Introduction
Obesity and estrogens have long been implicated in
the pathogenesis of breast cancer (1). Adipose tissue is known to be a site of
peripheral aromatization of adrenal androgens to estrogens, which
in turn induce mitogenic activity in breast tissue. Additionally,
obesity is strongly associated with insulin resistance
characterized by hyperinsulinemia, which is also suggested to play
a mitogenic role in the development of breast cancer (2).
Apart from aromatization of adrenal androgens,
adipose tissue secretes a variety of proteins, referred to as
adipocytokines, that may also play a role in breast carcinogenesis.
Multiple studies have been designed to elucidate the association
between breast cancer and adipocytokines, such as leptin,
adiponectin, visfatin and resistin. Although there are conflicting
results in the literature, high leptin (3,4), visfatin
(5) and resistin (6,7) levels and
low adiponectin (8) levels appear to
be associated with increased breast cancer risk.
Since adipocytokines are hormones, their levels in
the blood may be affected by several factors. It is of interest
whether the tumor mass itself or the various factors secreted into
the bloodstream by the tumor affect the blood levels of
adipocytokines. A recent study demonstrated that leptin is produced
by tumor cells, suggesting that removal of the tumor may decrease
the circulating levels of this adipocytokine (9). Thus, we hypothesized that the levels of
adipocytokines may be altered by surgical removal of the tumor mass
and medical treatment of the disease. If so, these cytokines may be
used as markers during the follow-up after breast cancer treatment.
The aim of this study was to compare the pre- and post-treatment
serum levels of leptin, adiponectin, visfatin, resistin and insulin
in patients with stage II and IIIA breast cancer.
Patients and methods
Patients
A total of 20 consecutive patients with operable
stage II and III breast cancer were included in this prospective
study. patients with metastatic disease, diabetes mellitus and
chronic medical conditions, such as obstructive pulmonary disease
and congestive heart disease, were not included in the study. All
the patients were newly diagnosed, histologically confirmed, adult
patients with breast cancer, who were treated at the Department of
General Surgery, Faculty of Medicine, Celal Bayar University
(Manisa, Turkey) between 2008 and 2012. The study protocol was
approved by the Ethics Committee of the Faculty and informed
consent was obtained from each patient prior to inclusion in the
study. The levels of adiponectin, leptin, visfatin, resistin,
glucose and insulin in the patients' blood were compared between
the preoperative period and at 6 months after surgery.
A total of 9 patients had stage II and 11 patients
had stage IIIA disease. The surgical treatment was
breast-conserving surgery (n=4) or total mastectomy (n=16). The
patients underwent axillary dissection if metastatic axillary lymph
nodes were identified during the preoperative examination or
sentinel lymph node biopsy. A total of 5 patients were
premenopausal at diagnosis. None of the patients had received
chemotherapy or radiotherapy prior to surgery. All the patients
received adjuvant chemotherapy and 15 patients received
radiotherapy following chemotherapy.
Demographic, clinical and anthropometric data were
collected upon recruitment. Staging was performed according to the
2009 American Joint Committee on Cancer staging system (10). Body mass index (BMI) was calculated as
weight (kg) divided by the square of height (m2) prior
to and following treatment for breast cancer. Insulin resistance
(IR) was calculated using the homeostatic model assessment index
(HOMA) as follows: (HOMA-IR) = [fasting glucose (mmol/l) × fasting
insulin (mU/l)/22.5] (11).
Blood samples were obtained from the antecubital
vein on the day prior to surgery between 8:00 and 9:00 am after an
overnight fast, and at 6 months after surgery. All the patients
completed their oncological treatment including chemo- and
radiotherapy within this 6-month period. For adiponectin, leptin,
resistin, visfatin and insulin studies, the blood was immediately
transferred into a tube containing EDTA and centrifuged. The serum
samples were then maintained at −80°C until further analysis. All
the samples from each patient were run in the same assay. Serum
adiponectin, leptin, visfatin and resistin levels were measured by
ELISA kits (Millipore Corp., Billerica, MA, USA). Serum insulin was
measured by an auto-analyzer (niCel DxI 800; Beckman Coulter, Brea,
CA, USA).
Statistical analysis
The Wilcoxon signed-rank test was used for the
analysis of biomedical data. Two-tailed probability values were
calculated. The correlation between post-treatment resistin levels
and insulin resistance was assessed by the Pearson's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis of the data was
performed using SPSS statistical software, version 17.0 for Windows
(SPSS, Chicago, IL, USA).
