Introduction
Solid pseudopapillary tumors (SPTs) are rare,
indolent tumors that mostly occur in the pancreas, and account for
0.1–3% of all exocrine pancreatic tumors (1). This type of neoplasm was first named
after V. K. Frantz, who identified its characteristics in 1959
(2). It was subsequently reported
under various names, until the term ‘solid pseudopapillary tumor
(or neoplasm)’ was finally adopted by the World Health Organization
(WHO) in 1996 (3). SPTs are defined
as low-grade malignant neoplasms of the exocrine pancreas in the
current WHO classification (4). There
have been 2,744 patients with SPTs identified in 484 studies
published between 1961 and 2012, according to a systematic review
of SPTs by Law et al (5). The
number of SPTs reported in the literature has increased 7-fold
since 2000 compared with the previous decades (5). The disease typically occurs in young
women, and possesses unique clinicopathological features. Most
patients initially present with abdominal pain or discomfort. SPTs
are associated with a favorable prognosis: After surgical
resection, 95% of patients are disease-free, and mortality is
<2%. However, SPTs also have a low potential for malignancy, and
carry a 6–15% rate of recurrence or metastasis (5), with the liver being the most common
metastatic site. There have been scattered reports of SPTs arising
in extrapancreatic sites, including the retroperitoneum (1,6,7), omentum (8,9), ovary
(10–12) and gastroduodenal area (13). The most common extrapancreatic site is
the mesocolon or omentum (1). In the
present study, two unusual extrapancreatic SPTs. One case is an
uncommon ovarian metastasis of an SPT, and the other is a
retroperitoneal SPT. Both cases had a good outcome, as is the case
with the majority of pancreatic SPTs.
Case reports
Clinical features
Case 1
A 22-year-old woman presented at the Emergency
Department of our hospital with sudden abdominal pain; two years
previously, the patient had been diagnosed with a pancreatic SPT
that had extensively metastasized into the abdominal cavity. A
review of her medical history revealed that the patient underwent
interventional treatment, including a distal pancreatectomy,
splenectomy, resection of liver segments IV and V, omental
metastasectomy, and other resections of metastases. On this
occasion, computed tomography (CT), performed on an Aquilon
64-layer Spiral CT scanner (Toshiba Medical Systems Corp., Tokyo,
Japan), revealed a cystic and low-signal tumor located in the left
ovary, measuring 12×9 cm. Levels of various tumor markers,
including carbohydrate antigen (CA) 19–9, carcinoembryonic antigen
and CA12.5, were normal. The patient underwent a left adnexectomy
and a bilateral distal fimbriectomy, and did not experience a
recurrence of the tumor. The patients remained alive over a
follow-up period of 12 months.
Case 2
A 47-year-old woman was referred to our hospital due
to slight pain in her upper left abdomen; the patient had lost 5 kg
of weight since the onset of her symptoms. CT revealed a 16×14 cm
heterogeneous, lobulated mass arising in the retroperitoneal area
that was compressing the adjacent organs, including the bowel, left
kidney and spleen. The pancreas appeared normal. Blood
biochemistry, routine blood counts, and associated tumor markers
were all within normal levels. The patient was scheduled for
abdominal surgery, and the tumor was completely excised from the
retroperitoneum. The patient has been without tumor recurrence for
14 months.
This study was approved by the Ethics Committees of
the First Affiliated Hospital of Bengbu Medical College, and was
conducted in accordance with the ethical guidelines of the
Declaration of Helsinki.
Immunohistochemical staining
The collected specimens were fixed with 10% neutral
buffered formalin, and embedded in paraffin blocks. Tissue blocks
were cut into 4 µm slides, deparaffinized in xylene, rehydrated
with a graded alcohol series, and immunostained with the following
mouse anti-human antibodies (all at a dilution of 1:100), against:
Vimentin, epithelial membrane antigen (EMA), cytokeratin (CK), CK7,
CD10, CD56, CD99, α1-antichymotrypsin (AACT), neuron-specific
enolase (NSE), synaptophysin (Syn), chromogranin A (CgA), S-100,
galectin-3 (GAL-3), calretinin, α-inhibin, β-catenin, E-cadherin,
cyclin D1, progesterone receptor (PR), estrogen receptor (ER) and
Ki-67. Sections were stained using a streptavidin-peroxidase system
(KIT-9720; Ultrasensitive TM S-P, Maixin Biotech, Inc., Fuzhou,
China). The chromogen used was diaminobenzidine tetrahydrochloride
substrate (employing a DAB kit; Maixin Biotech, Inc.), and sections
were slightly counterstained with hematoxylin, dehydrated and
mounted. Histochemical staining for Alcian Blue at pH 2.5 was
performed on sections from a block representing the predominant
histology of each case. Immunohistochemical data are summarized in
Table I.
