
(8,9).
However, there are few reports on the tolerability and
effectiveness of DC therapy in elderly patients with cancer.
Although there are some reports on DC therapy for elderly patients
with other types of cancer (10–12), none
of the previous reports have focused on endometrial cancer.
Therefore, we evaluated the tolerability and effectiveness of DC
chemotherapy for elderly patients with endometrial cancer.
Patients and methods
Patients
A total of 41 patients with endometrial cancer were
enrolled in this retrospective study between April, 2008 and March,
2015. The study protocol was approved by the Ethics Committee of
Saitama Medical University International Medical Center, and all
the patients provided written informed consent prior to the
procedure being performed. All the patients were staged according
to the International Federation of Gynecology and Obstetrics (FIGO)
staging system (13). Eligible
patients had histologically proven endometrial cancer, an Eastern
Cooperative Oncology Group (ECOG) performance status (PS) score of
≤2 and adequate baseline hematological (absolute neutrophil count
≥500/mm3, platelet count ≥100,000/mm3), renal
(serum creatinine ≤1.5 mg/dl) and liver functions (serum bilirubin
≤1.5 mg/dl). Patients were excluded if they had received previous
chemotherapy for endometrial cancer or a concurrent malignancy.
Treatment
Docetaxel (60 mg/m2) was administered
intravenously (i.v.) for 1 h, followed by carboplatin administered
i.v. for 1 h. The dose of carboplatin was calculated according to
the Calvert formula, with an area under the curve (AUC) of 6
mg/ml/min using a calculated glomerular filtration rate from the
Cockcroft-Gault formula (14).
Docetaxel and carboplatin were administered on day 1, and repeated
every 3 weeks. Dexamethasone (16.5 mg i.v.) and palonosetron (0.75
mg i.v.) were administered as premedications prior to docetaxel.
Treatment was continued until disease progression. Each
chemotherapy cycle was only delivered if the absolute neutrophil
and platelet counts on the day of treatment were at least 1,500 and
100,000/mm3, respectively. Otherwise, treatment was
delayed until this level was achieved.
Toxicity and chemotherapy
adjustments
Adverse events were graded at each cycle by study
investigators according to the National Cancer Institute Common
Terminology Criteria for Adverse Events, version 4.0 (15). Dose reductions were allowed at the
investigator's discretion, depending on the onset of hematological
toxicities, such as febrile neutropenia or grade 4
thrombocytopenia, or non-hematological toxicities ≥grade 3,
excluding alopecia, nausea and vomiting. If necessary, the dose of
both drugs was reduced as follows in the subsequent cycle:
docetaxel, 50 mg/m2 and carboplatin, AUC=5. Granulocyte
colony-stimulating factor (G-CSF) and prophylactic antibiotics were
permitted as clinically indicated in the presence of grade 4
neutropenia or febrile neutropenia.
Response evaluation
The treatment response was assessed every two or
three cycles by computed tomography scans according to the Response
Evaluation Criteria in Solid Tumors (16).
Statistical analysis
For this age-specific retrospective exploratory
analysis, patients were dichotomized according to the age split of
<65 or ≥65 years at the time of chemotherapy administration. All
the statistical tests were exploratory in nature. Categorical
variables were evaluated by the chi-square test or the Fisher's
exact test, as appropriate for category size. Kaplan-Meier curves
were produced for progression-free survival (PFS) and overall
survival (OS) by age group. Both PFS and OS were calculated from
the date of initial chemotherapy. The log-rank test was used to
compare survival between the two groups. Statistical significance
was set at P<0.05.
Results
Patient characteristics
A total of 41 patients were enrolled in this
retrospective study, of whom (63%) were aged <65 years and 15
(37%) were aged ≥65 years. The patient and tumor characteristics
were compared between the two groups (Table I). The median age in the younger group
was 56.5 years (range, 36–64 years) and in the older group 70.0
years (range, 65–78 years). There were no significant differences
in terms of PS score and FIGO stage between the two groups.
