Introduction
Prostate cancer remains the most frequently
diagnosed malignancy and the second leading cause of
cancer-associated mortality in men in Western industrialized
countries (1). Despite the notably
prolonged survival in patients with prostate cancer, improved
survival in patients with advanced disease has not significantly
contributed to this decline in mortality (2). Although intensive efforts have been made
in the field of prostate cancer research, androgen withdrawal
therapy is still the only effective treatment for men with advanced
prostate cancer. Initially, 80–90% of such patients favorably
respond to this therapy; however, disease progression to lethal
stage eventually occurs in the majority of these patients within a
few years under the low levels of serum testosterone, which is
recognized as castration-resistant prostate cancer (CRPC) (3).
Historically, prior to the introduction of
docetaxel, no agents demonstrated survival benefits in patients
with CRPC (4,5); therefore, the usefulness of different
types of hormonal therapy had been investigated to prolong the
duration until the appearance of a phenotype characteristic of CRPC
(6,7).
For example, anti-androgen withdrawal syndrome, a manifestation of
a prostate-specific antigen (PSA) decline, brought about by the
discontinuation of the administration of anti-androgen, has been
observed in 20–30% of patients receiving maximum androgen blockade
(MAB) (8,9). In addition, several previous studies
have reported a favorable PSA response to alternative anti-androgen
therapy in advanced prostate cancer, which relapsed following
initial MAB (8,10–14). Along
with docetaxel, several novel agents with different mechanisms of
action, including abiraterone, enzalutamide and cabazitaxel, have
been shown to yield positive results in phase III trials against
metastatic CRPC, and are already widely used in clinical practice
(15,16).
Considering these findings, it is important to
reevaluate the significance of the continuous treatment of patients
with advanced prostate cancer with secondary hormonal therapy
following the failure of initial MAB in order to determine whether
such a strategy remains suitable now that several novel agents
against CRPC have become available. The present study, therefore,
retrospectively reviewed the clinical outcomes in a total of 272
consecutive patients with advanced prostate cancer who underwent
anti-androgen withdrawal and/or subsequent alternative
anti-androgen therapy with flutamide following the failure of
initial MAB, using bicalutamide.
Patients and methods
Patients
The present study included a total of 272
consecutive patients with histologically diagnosed advanced
prostate cancer, who underwent anti-androgen withdrawal and/or
alternative anti-androgen therapy following the failure of initial
MAB using bicalutamide, between January 2010 and September 2014. In
all patients, serum PSA levels were measured at least every 12
weeks. Clinical variables were evaluated based on the findings of
digital rectal examination, transrectal ultrasonography (TRUS),
systematic TRUS-guided needle biopsy, pelvic computed tomography,
magnetic resonance imaging and bone scan, and were determined
according to the 2010 Tumor Node Metastasis classification system.
Treatment failure was defined as increased serum PSA levels on
three successive occasions, and the response duration was regarded
as the duration from the start of each treatment until failure.
Hormonal therapy
In this series, all patients were initially treated
with MAB consisting of either bilateral orchidectomy or medical
castration using a luteinizing hormone-releasing hormone agonist
(goserelin acetate or leuprorelin acetate) plus bicalutamide (80
mg/day) as first-line hormonal therapy. When the first-line therapy
was judged to have failed, bicalutamide was discontinued to
determine whether or not androgen withdrawal syndrome was observed
in certain patients without characteristics suggesting aggressive
disease, including the presence of bone metastasis at diagnosis,
high Gleason score, short PSA doubling time and/or short duration
of initial MAB therapy. Subsequent second-line MAB using flutamide
(375 mg/day) as an alternative anti-androgen was initiated in all
patients, irrespective of the evaluation of the androgen withdrawal
response.
Statistical analysis
All statistical analyses were performed using
Statview 5.0 software (Abacus Concepts, Inc., Berkeley, CA, USA).
Differences between the two groups were analyzed using the
chi-squared test or unpaired t-test. The overall survival (OS) and
cancer-specific survival (CSS) rates were calculated using the
Kaplan-Meier method, and the differences were determined by the
log-rank test. The prognostic significance of certain factors was
assessed using the Cox proportional hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Table I shows the
characteristics of the 272 patients included in the present study.