 | Table I.Sources of the antibodies used in the
immunohistochemistry analysis. |
Table I.
Sources of the antibodies used in the
immunohistochemistry analysis.
| Source | Antibodya |
|---|
| Vimentin | Monoclonal, clone
V9 |
| EMA | Monoclonal, clone
E29 |
| CK | Monoclonal, clone
AE1/AE3 |
| CK7 | Monoclonal, clone
OV-TL12/30 |
| CD10 | Monoclonal, clone
56C6 |
| CD56 | Monoclonal, clone
56C04 |
| CD99 | Monoclonal, clone
013 |
| AACT | Polyclonal |
| NSE | Monoclonal, clone
E27 |
| Syn | Monoclonal, clone
SYP02 |
| CgA | Monoclonal, clone
5p12 |
| S-100 | Monoclonal, clone
013 |
| GAL-3 | Monoclonal, clone
9C4 |
| Calretinin | Monoclonal, clone
SP13 |
| α-inhibin | Monoclonal, clone
R1 |
| β-catenin | Monoclonal, clone
CAT-5H10 |
| E-cadherin | Monoclonal, clone
4A2C7 |
| Cyclin D1 | Monoclonal, clone
DCS-6 |
| PR | Monoclonal, clone
1A6 |
| ER | Monoclonal, clone
6F11 |
| Ki-67 | Monoclonal, clone
MIB-1 |
Gross and histological features
Case 1
Macroscopically, the excised abdominal lesion