Patients older than 65 years were significantly more likely to have
serous or clear-cell histology and high-grade tumors compared with
the younger group (P=0.014 and 0.012, respectively). Patients older
than 65 years were also significantly more likely to have
hypertension (P=0.0017). There were no significant differences
between the two groups in the prevalence of diabetes mellitus,
cardiac disease or pulmonary disease.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Characteristics | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value |
|---|
| ECOG PS |
|
| 0.11 |
| 0 | 24 (92) | 10 (67) |
|
| 1 | 1 (4) | 3 (20) |
|
| 2 | 1 (4) | 2 (13) |
|
| Stage |
|
| 0.39 |
| IA | 1 (4) | 1 (7) |
|
| IB | 7 (27) | 3 (20) |
|
| II | 7 (27) | 1 (7) |
|
|
IIIA/B | 2 (8) | 2 (13) |
|
|
IIIC1 | 2 (8) | 0 (0) |
|
|
IIIC2 | 2 (8) | 1 (7) |
|
| IVB | 5 (19) | 7 (46) |
|
| Histology |
|
| 0.014 |
|
Endometrioid | 25 (96) | 9 (60) |
|
|
Serous | 0 (0) | 3 (20) |
|
| Clear
cell | 0 (0) | 1 (7) |
|
|
Mixed | 0 (0) | 2 (13) |
|
|
Undifferentiated | 1 (4) | 0 (0) |
|
| Grade |
|
| 0.012 |
| 1 | 13 (50) | 4 (26) |
|
| 2 | 9 (34) | 2 (13) |
|
| 3 | 4 (16) | 9 (60) |
|
| Comorbidities |
|
|
|
|
Hypertension | 4 (16) | 10 (67) | 0.0017 |
| Diabetes
mellitus | 7 (27) | 3 (20) | 0.72 |
| Cardiac
disease | 1 (4) | 2 (13) | 0.54 |
| Pulmonary
disease | 3 (12) | 1 (7) | >0.99 |
Feasibility
The feasibility of DC therapy was analyzed (Table II). The number of cycles received did
not differ between the two groups. Dose reductions were required in
6 (23%) patients from the younger group and 7 (46%) from the older
group. There was a trend toward more dose reductions among patients
aged >65 years (P=0.12), but the difference was not significant.
Hematological toxicity was the most common reason for dose
reduction in the older group (26%), whereas hematological as well
as non-hematological toxicities were responsible for dose
reductions in the younger group. There were no significant
differences in cycle delays or reasons for cycle delays between the
groups. The occurrence rate of treatment interruption also did not
differ significantly between the groups. Progressive disease was
the most common reason for treatment interruption in the younger
(19%) as well as in the older group (26%). There was no significant
difference in the number of patients who received G-CSF between the
two groups. G-CSF was used in 33 and 19% of the older and younger
patients, respectively.
 | Table II.Number of cycles and feasibility of
chemotherapy. |
Table II.
Number of cycles and feasibility of
chemotherapy.
| Variables | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value |
|---|
| Number of cycles |
|
| 0.33 |
| 1–3 | 8 (30) | 6 (40) |
|
| 4–6 | 16 (61) | 6 (40) |
|
|
>6 | 2 (8) | 3 (20) |
|
| Dose reduction | 6 (23) | 7 (46) | 0.12 |
|
Reasons |
|
|
|
|
Hematological
toxicity | 3 (11) | 4 (26) |
|
|
Non-hematological
toxicity | 3 (11) | 1 (7) |
|
|
Others | 0 (0) | 2 (13) |
|
| Cycle delay | 7 (27) | 5 (33) | 0.73 |
|
Reasons |
|
|
|
|
Hematological
toxicity | 7 (27) | 3 (20) |
|
|
Non-hematological
toxicity | 0 (0) | 1 (7) |
|
|
Others | 0 (0) | 1 (7) |
|
| Treatment
interruption | 12 (46) | 9 (60) | 0.39 |
|
Reasons |
|
|
|
|
Toxicity | 4 (15) | 1 (7) |
|
|
Progressive
disease | 5 (19) | 4 (26) |
|
|
Patient's
withdrawal | 3 (11) | 3 (20) |
|
|
Others | 0 (0) | 1 (7) |
|
Adverse effects
The incidence of hematological toxicities, such as
anemia, leukopenia, neutropenia and thrombocytopenia, did not
differ significantly between the two groups (Table III). The most common grade 3/4
hematological toxicity was neutropenia, occurring in 77% of the
younger patients and 80% of the older patients. The incidence of
non-hematological toxicities did not differ significantly between
the groups with regard to nausea, vomiting, neurotoxicity and
myalgia (Table IV). The incidence of
grade 3/4 diarrhea was significantly higher in the older group
compared with that in the younger group (P=0.014). Hypersensitivity
was significantly more frequent in the younger group (P=0.035).