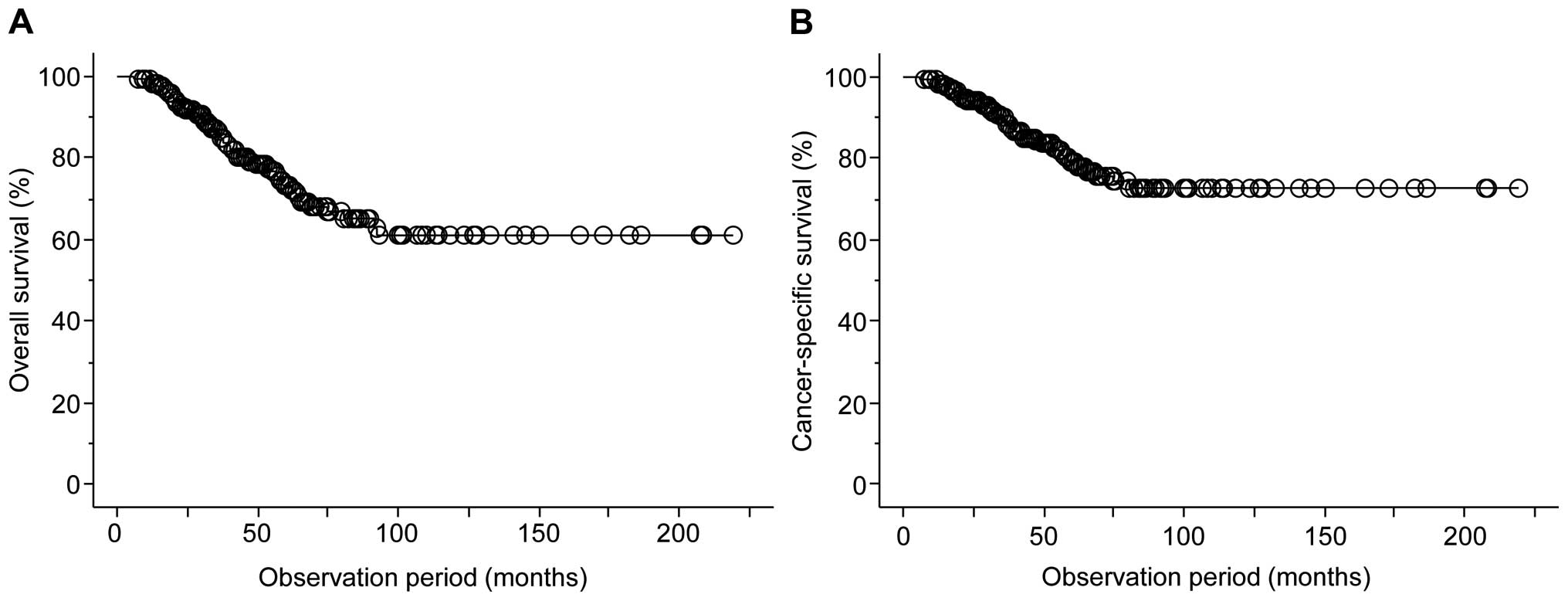
As shown in Fig. 1, during the
observation period of the present study from the introduction of
initial MAB (median, 42.3 months), overall and cancer-specific
mortality occurred in 58 (21.3%) and 41 (15.0%), respectively, and
the 5- and 10-year OS rates were 73.3 and 66.8%, respectively,
while the 5- and 10-year CSS rates were 79.2 and 72.5%,
respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Patient details |
|---|
| Median age
(years) | 72
(47–89)a |
| Median initial PSA
(ng/ml) |
120.9
(1.3–22,412.1)a |
| Biopsy Gleason
score |
|
| ≤7 | 49
(18.0)b |
| 8–10 | 223
(82.0)b |
| T category |
|
| T1 or
T2 | 45
(16.6)b |
| T3 | 169
(62.1)b |
| T4 | 58
(21.3)b |
| N category |
|
| N0 | 145
(53.3)b |
| N1 | 127
(46.7)b |
| M category |
|
| M0 | 91
(33.5)b |
| M1 | 181
(66.5)b |
| Median PSA nadir
during initial MAB (ng/ml) |
0.30
(<0.001–550.3)a |
| Median duration of
initial MAB (months) | 15.0
(1–180)a |
Once the initial MAB using bicalutamide had failed,
the incidence of anti-androgen withdrawal syndrome was assessed in
231 (84.9%) of the 272 patients; however, the observation of
anti-androgen withdrawal syndrome was omitted in the remaining 41
(15.1%) due to characteristics suggesting the presence of
aggressive diseases. A decline in the serum PSA level following
anti-androgen withdrawal was observed in 83 (35.9%) of the 231
patients, and >50% decline from the baseline serum PSA level was
observed in 18 patients (7.8%). Of several factors examined by
univariate analysis, no factor significantly correlated with PSA
decline by anti-androgen withdrawal therapy (data not shown).