measured 5×4×1 cm and was well-circumscribed, encapsulated, and
exhibited a loss of content of its cystic mass.
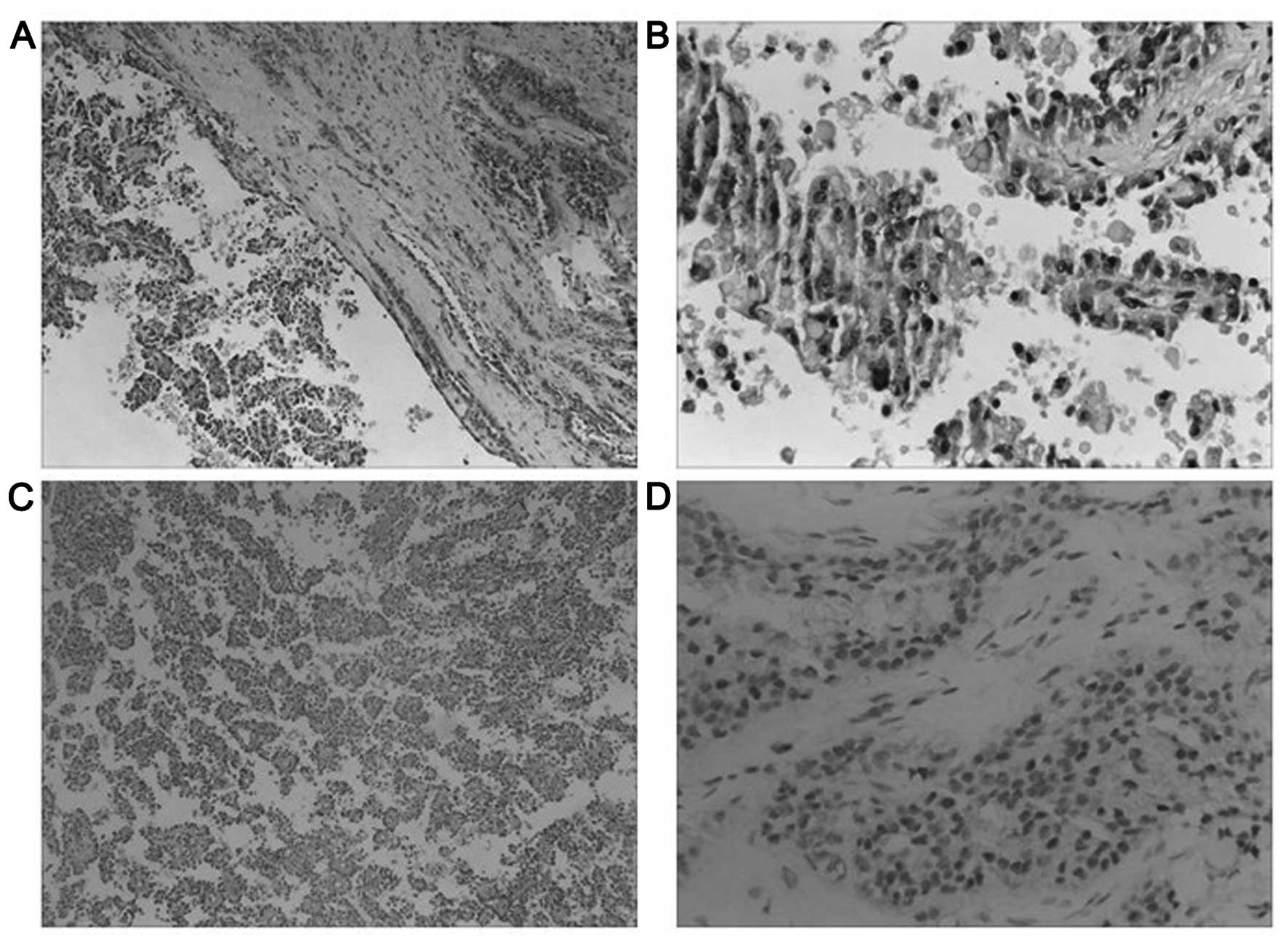
Microscopically, the tumor had a fibrous capsule,
and exhibited a combination of solid and cystic components, with
cellular degenerative changes as well as pseudopapillary formation
(Fig. 1A). Necrobiotic nests were
readily visible. Tumor cells were monomorphic with ill-defined
cytoplasmic limits, with pale or eosinophilic cytoplasm. The nuclei
were irregular, with nuclear membrane shrinkage, and small
eccentric nucleoli were readily observed. The tumor exhibited an
increased nuclear-to-cytoplasm (N/C) ratio and focal necrosis
compared with primary SPTs of the pancreas. Focally, large numbers
of intra- and extracellular hyaline globules were present (Fig. 1B). Pseudopapillary structures with a
fibrovascular core were observed (Fig.
1C). Foamy histiocytes, cholesterol crystals and foreign body
giant cell reactions were absent from this specimen.