Results
Patient characteristics
The mean age of the patients was 51 years (range,
26–71 years). of the 20 patients, 13 were considered as obese
(BMI>30 kg/m2). Oncological treatment did not alter
the BMI values of the patients (P>0.05).
Pre- and post-treatment serum
adipocytokine and insulin levels
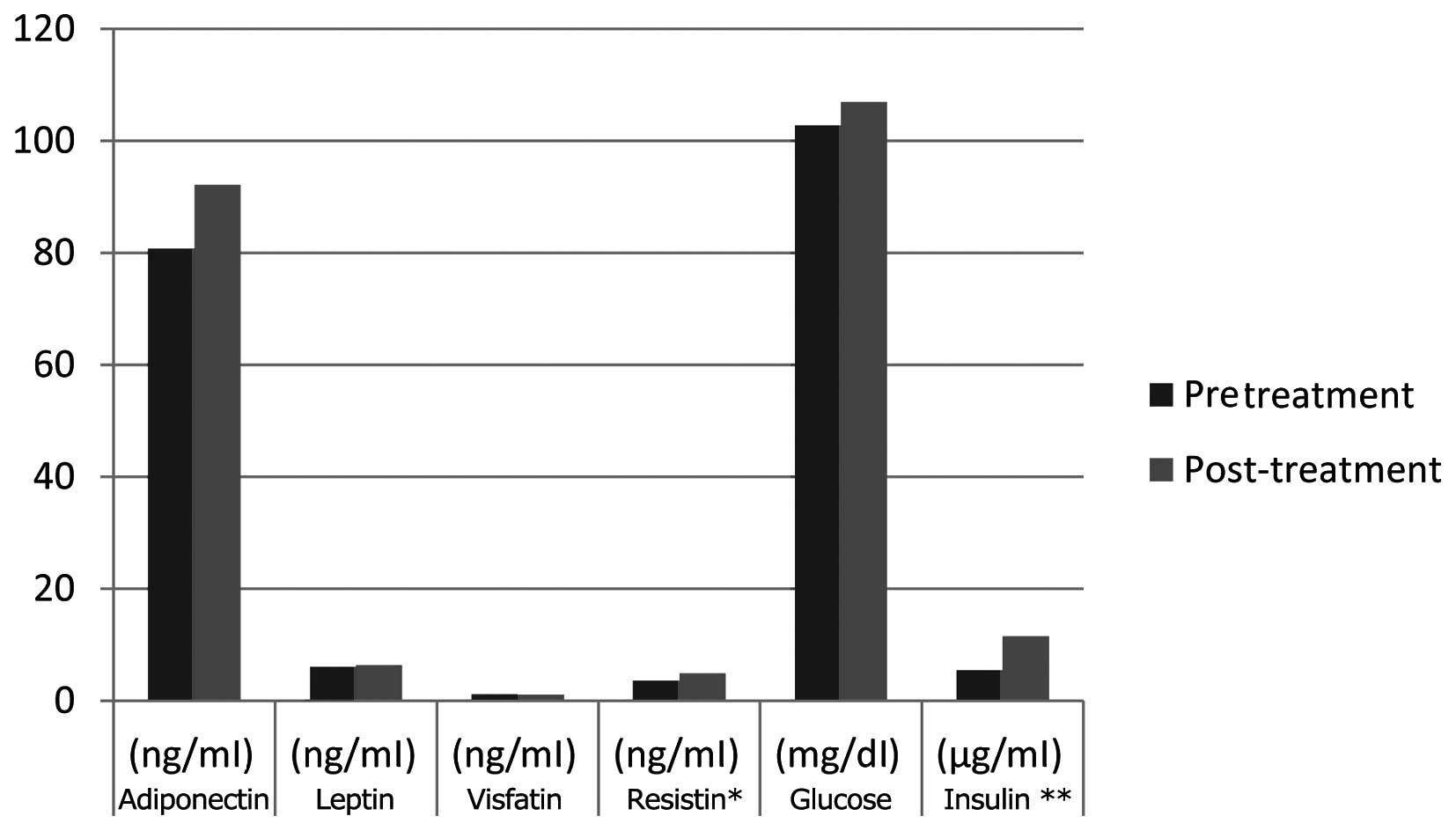
There was no statistically significant difference
between the pre- and post-treatment levels of visfatin, adiponectin
and leptin. However, the serum insulin and resistin levels and
insulin resistance were found to be statistically significantly
increased following treatment (P<0.05) (Table I and Fig.