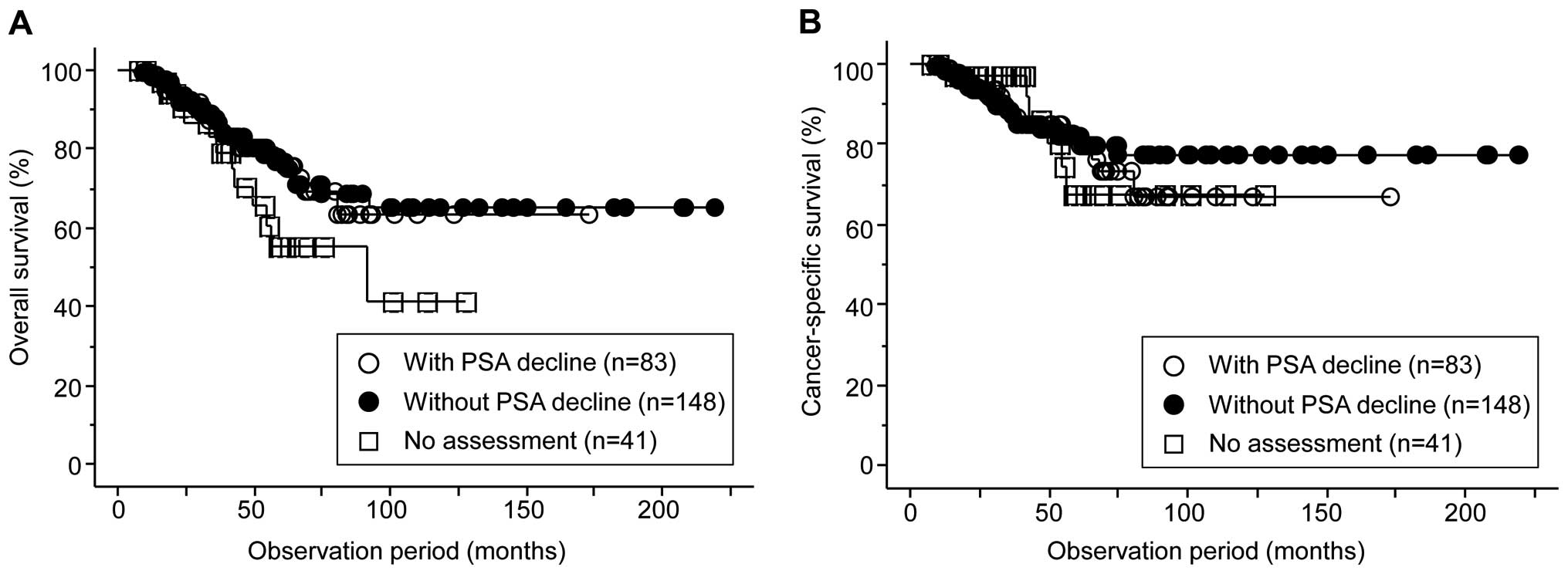
Furthermore, when the included patients were classified into the
following three groups: i) 41 patients without assessment of
anti-androgen withdrawal syndrome; ii) 83 with PSA decline; iii)
148 without PSA decline following anti-androgen withdrawal therapy,
no significant difference in the OS or CSS was observed among the
groups (Fig. 2).