Case 2
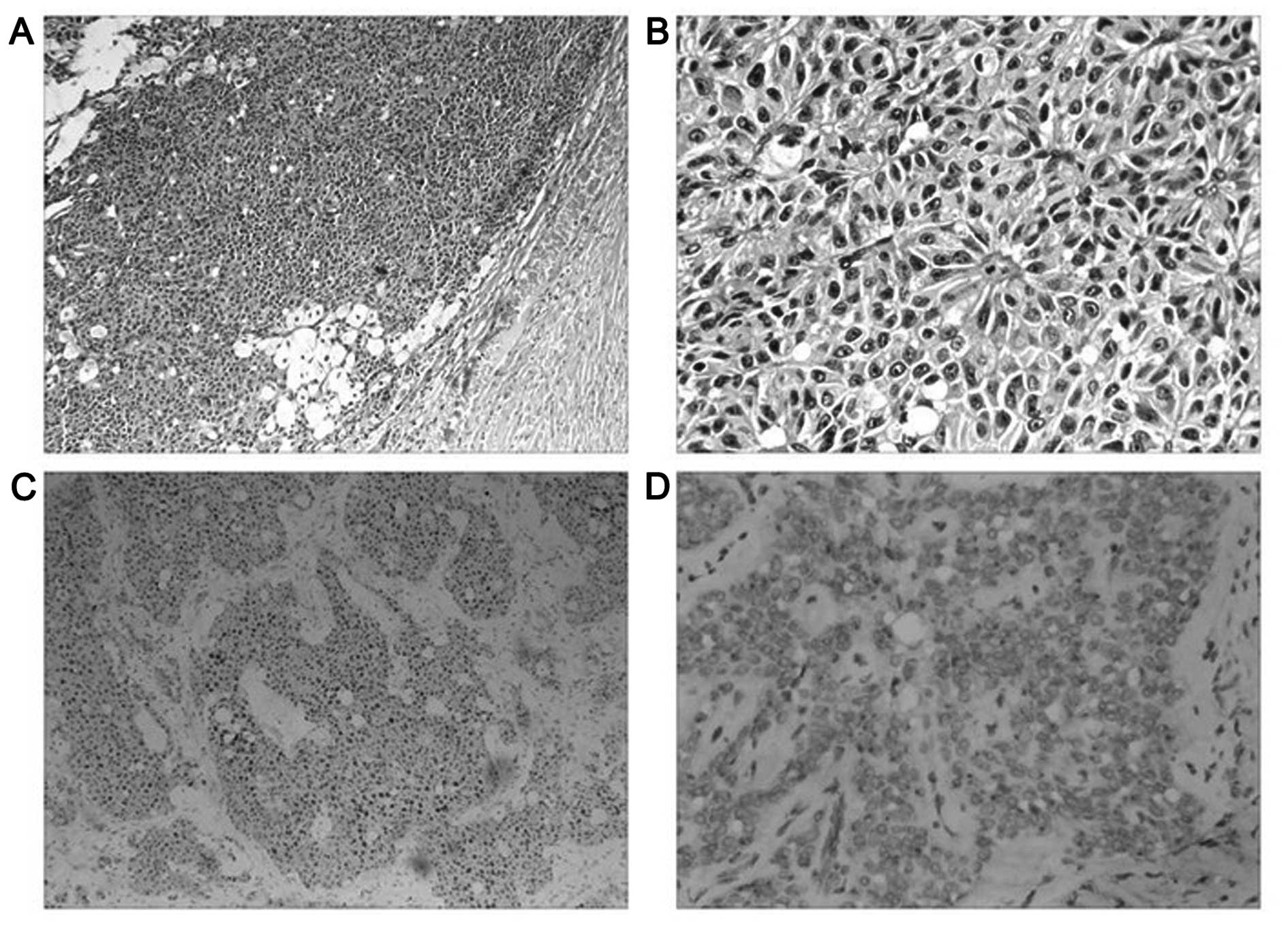
Macroscopically, the lesion was a lobulated large
tumor accompanied by necrosis within a complete fibrous capsule.
The cut surface was grey-red to yellow and multinodular, with
slight hemorrhaging.
Microscopically, no clear pseudopapillae were
observed. The solid area constituted a major portion, was rich in
microvasculature, and revealed sheets of cells arranged around
fibrovascular septa, forming a grid-like appearance. Few tumor
cells with intracytoplasmic vacuoles were readily observed, and
foamy tissue surrounding the solid area was evident (Fig. 2A). Certain areas exhibited monotonous
cells with abundant eosinophilic cytoplasm, surrounded by delicate
vessels resembling ependymal rosettes (Fig. 2B). Tumor cells revealed empty,
centrally localized nuclei, and vacuolated or faintly eosinophilic
cytoplasm. Cellular atypia and mitotic figures were absent. In this
case, an ectopic pancreatic component in the lesion was not
identified.
Immunohistochemical features
Case 1
The immunohistochemical phenotype of the primary
tumor was comparable with that of the ovarian metastasis.
Immunohistochemical staining revealed that the tumor cells were
positive for vimentin, CK, CK7, CD10, CD56, CD99 (perinuclear
punctate staining pattern), AACT, NSE, CgA, GAL-3, β-catenin
(cytoplasmic and nuclear pattern), cyclin D1, and PR (Fig. 1D). The Ki-67 index was <5%. Other
markers, including EMA, Syn, E-cadherin, calretinin, α-inhibin, ER,
and S-100 protein, were negative (Table
II). The histological findings were compatible with the
diagnosis of an ovarian metastasis of an SPT.
 | Table II.Immunohistochemistry profiles of
cases 1 and 2. |
Table II.