1).
 | Table I.Comparison between pre- and
post-treatment levels of adiponectin, leptin, visfatin, resistin,
glucose and insulin in the serum of 20 breast cancer patients. |
Table I.
Comparison between pre- and
post-treatment levels of adiponectin, leptin, visfatin, resistin,
glucose and insulin in the serum of 20 breast cancer patients.
| Timepoint | Adiponectin
(ng/ml) | Leptin (ng/ml) | Visfatin (ng/ml) | Resistina (ng/ml) | Glucose (mg/dl) | Insulinb (µg/ml) |
|---|
| Pretreatment | 80.8±28.7 | 6.0±4.7 | 1.1±0.9 | 3.5±1.0 | 102.7±11.9 | 5.4±2.5 |
| Post-treatment | 92.1±44.2 | 6.3±3.9 | 1.0±0.8 | 4.9±1.9 | 106.9±14.5 | 11.5±7.3 |
In addition, the post-treatment resistin levels were
positively correlated with insulin resistance (r=0.45,
P<0.05).
Discussion
Adipose tissue is a complex and metabolically active
endocrine organ. Adipocytes, as well as the non-adipocyte fraction
of the adipose tissue, synthesize and secrete several hormones,
such as leptin, visfatin, resistin and adiponectin, referred to as
adipocytokines.
In brief, leptin regulates the body's fat stores,
induces cell proliferation, promotes angiogenesis and inhibits
cellular apoptosis (8). The role of
visfatin and resistin in carcinogenesis is not as clear as that of
leptin. A basic research study revealed that visfatin may
contribute to breast cancer etiopathogenesis by augmenting cell
proliferation through stimulation of cell cycle progression and by
increasing the expression of genes that are crucial for
angiogenesis and metastasis (12).
Resistin is secreted from adipocytes and, particularly, from
monocytes in the adipose tissue and is suggested to be involved in
inflammatory processes, including obesity-related subclinical
inflammation, atherosclerosis, cardiovascular disease and rheumatic
disease (13); its role in malignant
tumors has not been yet established, but it was suggested to be
involved in carcinogenesis. Adiponectin has been found to exert an
antiproliferative effect on various breast cancer cell lines
(14) and has been suggested to be an
anticarcinogenic hormone.
Although the results reported in the literature are
conflicting, it appears that the circulating levels of leptin,
visfatin and resistin are increased and the levels of adiponectin
are decreased in patients with breast cancer compared with those in
healthy controls. However, the cause of the differences in
adipocytokine levels between patients and controls has not been
fully elucidated. adipocytokines secreted by the tumor mass itself
or the biologically active substances secreted by the tumor, which
may stimulate or inhibit the production of adipocytokines by the
adipose tissue, may be the cause of this difference. If so, removal
of the tumor will obviously decrease the levels of visfatin, leptin
and resistin and increase the levels of adiponectin in cancer
patients.
To the best of our knowledge, this is the first
study to evaluate the effect of surgical and medical treatment of
breast cancer on the circulating levels of adipocytokines in the
blood. In one of our recent studies, we investigated the effect of
the surgical removal of the tumor on adipocytokine levels in
patients with colorectal cancer and observed a statistically
significant decrease in only the adiponectin levels following
treatment (15); serum ghrelin,
resistin and visfatin levels, however, were found to be unchanged,
despite cancer treatment. In the present study, despite radical
treatment, we did not identify any significant differences between
the pre- and post-treatment levels of visfatin, leptin and
adiponectin in breast cancer patients. Only resistin levels were
found to be significantly increased following treatment. It appears
that the tumor mass itself exerted no or little effect on the
levels of visfatin, leptin and adiponectin in our study.
The main difference between the two measurements of
resistin levels in our study is the oncological treatment that the
patients received, which included chemotheraphy and radiotherapy.
Resistin, as previously mentioned, is closely associated with
various inflammatory processes. Radiation is one of the main causes
that activate inflammation in the tissues undergoing radiotherapy.