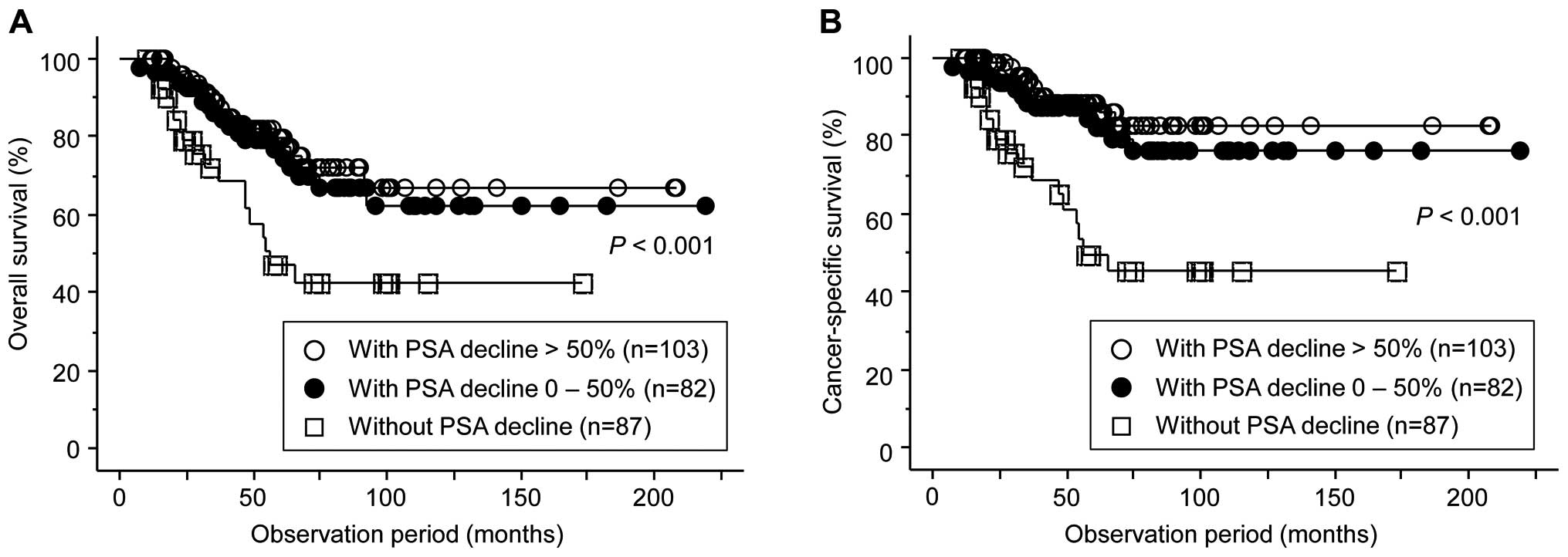
Following the introduction of alternative
anti-androgen therapy using flutamide, PSA decline was observed in
185 patients (68.0%), among whom 103 (37.9%) were regarded as
responders exhibiting a reduction of PSA >50%; however, the PSA
level continued to increase in the remaining 87 (32.0%). Although
no significant difference was observed in the OS or CSS between 103
patients with PSA decline >50% and 82 with that of 0–50%, both
the OS and CSS in 87 patents without PSA decline were significantly
poorer compared with the 103 and 83 patients showing PSA decline
>50% and 0–50%, respectively (Fig.
3). Furthermore, as shown in Table
II, of several factors examined, only the duration of initial
MAB therapy was shown to be significantly correlated with whether
or not PSA declined following alternative anti-androgen
therapy.
 | Table II.Association between several factors
and PSA decline following alternative anti-androgen therapy. |
Table II.
Association between several factors
and PSA decline following alternative anti-androgen therapy.
|
| PSA decline following
alternative anti-androgen withdrawal |
|
|---|
|
|
|
|
|---|
|
| Yes (n=185) | No (n=87) | P-value |
|---|
| Median age
(years) | 72
(47–89)a | 72
(54–87)a | 0.79 |
| Median initial PSA
(ng/ml) | 167.3
(1.3–22,412.1)a | 92.4
(3.4–6,305.8)a | 0.35 |
| Biopsy gleason
score |
|
| 0.63 |
| ≤7 | 33
(17.8)b | 16
(18.4)b |
|
| 8–10 | 152
(82.2)b | 71
(81.6)b |
|
| T category |
|
| 0.34 |
| T1 or
T2 | 31
(16.8)b | 14
(16.1)b |
|
| T3 or
T4 | 154
(83.2)b | 73
(83.9)b |
|
| N category |
|
| 0.71 |
| N0 | 99