Immunohistochemistry profiles of
cases 1 and 2.
|
| Case |
|---|
|
|
|
|---|
| Antibody
source | 1 | 2 |
|---|
| Vimentin | + | + |
| CK | + | − |
| CK7 | + | − |
| CD10 | + | + |
| CD56 | + | + |
| CD99 | + | + |
| AACT | + | + |
| NSE | + | − |
| CgA | + | − |
| GAL-3 | + | + |
| β-catenin | + | + |
| Cyclin D1 | + | + |
| PR | + | − |
| Ki-67 | <5% | <2% |
| EMA | − | − |
| Syn | − | + |
| E-cadherin | − | − |
| Calretinin | − | − |
| α-inhibin | − | − |
| ER | − | − |
| S-100 | − | − |
Case 2
Immunohistochemical findings revealed that the tumor
cells were SPT cells, as they were characteristically positive for
β-catenin (cytoplasmic and nuclear patterns; Fig. 2C), vimentin, CD10, CD56, CD99
(perinuclear punctate staining pattern; Fig. 2D), AACT, Syn, GAL-3 and cyclin D1. The
Ki-67 index was <2%. Other markers, including CK, CK7, EMA, NSE,
E-cadherin, α-inhibin, CgA, calretinin, PR, ER and S-100, were
negative (Table II). The final
diagnosis was a primary retroperitoneal SPT.
Discussion
Solid pseudopapillary tumors of low malignant
potential usually occur in the pancreas, and predominantly affect
young females in their second or third decade of life (14). They occasionally occur in children
(2,14)
and older females (13) or males
(6). A review of 718 well-documented
cases of pancreatic SPTs by Papavramidis et al (15) reported a male-to-female ratio of
1:9.78, with a mean age of 21.97 years (range, 2–85 years). The
most common location was the tail of the pancreas. Patients
generally presented with a palpable abdominal mass, and few
patients presented with a ruptured pancreatic SPT following blunt
abdominal trauma (14). Recurrence or
metastasis is present in 6–15% of SPT cases (5,15). The
liver is the most common site of metastasis, followed by the lymph
nodes and peritoneum (16). Lung
metastasis was also reported in one previous study (17). In the present study, a rare metastatic
ovarian tumor arising from an SPT was presented. A review of the
English literature published between 1990 and 2013 revealed only 13
cases of extrapancreatic SPTs (1).
There are only three reported cases of primary extrapancreatic SPTs
arising in the retroperitoneal area (1,6,7). Only one extrapancreatic SPT arising in
the retroperitoneum without ectopic pancreatic tissue has been
reported previously, by Miyazaki et al (7). A review of all the reports on
extrapancreatic SPTs reveals that the tumors are normally benign,
and are likely to have a favorable clinical course, similar to
their pancreatic counterparts.
The mean SPT size is 6.08 cm, and ranges from
0.5–34.5 cm (15). Microscopically,
the tumor is characteristically a mixture of solid-cystic and
pseudopapillary structures, and tumor cells are totally monomorphic
with ill-defined cytoplasmic limits. The nuclei are uniform, and
seldom exhibit nucleoli, whereas chromatin is finely dispersed. The
chromatin may occasionally contain a single clear cytoplasmic
vacuole (10), whereas nuclear
pleomorphisms are rare. Intra- and extracellular eosinophilic
globules, foamy histiocytes, cholesterol crystals and foreign body
giant cell reactions are commonly present in SPTs (6,12,17). One SPT with prominent, atypical
multinucleated giant tumor cells has been reported (18), and Tang et al (19) reported two cases of clinically
aggressive SPTs of the pancreas with sarcomatoid carcinoma
components. The authors indicated that unusual pathological
features, including solid and diffuse growth patterns with
extensive tumor necrosis and a high mitotic rate, may predict
aggressiveness and clinical outcomes. Only three extrapancreatic
SPTs that were associated with malignant behavior and resulted in
metastasis have previously been reported (9,11,13). They exhibited an increased N/C ratio,
hyperchromasia, greater nuclear atypia and pleomorphism. Mitotic
figures were detected more frequently compared with the primary
tumor. One primary SPT of the ovary was even fatal (11). However, the mitotic indexes of
metastatic and non-metastatic SPTs are not markedly different
(19). Case 1 in the current study
presented with evident necrotic nests, but without mitotic figures.