The ionization events and free radicals produced by radiation cause
damage to vital cellular components. DNA damage from radiation
commonly leads to cell death during the first cell division, or
within the first few divisions following irradiation, leading to
inflammation (16). Additionally,
radiation activates various cellular signalling pathways (17) that lead to the expression and
activation of proinflammatory and profibrotic cytokines (18–20),
vascular injury (21) and activation
of the coagulation cascade (22).
These changes may be involved in the development of oedema,
inflammatory responses and the initiation of wound-healing
processes. A total of 75% of the patients received radiotherapy in
our study. The blood samples for adipocytokine analysis in our
patients were collected immediately following completion of the
radiation therapy, as determined by the study design. Therefore, we
hypothesized that radiotherapy-induced inflammation may be the
cause of the increase in resistin levels in the patients in our
study. However, since this is only a hypothesis, prospective
randomized studies are required to determine the effect of
radiotherapy on the serum resistin levels of breast cancer
patients.
In the literature, it has been reported that the
risk of breast cancer is increased in association with obesity and
diabetes, both of which are characterized by increased insulin
resistance, with consequent increases in the circulating levels of
insulin and glucose (23,24). Insulin promotes cell proliferation
(25,26) and enhances breast tumor growth in
animal models (27,28). Therefore, we may hypothesize that high
levels of insulin or glucose may be involved in the etiology of
breast cancer. The few prospective studies to date that have
directly investigated the association between fasting glucose and
insulin levels and the risk of incident breast cancer have yielded
conflicting results (29–32). the aim of our study was not to
determine whether high insulin levels induce breast cancer, but
rather to identify any changes in fasting insulin levels between
pre- and post-treatment measurements. There was a statistically
significant increase in insulin levels and insulin resistance
during the post-treatment period in our patients, whereas there was
no change in glucose levels.
Certain factors may have induced an increase in the
insulin levels in our study. One of these factors may be the
metabolic disturbance observed following adjuvant breast cancer
therapy. Guinan et al (33)
investigated the alterations in metabolic and insulin resistance
parameters from the time of breast cancer diagnosis to completion
of adjuvant breast cancer treatment, and reported that patients
with breast cancer, without other serious comorbidities,
demonstrated a significant deterioration in their metabolic
profiles, characterized by increases in fasting insulin and
associated insulin resistance. Despite the observed increased
fasting insulin levels, fasting glucose remained unchanged in their
study, suggesting that insulin resistance had developed, but that
the pancreas was able to compensate sufficiently to maintain
glucose homeostasis (34). There were
also no differences regarding the weight and BMI of the patients
following completion of the adjuvant therapy. All these results are
consistent with the results of our study. The increase in
post-treatment insulin levels and insulin resistance was suggested
by Guinan et al (33) to be
due to obesity, age and estrogen deficiency. Obesity is the most
common underlying cause of insulin resistance. It is commonly
reported that breast cancer patients frequently gain weight during
adjuvant treatment for breast cancer (35). However, we observed no differences in
body weight or BMI in our patients following adjuvant treatment.
The other factor that may have contributed to the increase in
post-treatment insulin levels and insulin resistance is estrogen
deficiency. Insulin resistance is rarely observed in premenopausal
women, but emerges with the onset of menopause due to estrogen
deficiency (36). Adjuvant
chemotheraphy and tamoxifen treatment may induce ovarian ablation
in young patients, leading to early-onset menopause and related
metabolic changes (37). Only 5
premenopausal women were included in our study, whose menopausal
status could be affected by oncological treatment. Therefore, the
effect of chemotherapy-induced menopause on insulin levels in our
study would be minimal, if any.
Another cause that may increase insulin levels and
insulin resistance in patients who undergo oncological treatment
may be the increased levels of resistin during the post-treatment
period. Resistin was first identified in 2001 and considered to
contribute to insulin resistance. The administration of resistin to
healthy mice impaired glucose tolerance and insulin action, and
antibody against resistin improved blood sugar levels and insulin
action (38). We observed
statistically increased resistin levels in our patients during the
post-treatment period. In addition, the increase in resistin levels
was positively correlated with the increase in insulin resistance
in our study. These findings suggest that high resistin levels
induced by the oncological treatment may be the cause underlying
the development of metabolic disturbances, such as insulin
resistance, in treated cancer patients.