(53.5)b | 46
(52.9)b |
|
| N1 | 86
(46.5)b | 41
(47.1)b |
|
| M category |
|
| 0.65 |
| M0 | 61
(33.0)b | 30
(34.5)b |
|
| M1 | 124
(67.0)b | 57
(65.5)b |
|
| Median PSA nadir
during initial MAB (ng/ml) | 0.25
(<0.003–47.6)a | 0.63
(0.007–476.4)a | 0.16 |
| Median duration of
initial MAB (months) | 16.9
(2.3–179.1)a | 11.7
(1.1–135.9)a | 0.016 |
| PSA decline following
anti-androgen withdrawal |
|
| 0.31 |
| Yes | 54
(29.2)b | 29
(33.3)b |
| No | 114
(61.6)b | 34
(39.1)b |
| Not
assessed | 17 (9.2)b | 24
(27.6)b |
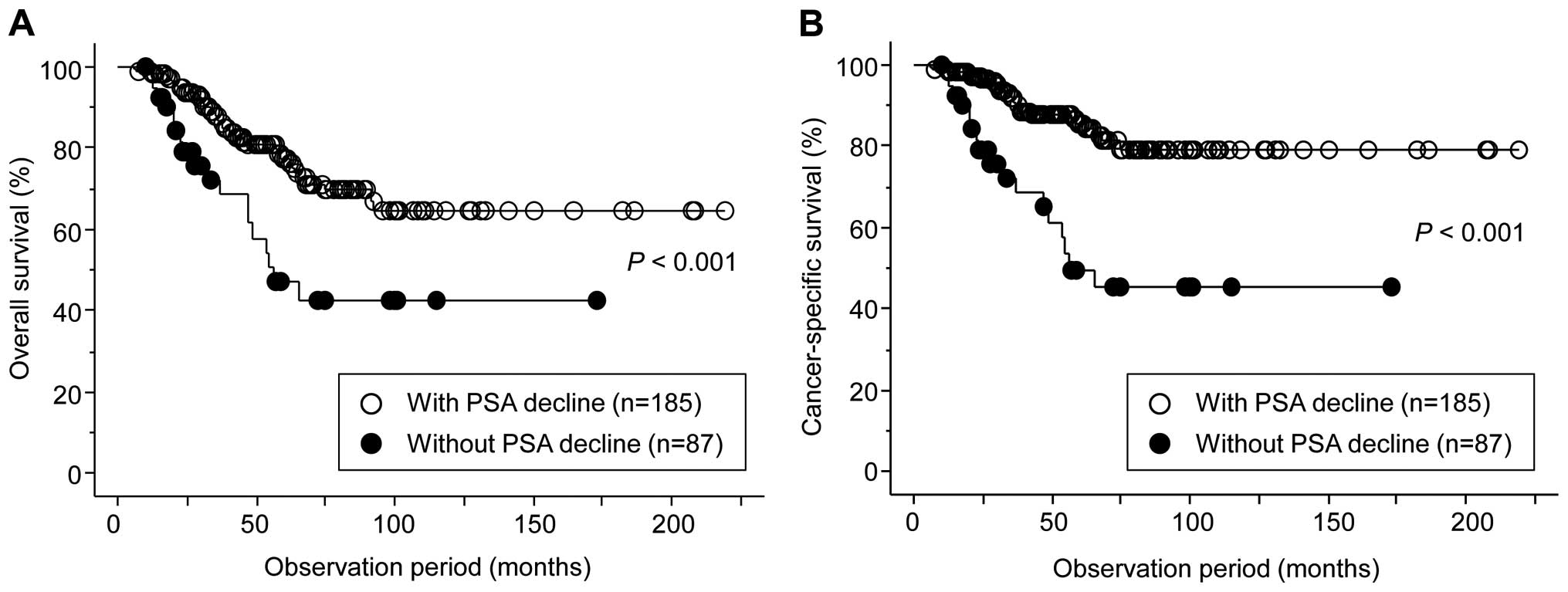
The present study subsequently performed uni- and
multivariate analyses to identify factors predicting the OS and CSS
in the 272 patients. Univariate analyses identified the following
significant prognostic predictors: PSA nadir during initial MAB,
duration of initial MAB therapy and PSA decline following
alternative anti-androgen therapy for OS, and positive for M
category, PSA nadir during initial MAB, duration of initial MAB
therapy and PSA decline following alternative anti-androgen therapy
for CSS. However, only PSA decline following alternative
anti-androgen therapy was shown to be independently associated with
both the OS and CSS by multivariate analysis (Table III). Figure 4 highlights the OS and CSS curves,
according to PSA decline after alternative anti-androgen
therapy.
 | Table III.Univariate and multivariate analyses
of associations between various parameters with cancer-specific and
overall survival in 272 patients with advanced prostate cancer who
underwent anti-androgen withdrawal and/or alternative anti-androgen
therapy. |
Table III.