Therefore, we hypothesized that cellular pleomorphism, as well as a
slightly elevated mitotic rate, do not appear to have a marked
impact on the behavior of SPTs. In case 2, the tumor lacked a
pseudopapillary structure, which is otherwise common in SPTs of the
pancreas. However, the solid area featured monotonous cells with
abundant eosinophilic cytoplasm, surrounded by vessels resembling
ependymal rosettes; this is consistent with the SPTs previously
reported (20,21).
According to the literature, the presence of
positive N/C β-catenin in conjunction with the membrane loss of
E-cadherin and perinuclear punctate staining pattern of CD99 are
major diagnostic indicators (20,21). CD99
as an immunohistological marker for SPT was first studied by Li
et al (22) in 2011. All of
the SPTs in that study presented unique perinuclear punctate CD99
staining. Nuclear accumulation of β-catenin protein was present in
95% of cases, and activating β-catenin oncogenic mutations were
identified in 90% of SPTs (21). This
was also true of β-catenin in the two presented cases in the
current study. Previously, Wnt signaling, mostly associated with
β-catenin mutations, has been suggested to occupy a major role in
the tumorigenesis of SPTs (12,17,21).
Immunoreactivity for nuclear PR was robust in >80% of the cells
(23,24). Furthermore, a possible role for sex
hormones in the histogenesis of SPTs has been suggested, although
this is controversial, since 12.2% of SPTs occur in men (5) and PR is not always detected (6,11). One of
our own cases was negative for PR staining. Another report by Geers
et al (23) revealed that SPT
cells exhibit positive immunoreactivity for GAL-3, similarly to
pancreatic duct cells. This led to the proposal of an hypothesis
that SPTs have a duct cell origin (24). GAL-3 was detected in both case studies
in the present study. The marker pattern, comprising the frequent
expression of vimentin, NSE and α1-antitrypsin, together with
negativity for CK, leads to the question of whether SPTs are
mesenchymal, rather than epithelial tumors (23). In view of the striking female
predominance of SPTs and the known approximation of the genital
ridges to the pancreatic anlage during embryogenesis, it is
possible that SPTs, including certain extrapancreatic SPTs
identified in the retroperitoneal space, may arise from genital
ridge/ovarian anlage-associated cells (10,23).
Regarding the morphological overlap between SPTs and
other neoplasms, the major differential diagnosis in case 1 of the
present study is a yolk sac tumor (YST) of the ovary. YST, also
known as an endodermal sinus tumor, is a rare malignant germ cell
tumor that is able to emulate an SPT with multiple patterns of
solid, as well as cystic, components, in addition to intra- and
extracellular hyaline globules. However, Schiller-Duval bodies have
only been identified in YSTs (25),
and a key diagnostic feature of a YST is an elevated level of
α-fetoprotein, which distinguishes it from a metastatic ovarian
tumor of an SPT. Mete et al (16) reported the first pancreatic SPT with
ovarian metastasis, which was initially misdiagnosed as a bilateral
sex-cord stromal tumor. Therefore, sex-cord stromal tumors should
also be considered; however, it was possible to exclude this in the
present study, since such tumors are positive for α-inhibin and
calretinin (which were negative in our cases) and lack
pseudopapillary patterns (which were observed in one of our cases).
In case 2 of the present study, the nested pattern of fibrovascular
septa with a grid-like appearance may indicate paraganglioma;
however, paragangliomas are positive for neuroendocrine markers,
such as Syn and CgA, as well as for S-100. In addition, they have
no pseudopapillary patterns and do not exhibit vimentin or AACT
staining, and may therefore be ruled out.