 | Table III.Worst degree of hematological
toxicities by patient according to NCI-CTC 4.0. |
Table III.
Worst degree of hematological
toxicities by patient according to NCI-CTC 4.0.
|
| All grades | Grade ≥3 |
|---|
|
|
|
|
|---|
| Toxicities | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value |
|---|
| Anemia | 23 (88) | 14 (88) | >0.99 | 9 (35) | 6 (40) | 0.73 |
| Leukopenia | 21 (81) | 13 (87) | 0.99 | 15 (58) | 11 (73) | 0.50 |
| Neutropenia | 21 (81) | 13 (87) | >0.99 | 20 (77) | 12 (80) | >0.99 |
|
Thrombocytopenia | 19 (73) | 10 (67) | 0.73 | 3 (12) | 0 (0) | 0.29 |
| Febrile
neutropenia | 2 (8) | 3 (20) | 0.34 | 2 (8) | 3 (20) | 0.34 |
 | Table IV.Worst degree of non-hematological
toxicities by patient according to NCI-CTC 4.0. |
Table IV.
Worst degree of non-hematological
toxicities by patient according to NCI-CTC 4.0.
|
| All grades | Grade ≥3 |
|---|
|
|
|
|
|---|
| Toxicities | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value |
|---|
| Nausea | 13 (50) | 11 (73) | 0.20 | 2 (8) | 0 (0) | 0.52 |
| Vomiting | 6 (23) | 4 (27) | >0.99 | 0 (0) | 0 (0) | >0.99 |
| Diarrhea | 5 (19) | 7 (47) | 0.083 | 0 (0) | 4 (27) | 0.014 |
| Neurotoxicity | 14 (54) | 9 (60) | 0.70 | 1 (4) | 1 (7) | >0.99 |
| Myalgia | 7 (27) | 2 (13) | 0.45 | 1 (4) | 0 (0) | >0.99 |
|