Univariate and multivariate analyses
of associations between various parameters with cancer-specific and
overall survival in 272 patients with advanced prostate cancer who
underwent anti-androgen withdrawal and/or alternative anti-androgen
therapy.
|
| Overall survival | Cancer-specific
survival |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Variables | Hazard ratio | P-value | Hazard ratio | P-value | Hazard ratio | P-value | Hazard ratio | P-value |
|---|
| Agea (<70, vs. 70≤) | 0.56 | 0.770 | – | – | 0.80 | 0.2300 | – | – |
| Initial
PSAb (<100, vs.
100≤) | 1.11 | 0.530 | – | – | 1.37 | 0.3000 | – | – |
| Biopsy Gleason
score (≤7, vs. 8–10) | 1.39 | 0.390 | – | – | 1.13 | 0.2900 | – | – |
| T category (T1/T2,
vs. T3/T4) | 1.20 | 0.680 | – | – | 1.14 | 0.4800 | – | – |
| N category (N0, vs.
N1) | 1.13 | 0.490 | – | – | 1.20 | 0.4400 | – | – |
| M category (M0, vs.
M1) | 1.52 | 0.055 | – | – | 2.65 | 0.0097 | 2.33 | 0.210 |
| PSA nadir during
initial MABb (<2, vs.
2≤) | 0.47 | 0.031 | 1.21 | 0.11 | 0.99 | 0.0130 | 0.97 | 0.260 |
| Duration of initial
MABc (<24, vs.
24≤) | 0.53 | 0.019 | 0.72 | 0.38 | 0.25 | 0.0300 | 0.70 | 0.310 |
| PSA decline after
anti-androgen withdrawal (yes, vs. no/not assessed). | 0.88 | 0.500 |
|
| 0.99 | 0.8500 | – | – |
| PSA decline after
alternative anti-androgen therapy (yes, vs. no) | 0.31 | <0.001 | 0.33 | 0.029 | 0.21 | <0.001 | 0.28 | 0.001 |
Discussion
Until the introduction of docetaxel (4,5), no
standard approach existed for patients with advanced prostate
cancer if first-line hormonal therapy failed. Therefore, whether
several secondary approaches using hormonal agents improved the
prognosis of such patients was previously investigated (6,7). Of these,
anti-androgen withdrawal and alternative anti-androgen therapy
became regarded as comparatively useful options for prostate cancer
patients following the failure of initial MAB (8–14). In
recent years, however, novel agents with different mechanisms of
action against CRPC have been approved and widely introduced into
clinical practice, resulting in a marked paradigm shift in the
therapeutic strategy for advanced prostate cancer (15,16). Taken
together, it is important to re-analyze the significance of
secondary hormonal therapy, which, despite the lack of high levels
of evidence, have been reported to exhibit certain beneficial
effects on the prognosis of patients with advanced prostate cancer.
The present study retrospectively assessed the clinical outcomes in
a total of 272 Japanese men with advanced prostate cancer who
underwent anti-androgen withdrawal and/or subsequent alternative
anti-androgen therapy following the failure of initial MAB.
In this series, considering the relatively low
incidences of anti-androgen withdrawal syndrome in previous studies
(8–10), 12.9% of patients judged to have
aggressive disease did not undergo anti-androgen withdrawal
therapy. However, the proportion of patients exhibiting
anti-androgen withdrawal syndrome remained low even following the
exclusion of patients without the assessment of anti-androgen
withdrawal syndrome; that is, PSA decline >50% following
anti-androgen withdrawal was observed in only 7.8% of patients.
Furthermore, the present study failed to identify any parameter
significantly correlated with the PSA decline by anti-androgen
withdrawal therapy, suggesting that it is difficult to identify
patients who are likely to benefit from this therapy. Collectively,
these findings suggested that confirming the anti-androgen
withdrawal response in patients with prostate cancer showing
relapse following initial MAB cannot be positively recommended.
In the present study, MAB using flutamide was
applied to all patients as alternative anti-androgen therapy, and a
reduction in the PSA level was observed in 68.0% of the patients.