In conclusion, SPTs with ovarian metastasis or
primary retroperitoneal SPTs are rarely reported. The two cases in
the current study presented with the identical pathological
features and immunohistological phenotypes as pancreatic SPTs, with
the exception of necrosis and an increased N/C ratio, which are
more readily detected in metastatic ovarian neoplasms. The two
cases exhibited nuclear and cytoplasmic reactivity with β-catenin,
and complete loss of membrane E-cadherin staining. Furthermore,
CD99 exhibited a unique perinuclear punctate staining pattern that
was easily distinguishable from morphologically overlapping tumors,
including YSTs of the ovary, sex-cord stromal tumors and
paragangliomas. The good prognosis of the two unusual SPTs
indicated that extrapancreatic SPTs are likely to have a favorable
clinical course similar to their pancreatic counterparts; however,
considering the 6.5% recurrence rate following resection in
patients (14), a five-year follow-up
period is recommended (5).
Acknowledgements
We would like to thank Editage Editing Services for
their English language editing. We also thank Dr Di-chen Li and
Professor Qun Xie (Department of Pathology, the First Affiliated
Hospital of Bengbu Medical College) for their assistance with the
histopathological and immunohistochemical stain evaluations. The
present study was supported by the Natural Science Key Foundation
of the Education Department in Anhui province, no. KJ2014A160.
Glossary
Abbreviations
Abbreviations:
|
SPT
|
solid pseudopapillary tumor
|
|
CA
|
carbohydrate antigen
|
|
N/C
|
nuclear-to-cytoplasm
|
|
YST
|
yolk sac tumor
|
|
EMA
|
epithelial membrane antigen
|
|
AACT
|
α1-antichymotrypsin
|
|
Syn
|
synaptophysin
|
|
CgA
|
chromogranin A
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
References
|
1
|
Zhu H, Xia D, Wang B and Meng H:
Extrapancreatic solid pseudopapillary neoplasm: Report of a case of
primary retroperitoneal origin and review of the literature. Oncol
Lett. 5:1501–1504. 2013.PubMed/NCBI
|
|
2
|
Frantz VK: Tumor of the pancreas. Atlas of
Tumor Pathology. Bumberg CW: (1st). Armed Forces Institute of
Pathology. (Washington). 32–33. 1959.
|
|
3
|
Klöppel G, Solcia E, Longnecker DS,
Capella C and Sobin LH: World Health Organization International
Histological Classification of Tumours: Histological Typing of
Tumors of the Exocrine Pancreas (2nd). Springer. Berlin: 15–21.
1996.
|
|
4
|
Klöppel G, Hruban RH, Klimstra DS, et al:
Solid-pseudopapillary tumor of pancreas. Bosman FT, Carneiro F,
Hruban RH and Theise ND: World Health Organization classification
of tumours of the digestive system. IARC. (Lyon). 327–330.
2010.
|
|
5
|
Law JK, Ahmed A, Singh VK, Akshintala VS,
Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, et al: A
systematic review of solid-pseudopapillary neoplasms: Are these
rare lesions? Pancreas. 43:331–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klöppel G, Maurer R, Hofmann E, Lüthold K,
Oscarson J, Forsby N, Ihse I, Ljungberg O and Heitz PU:
Solid-cystic (papillary-cystic) tumours within and outside the
pancreas in men: Report of two patients. Virchows Arch A Pathol
Anat Histopathol. 418:179–183. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazaki Y, Miyajima A, Maeda T, Yuge K,
Hasegawa M, Kosaka T, Kikuchi E, Kameyama K, Jinzaki M, Nakagawa K
and Oya M: Extrapancreatic solid pseudopapillary tumor: Case report
and review of the literature. Int J Clin Oncol. 17:165–168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukunaga M: Pseudopapillary solid cystic
tumor arising from an extrapancreatic site. Arch Pathol Lab Med.
125:1368–1371. 2001.PubMed/NCBI
|
|
9
|
Hibi T, Ojima H, Sakamoto Y, Kosuge T,
Shimada K, Sano T, Sakamoto M, Kitajima M and Yamasaki S: A solid
pseudopapillary tumor arising from the greater omentum followed by
multiple metastases with increasing malignant potential. J
Gastroenterol. 41:276–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deshpande V, Oliva E and Young RH: Solid
pseudopapillary neoplasm of the ovary: A report of 3 primary
ovarian tumors resembling those of the pancreas. Am J Surg Pathol.