In conclusion, removal of the tumor mass and
adjuvant oncological treatment did not alter the serum levels of
visfatin, adiponectin or leptin in patients with stage II–III
breast cancer in our study. However, the resistin levels were found
to be increased. Radiotheraphy-induced inflammation may be the
cause underlying the increase in resistin levels during the
post-treatment period in our study. The effect of radiotherapy on
resistin levels following cancer treatment should be further
investigated in well-designed studies. Post-treatment resistin
levels were also found to be positively correlated with insulin
resistance in our study, which, in turn, suggests that high
resistin levels may have affected insulin resistance in our breast
cancer patients following treatment.
References
|
1
|
Stoll BA: Upper abdominal obesity, insulin
resistance and breast cancer risk. Int J Obes Relat Metab Disord.
26:747–753. 2002.PubMed/NCBI
|
|
2
|
Macciò A, Madeddu C and Mantovani G:
Adipose tissue as target organ in the treatment of
hormone-dependent breast cancer: New therapeutic perspectives. Obes
Rev. 10:660–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo
FC, Fu OY, Chen HY, Hou MF and Yuan SS: Serum adiponectin and
leptin levels in Taiwanese breast cancer patients. Cancer Lett.
237:109–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH,
Yu CP, Yu JC and Sun CA: Circulating levels of leptin, adiposity
and breast cancer risk. Br J Cancer. 100:578–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalamaga M, Archondakis S, Sotiropoulos G,
Karmaniolas K, Pelekanos N, Papadavid E and Lekka A: Could serum
visfatin be a potential biomarker for postmenopausal breast cancer?
Maturitas. 71:301–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang JH, Yu BY and Youn DS: Relationship
of serum adiponectin and resistin levels with breast cancer risk. J
Korean Med Sci. 22:117–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalamaga M, Sotiropoulos G, Karmaniolas K,
Pelekanos N, Papadavid E and Lekka A: Serum resistin: A biomarker
of breast cancer in postmenopausal women? Association with
clinicopathological characteristics, tumor markers, inflammatory
and metabolic parameters. Clin Biochem. 46:584–590. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jardé T, Perrier S, Vasson MP and
Caldefie-Chézet F: Molecular mechanisms of leptin and adiponectin
in breast cancer. Eur J Cancer. 47:33–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tessitore L, Vizio B, Jenkins O, De
Stefano I, Ritossa C, Argiles JM, Benedetto C and Mussa A: Leptin
expression in colorectal and breast cancer patients. Int J Mol Med.
5:421–426. 2000.PubMed/NCBI
|
|
10
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual (7th). Springer.
New York, NY: 2010.
|
|
11
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JG, Kim EO, Jeong BR, Min YJ, Park JW,
Kim ES, Namgoong IS, Kim YI and Lee BJ: Visfatin stimulates
proliferation of MCF-7 human breast cancer cells. Mol Cells.
30:341–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Filková M, Haluzík M, Gay S and Senolt L:
The role of resistin as a regulator of inflammation: Implications
for various human pathologies. Clin Immunol. 133:157–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo
RL, Wu D, Cooper GJ and Xu A: Adiponectin modulates the glycogen
synthase kinase-3beta/beta-catenin signaling pathway and attenuates
mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res.
66:11462–11470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kosova F, Coskun T, Kaya Y, Kara E and Ari
Z: Adipocytokine levels of colon cancer patients before and after
treatment. Bratisl Lek Listy. 114:394–397. 2013.PubMed/NCBI
|
|
16
|
Thompson LH and Suit HD: Proliferation
kinetics of x-irradiated mouse L cells studied WITH TIME-lapse
photography. II. Int J Radiat Biol Relat Stud Phys Chem Med.