Hypersensitivity | 7 (27) | 0 (0) | 0.035 | 0 (0) | 0 (0) | >0.99 |
Response and survival
Of the 8 patients with target lesions assessable for
response in the younger group, 4 achieved a partial response. Of
the 7 patients with target lesions assessable for response in the
older group, 1 achieved a complete response and 2 a partial
response (Table V). The tumor
response to DC therapy did not differ significantly between the two
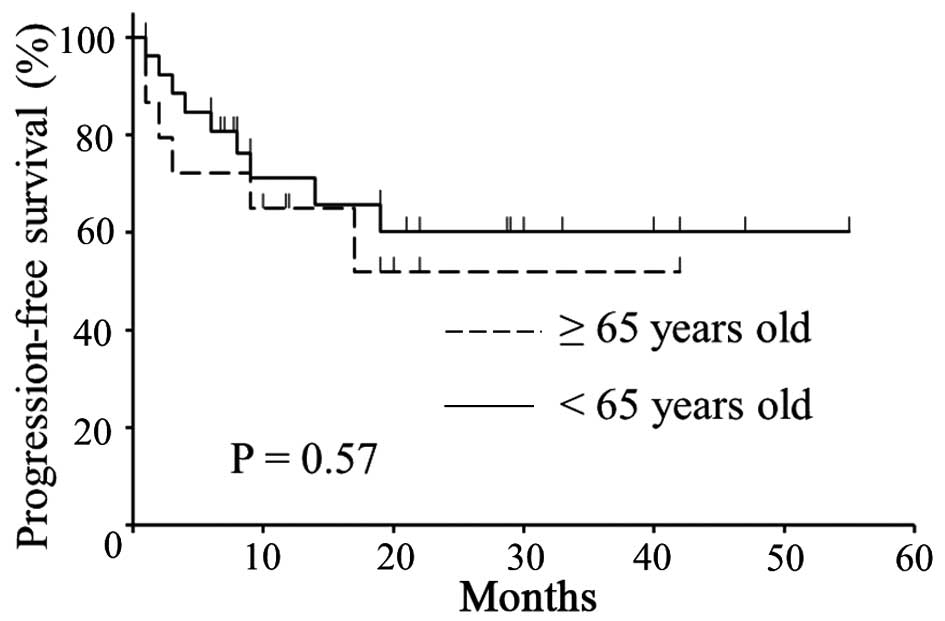
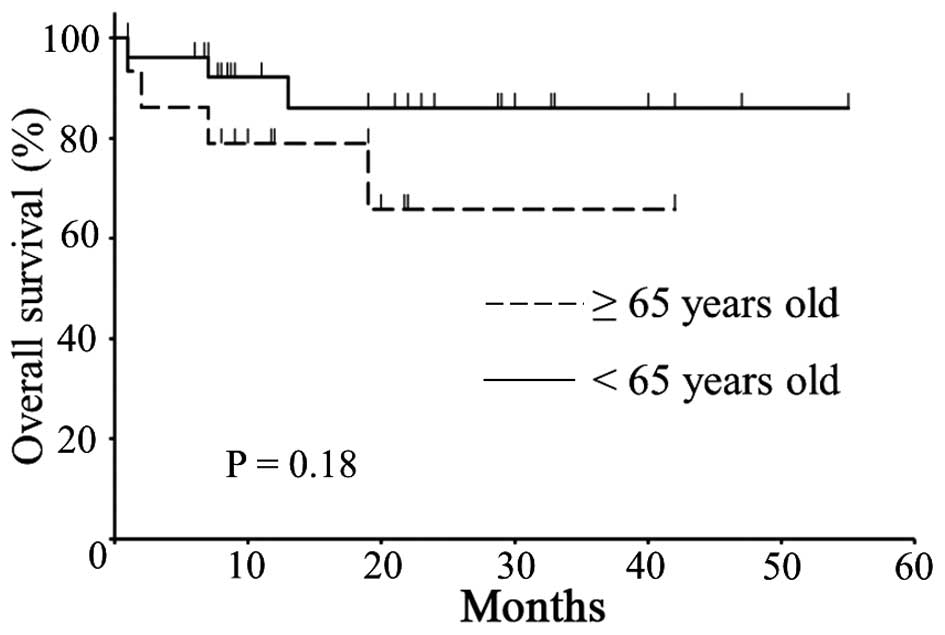
groups. There was no significant difference in PFS (Fig. 1, P=0.57) or OS (Fig. 2, P=0.18) between the two groups.
 | Table V.Response to chemotherapy. |
Table V.
Response to chemotherapy.
| Type of
response | <65 years, n (%)
(n=26) | ≥65 years, n (%)
(n=15) | P-value |
|---|
| Complete
response | 0 (0) | 1 (7) | 0.54 |
| Partial
response | 4 (15) | 2 (13) |
|
| Stable disease | 2 (8) | 1 (7) |
|
| Progressive
disease | 2 (8) | 3 (20) |
|
| Not evaluable
disease | 18 (69) | 8 (53) |
|
Discussion
As the aging population continues to grow, there is
a consequent increase in the life expectancy worldwide. Thus, the
incidences of diseases that affect the elderly, such as cancer, are
also expected to increase. Therefore, the use of chemotherapeutic
agents for elderly patients with cancer may also increase.