34:1514–1520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Syriac S, Kesterson J, Izevbaye I, de Mesy
Bentley KL, Lele S and Mhawech-Fauceglia P: Clinically aggressive
primary solid pseudopapillary tumor of the ovary in a 45-year-old
woman. Ann Diagn Pathol. 16:498–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kominami A, Fujino M, Murakami H and Ito
M: β-catenin mutation in ovarian solid pseudopapillary neoplasm.
Pathol Int. 64:460–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walter T, Hommell-Fontaine J, Hervieu V,
Adham M, Poncet G, Dumortier J, Lombard-Bohas C and Scoazec JY:
Primary malignant solid pseudopapillary tumors of the
gastroduodenal area. Clin Res Hepatol Gastroenterol. 35:227–233.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tajima Y, Kohara N, Maeda J, Inoue K,
Kitasato A, Natsuda K, Irie J, Adachi T, Kuroki T, Eguchi S and
Kanematsu T: Peritoneal and nodal recurrence 7 years after the
excision of a ruptured solid pseudopapillary neoplasm of the
pancreas: Report of a case. Surg Today. 42:776–780. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papavramidis T and Papavramidis S: Solid
pseudopapillary tumors of the pancreas: Review of 718 patients
reported in English literature. J Am Coll Surg. 200:965–972. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mete Ö, Yegen G, Güllüoglu MG, Kapran Y
and Klöppel G: An unusual clinical presentation of pancreatic solid
pseudopapillary tumor with ovarian metastases: A diagnostic
dilemma. Int J Surg Pathol. 19:342–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takahashi Y, Fukusato T, Aita K, Toida S,
Fukushima J, Imamura T, Tanaka F, Amano H, Takada T and Mori S:
Solid pseudopapillary tumor of the pancreas with metastases to the
lung and liver. Pathol Int. 55:792–796. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Othman M, Rashid A and Wang H, Li Z,
Katz MH, Lee JE, Pisters PW, Abbruzzese JL, Fleming JB and Wang H:
Solid pseudopapillary neoplasm of the pancreas with prominent
atypical multinucleated giant tumour cells. Histopathology.
62:465–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang LH, Aydin H, Brennan MF and Klimstra
DS: Clinically aggressive solid pseudopapillary tumors of the
pancreas: A report of two cases with components of undifferentiated
carcinoma and a comparative clinicopathologic analysis of 34
conventional cases. Am J Surg Pathol. 29:512–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhatnagar R, Olson MT, Fishman EK, Hruban
RH, Lennon AM and Ali SZ: Solid-pseudopapillary neoplasm of the
pancreas: Cytomorphologic findings and literature review. Acta
Cytol. 58:347–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abraham SC, Klimstra DS, Wilentz RE, Yeo
CJ, Conlon K, Brennan M, Cameron JL, Wu TT and Hruban RH:
Solid-pseudopapillary tumors of the pancreas are genetically
distinct from pancreatic ductal adenocarcinomas and almost always
harbor beta-catenin mutations. Am J Pathol. 160:1361–1369. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Li J, Hao C, Zhang C, Mu K, Wang Y
and Zhang T: Immunohistochemical evaluation of solid
pseudopapillary tumors of the pancreas: The expression pattern of
CD99 is highly unique. Cancer Lett. 310:9–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geers C, Moulin P, Gigot JF, Weynand B,
Deprez P, Rahier J and Sempoux C: Solid and pseudopapillary tumor
of the pancreas-review and new insights into pathogenesis. Am J
Surg Pathol. 30:1243–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kosmahl M, Seada LS, Jänig U, Harms D and
Klöppel G: Solid-pseudopapillary tumor of the pancreas: Its origin
revisited. Virchows Arch. 436:473–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Thielen T, Degryse H and Coeman D:
Yolk sac tumor of the ovary. JBR-BTR. 96:256–7. 2013.PubMed/NCBI
|