15:347–362. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dent P, Yacoub A, Contessa J, Caron R,
Amorino G, Valerie K, Hagan MP, Grant S and Schmidt-Ullrich R:
Stress and radiation-induced activation of multiple intracellular
signaling pathways. Radiat Res. 159:283–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Williams J, Ding I, Hernady E, Liu
W, Smudzin T, Finkelstein JN, Rubin P and Okunieff P: Radiation
pneumonitis and early circulatory cytokine markers. Semin Radiat
Oncol. 12(Suppl 1): 26–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rubin P, Johnston CJ, Williams JP,
McDonald S and Finkelstein JN: A perpetual cascade of cytokines
postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol
Biol Phys. 33:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu XL, Huang H, Bentel G, Clough R, Jirtle
RL, Kong FM, Marks LB and Anscher MS: Predicting the risk of
symptomatic radiation-induced lung injury using both the physical
and biologic parameters V30 and transforming growth factor β. Int J
Radiat Oncol Biol Phys. 50:899–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paris F, Fuks Z, Kang A, Capodieci P, Juan
G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C and Kolesnick
R: Endothelial apoptosis as the primary lesion initiating
intestinal radiation damage in mice. Science. 293:293–297. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hauer-Jensen M, Kong FM, Fink LM and
Anscher MS: Circulating thrombomodulin during radiation therapy of
lung cancer. Radiat Oncol Investig. 7:238–242. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaaks R: Nutrition, hormones, and breast
cancer: Is insulin the missing link? Cancer Causes Control.
7:605–625. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vona-Davis L, Howard-McNatt M and Rose DP:
Adiposity, type 2 diabetes and the metabolic syndrome in breast
cancer. Obes Rev. 8:395–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chappell J, Leitner JW, Solomon S,
Golovchenko I, Goalstone ML and Draznin B: Effect of insulin on
cell cycle progression in MCF-7 breast cancer cells. Direct and
potentiating influence. J Biol Chem. 276:38023–38028.
2001.PubMed/NCBI
|
|
26
|
Ish-Shalom D, Christoffersen CT, Vorwerk
P, Sacerdoti-Sierra N, Shymko RM, Naor D and De Meyts P: Mitogenic
properties of insulin and insulin analogues mediated by the insulin
receptor. Diabetologia. 40(Suppl 2): S25–S31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shafie SM and Hilf R: Insulin receptor
levels and magnitude of insulin-induced responses in
7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats.
Cancer Res. 41:826–829. 1981.PubMed/NCBI
|
|
28
|
Shafie SM and Grantham FH: Role of
hormones in the growth and regression of human breast cancer cells
(MCF-7) transplanted into athymic nude mice. J Natl Cancer Inst.
67:51–56. 1981.PubMed/NCBI
|
|
29
|
Mink PJ, Shahar E, Rosamond WD, Alberg AJ
and Folsom AR: Serum insulin and glucose levels and breast cancer
incidence: The atherosclerosis risk in communities study. Am J
Epidemiol. 56:349–352. 2002. View Article : Google Scholar
|
|
30
|
Eliassen AH, Tworoger SS, Mantzoros CS,
Pollak MN and Hankinson SE: Circulating insulin and c-peptide
levels and risk of breast cancer among predominately premenopausal
women. Cancer Epidemiol Biomarkers Prev. 16:161–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gunter MJ, Hoover DR, Yu H,
Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X,
Anderson GL, et al: Insulin, insulin-like growth factor-I, and risk
of breast cancer in postmenopausal women. J Natl Cancer Inst.
101:48–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kabat GC, Kim M, Caan BJ, Chlebowski RT,
Gunter MJ, Ho GY, Rodriguez BL, Shikany JM, Strickler HD, Vitolins
MZ, et al: Repeated measures of serum glucose and insulin in
relation to postmenopausal breast cancer. Int J Cancer.
125:2704–2710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guinan E, Connolly EM, Healy LA, Carroll
PA, Kennedy MJ and Hussey J: The development of the metabolic
syndrome and insulin resistance after adjuvant treatment for breast
cancer. Cancer Nurs. 37:355–362. 2014.PubMed/NCBI
|
|
34
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 35(Suppl
1): S62–S69. 2010.
|
|
35
|
Demark-Wahnefried W, Peterson BL, Winer
EP, Marks L, Aziz N, Marcom PK, Blackwell K and Rimer BK: Changes
in weight, body composition, and factors influencing energy balance
among premenopausal breast cancer patients receiving adjuvant
chemotherapy. J Clin Oncol. 19:2381–2389. 2001.PubMed/NCBI
|
|
36
|
Carr MC: The emergence of the metabolic
syndrome with menopause. J Clin Endocrinol Metab. 88:2404–2411.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaaja RJ: Metabolic syndrome and the
menopause. Menopause Int. 14:21–25. 2008.PubMed/NCBI
|
|
38
|
Steppan CM, Bailey ST, Bhat S, Brown EJ,
Banerjee RR, Wright CM, Patel HR, Ahima RS and Lazar MA: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View
Article : Google Scholar : PubMed/NCBI
|