Nonetheless, there is no definitive evidence of the tolerability
and effectiveness of chemotherapeutic drugs among elderly patients
with cancer. Previous reports have concluded that elderly patients
experience more chemotherapy-related adverse events compared with
younger patients, due to the deteriorating organ function and poor
nutritional status. For example, a limited number of studies
reported an increased incidence of myelosuppression due to
chemotherapy, and hypertension as a result of bevacizumab therapy
in elderly patients with colon cancer compared with younger
patients (17,18). Additionally, cardiac toxicity and
treatment-related deaths with anthracycline-based drugs were more
common among elderly patients with breast cancer compared with
younger patients (19,20).
With regard to elderly patients with gynecological
cancer, a previous study reported that the completion rate of
chemotherapy was reduced in elderly patients with ovarian cancer
(aged ≥65 years) with >2 comorbidities (21). Additionally, compared with younger
patients, the disease is generally more advanced at diagnosis and
tends to progress more quickly in elderly patients with ovarian
cancer aged >70 years. This results in a higher risk of
mortality and a significantly lower 5-year survival rate among
these patients (22). By contrast, in
a clinical trial by Eisenhauer et al, there was no
difference in the frequency of dose reduction, treatment
interruption, response rate, or prognosis among patients aged ≥65
or <65 years who received combination chemotherapy with a
platinum-containing drug and a taxane agent as an initial
postoperative chemotherapy for advanced ovarian cancer (23).
Only few studies have reported on the tolerability
and effectiveness of DC therapy in elderly patients with cancer. A
comparison of patients with cancer older and younger than 65 years
of age treated with docetaxel revealed no differences in docetaxel
clearance, incidence of grade 4 neutropenia, or incidence of
febrile neutropenia between the two groups (24). Conversely, in a clinical trial where
docetaxel was administered to prostate cancer patients older than
75 years, the chemotherapy regimen was modified (e.g., dose
reduction) in 46% of the patients (25). Additionally, in a clinical trial of DC
therapy for non-small-cell lung cancer, subgroup analyses of
patients aged >65 years revealed that 86% of the patients
developed grade ≥3 neutropenia, the incidence of febrile
neutropenia was 7%, and the incidence of grade ≥3 non-hematological
toxicities was <17.5% (26).
In this trial, there was no difference in the
frequency of treatment delay or interruption between older and
younger patients with endometrial cancer who received DC therapy.
The frequency of dose reduction tended to be higher among older
patients (46% in older and 23% in younger patients), but this
difference was not significant. Hematological toxicities were the
main cause of dose reductions in older patients. In younger
patients, hematological and non-hematological toxicities were
equally responsible for the dose reductions. However, no difference
in the incidence of hematological toxicities was observed between
older and younger patients. Among patients who developed grade ≥3
hematological toxicities, neutropenia was the most frequent
complication in both age groups. As regards non-hematological
toxicities, the incidence of grade ≥3 diarrhea was significantly
higher among older patients (0 vs. 27%, P=0.014), whereas the
incidence of hypersensitivity was significantly higher among
younger patients (27 vs. 0%, P=0.035). In any case, in the older
and younger groups, DC therapy-related hematological and
non-hematological toxicities were manageable, and DC therapy was
generally well-tolerated in both groups, with acceptable
effectiveness; however, it was difficult to evaluate the
effectiveness of DC therapy due to the rarity of assessable
lesions. There were no significant differences in prognosis between
the two groups. Therefore, we consider DC therapy to have an
acceptable toxicity profile and efficacy in endometrial cancer
patients aged >65 years, when compared with younger patients.
However, as this study included relatively few cases, a larger
prospective study is required in the future.
Fader et al compared the effectiveness and
toxicity of TC therapy in elderly patients with ovarian cancer aged
≥70 years, who were subdivided into a standard-dose and a
dose-reduction group (27).
Carboplatin (AUC=5–6) and paclitaxel (175 mg/m2) were
administered to patients in the standard-dose group, whereas
carboplatin (AUC=4–5) and paclitaxel (135 mg/m2) were
administered to those in the dose-reduction group. The results
revealed a significantly lower frequency of neutropenia in the
dose-reduction group and no significant differences in the response
rate or prognosis between the two groups, suggesting that
chemotherapy may be safely administered without becoming less
effective, even when reducing dosage for elderly patients.
Furthermore, in this study, dose reduction tended to be more
frequent among older patients; however, there were no differences
in the response rate or prognosis between the two age groups. Thus,
the optimal dose for elderly patients may be lower than the
standard dose.
In our aging society, the number of elderly patients
receiving chemotherapy is expected to increase in the future.
Determining an appropriate chemotherapy regimen and the optimal
dose is crucial for elderly patients due to their vulnerability.
However, there are few clearly determined indices for evaluating
the vulnerability of elderly cancer patients and estimating the
optimal doses of chemotherapeutic agents to be administered to such
patients. Further studies are required to design safe and effective
chemotherapeutic regimens to elderly cancer patients.
References
|
1
|
Monson K, Litvak DA and Bold RJ: Surgery
in the aged population: Surgical oncology. Arch Surg.
138:1061–1067. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Platz CE and Benda JA: Female genital
tract cancer. Cancer. 75:270–294. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Randall ME, Filiaci VL, Muss H, Spirtos
NM, Mannel RS, Fowler J, Thigpen JT and Benda JA: Gynecologic
Oncology Group Study: Randomized phase III trial of whole-abdominal
irradiation versus doxorubicin and cisplatin chemotherapy in
advanced endometrial carcinoma: A Gynecologic Oncology Group study.
J Clin Oncol. 24:36–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fleming GF, Brunetto VL, Cella D, Look KY,
Reid GC, Munkarah AR, Kline R, Burger RA, Goodman A and Burks RT:
Phase III trial of doxorubicin plus cisplatin with or without
paclitaxel plus filgrastim in advanced endometrial carcinoma: A
gynecologic oncology group study. J Clin Oncol. 22:2159–2166. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller D, Fillaci V, Fleming G, Mannel R,
Cohn D, Matsumoto T, Tewari K, DiSilvestro P, Pearl M and Zaino R:
Randomized phase III noninferiority trial of first-line
chemotherapy for metastatic or recurrent endometrial carcinoma: A
Gynecologic Oncology Group study. Gynecol Oncol. 125:771–773. 2012.
View Article : Google Scholar
|
|
7
|
Nomura H, Aoki D, Takahashi F, Katsumata
N, Watanabe Y, Konishi I, Jobo T, Hatae M, Hiura M and Yaegashi N:
Randomized phase II study comparing docetaxel plus cisplatin,
docetaxel plus carboplatin, and paclitaxel plus carboplatin in
patients with advanced or recurrent endometrial carcinoma: A
Japanese Gynecologic Oncology Group study (JGOG2041). Ann Oncol.
22:636–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
ScribnerDR Jr, Puls LE and Gold MA: A
phase II evaluation of docetaxel and carboplatin followed by tumor
volume directed pelvic plus or minus paraaortic irradiation for
stage III endometrial cancer. Gynecol Oncol. 125:388–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geller MA, Ivy JJ, Ghebre R, Downs LS Jr,
Judson PL, Carson LF, Jonson AL, Dusenbery K, Vogel RI, Boente MP,
et al: A phase II trial of carboplatin and docetaxel followed by
radiotherapy given in a ‘sandwich’ method for stage III, IV and
recurrent endometrial cancer. Gynecol Oncol. 121:112–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimura N, Kudoh S, Kimura T, Mitsuoka
S, Kyoh S, Tochino Y, Asai K, Kodama T, Ichimaru Y, Yana T and
Hirata K: Phase II study of docetaxel and carboplatin in elderly
patients with advanced non-small cell lung cancer. J Thorac Oncol.
4:371–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HJ, Kim TG, Lee HJ, Kim JH, Lim BH,
Seo JW, Kang EM, Lee BU, Ahn YM, Roh YH, et al: A phase II study of
combination chemotherapy with docetaxel and carboplatin for elderly
patients with advanced non-small cell lung cancer. Lung Cancer.
68:248–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurt E, Cubukcu E, Karabulut B, Olmez OF,
Kurt M, Avci N, Ozdemir F, Tunali D, Evrensel T and Manavoglu O: A
multi-institutional evaluation of carboplatin plus docetaxel
regimen in elderly patients with advanced gastric cancer. J BUON.
18:147–153. 2013.PubMed/NCBI
|
|
13
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cockcroft DW and Gault MH: Prediction of
clearance from serum creatinine. Nephron. 16:31–41. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE) version 4.0.
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40Accessed.
June 21–2015
|
|
16
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldberg RM, Tabah-Fisch I, Bleiberg H, de
Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E and
Sargent DJ: Pooled analysis of safety and efficacy of oxaliplatin
plus fluorouracil/leucovorin administered bimonthly in elderly
patients with colorectal cancer. J Clin Oncol. 24:4085–4091. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kabbinavar FF, Schulz J, McCleod M, Patel
T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B and Novotny WF:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: Results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pinder MC, Duan Z, Goodwin JS, Hortobagyi
GN and Giordano SH: Congestive heart failure in older women treated
with adjuvant anthracycline chemotherapy for breast cancer. J Clin
Oncol. 25:3808–3815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muss HB, Woolf S, Berry D, Cirrincione C,
Weiss RB, Budman D, Wood WC, Henderson IC, Hudis C, Winer E, et al:
Adjuvant chemotherapy in older and younger women with lymph
node-positive breast cancer. JAMA. 293:1073–1081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fairfield KM, Murray K, Lucas FL, Wierman
HR, Earle CC, Trimble EL, Small L and Warren JL: Completion of
adjuvant chemotherapy and use of health services for older women
with epithelial ovarian cancer. J Clin Oncol. 29:3921–3926. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sundararajan V, Hershman D, Grann VR,
Jacobson JS and Neugut AI: Variations in the use of chemotherapy
for elderly patients with advanced ovarian cancer: A
population-based study. J Clin Oncol. 20:173–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eisenhauer EL, Tew WP, Levine DA, Lichtman
SM, Brown CL, Aghajanian C, Huh J, Barakat RR and Chi DS: Response
and outcomes in elderly patients with stages IIIC-IV ovarian cancer
receiving platinum-taxane chemotherapy. Gynecol Oncol. 106:381–387.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
ten Tije AJ, Verweij J, Carducci MA,
Graveland W, Rogers T, Pronk T, Verbruggen MP, Dawkins F and Baker
SD: Prospective evaluation of the pharmacokinetics and toxicity
profile of docetaxel in the elderly. J Clin Oncol. 23:1070–1077.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Italiano A, Ortholan C, Oudard S, Pouessel
D, Gravis G, Beuzeboc P, Bompas E, Fléchon A, Joly F, Ferrero JM
and Fizazi K: Docetaxel-based chemotherapy in elderly patients (age
75 and older) with castration-resistant prostate cancer. Eur Urol.
55:1368–1375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belani CP and Fossella F: Elderly subgroup
analysis of a randomized phase III study of docetaxel plus platinum
combinations versus vinorelbine plus cisplatin for first-line
treatment of advanced nonsmall cell lung carcinoma (TAX 326).
Cancer. 104:2766–2774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fader AN, von Gruenigen V, Gibbons H,
Abushahin F, Starks D, Markman M, Belinson J and Rose P: Improved
tolerance of primary chemotherapy with reduced-dose carboplatin and
paclitaxel in elderly ovarian cancer patients. Gynecol Oncol.
109:33–38. 2008. View Article : Google Scholar : PubMed/NCBI
|