When the response was defined as a decrease of >50% in the PSA
level, 30.1% of patients were regarded as responders. This outcome
of alternative anti-androgen therapy is comparable with those
previously reported (8,10–14). For
example, Kassouf et al (14)
noted a PSA decrease >50% in 29% of patients following
second-line hormonal therapy with nilutamide, while Suzuki et
al (8) reported that 35.8% of
patients treated with non-steroidal anti-androgens as alternative
therapy, achieved PSA decline >50%. These findings indicated the
definitive effect of alternative anti-androgen therapy,
irrespective of introduced agents, on the PSA decline in patients
with advanced prostate cancer.
In the present series, no significant difference was
observed in the OS or CSS between 103 patients with PSA decline
>50% and 82 with that of 0–50% following alternative
anti-androgen therapy. The present study, therefore, assessed the
impacts of several parameters on whether or not the PSA decline
could be achieved by alternative anti-androgen therapy. Of several
factors examined, only the duration of initial MAB was shown to be
significantly correlated with the induction of PSA decline by
alternative anti-androgen therapy. Previous studies identified
certain variables associated with the response to alternative
anti-androgen therapy, including the baseline serum PSA level,
presence of bone metastasis and anti-androgen withdrawal response,
in addition to the duration of initial MAB (8,10,11,14). These
findings suggested that alternative anti-androgen therapy can
promote a favorable response in patients with prostate cancer with
comparatively indolent characteristics and limited disease
extension. Furthermore, whether or not PSA decline was achieved
following alternative anti-androgen therapy was identified as the
only independent predictor of both the OS and CSS in the 272
patients included in the present study. This finding was supported
by a previous study demonstrating the potential value of the
response to alternative anti-androgen therapy to assess the
prognosis following the failure of initial MAB (8).
The present study had several limitations. Firstly,
despite being one of the largest series evaluating the outcomes of
second-line hormonal therapy using alternative anti-androgen, this
was a retrospective study including only Japanese men. Secondly,
the indication of anti-androgen withdrawal therapy was not
determined based on a strict criterion, which may have affected the
present outcomes, even slightly. Finally, the outcomes of the
present study should be carefully interpreted considering that no
patient was included who received agents demonstrated to have
prognostic benefits in CRPC patients, with the exception of
docetaxel.
In conclusion, following the failure of initial MAB,
it may not be necessary to evaluate anti-androgen withdrawal
irrespective of the characteristics of patients with advanced
prostate cancer, whereas if initial maximum androgen blockade is
effective, alternative anti-androgen therapy may be considered.
However, it should be prospectively investigated whether or not
alternative anti-androgen therapy would be superior to the direct
introduction of currently approved agents against CRPC followed by
initial MAB.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu JN, Fish KM, Evans CP, White Devere RW
and Dall'Era MA: No improvement noted in overall or cause-specific
survival for men presenting with metastatic prostate cancer over a
20-year period. Cancer. 120:818–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris WP, Mostaghel EA, Nelson PS and
Montgomery B: Androgen deprivation therapy: Progress in
understanding mechanisms of resistance and optimizing androgen
depletion. Nat Clin Pract Urol. 6:76–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharifi N, Dahut WL and Figg WD: Secondary
hormonal therapy for prostate cancer: What lies on the horizon? BJU
Int. 101:271–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyamoto H, Messing EM and Chang C:
Androgen deprivation therapy for prostate cancer: Current status
and future prospects. Prostate. 61:332–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki H, Okihara K, Miyake H, Fujisawa M,
Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, et
al: Alternative nonsteroidal antiandrogen therapy for advanced
prostate cancer that relapsed after initial maximum androgen
blockade. J Urol. 180:921–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sartor AO, Tangen CM, Hussain MH,
Eisenberger MA, Parab M, Fontana JA, Chapman RA, Mills GM, Raghavan
D and Crawford ED: Southwest Oncology Group: Antiandrogen
withdrawal in castrate-refractory prostate cancer: A Southwest
Oncology Group trial (SWOG 9426). Cancer. 112:2393–2400. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyake H, Hara I and Eto H: Clinical
outcome of maximum androgen blockade using flutamide as second-line
hormonal therapy for hormone-refractory prostate cancer. BJU Int.
96:791–795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kojima S, Suzuki H, Akakura K, Shimbo M,
Ichikawa T and Ito H: Alternative antiandrogens to treat prostate
cancer relapse after initial hormone therapy. J Urol. 171:679–683.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okihara K, Ukimura O, Kanemitsu N,
Mizutani Y, Kawauchi A and Miki T: Kyoto Prefectural University of
Medicine Prostate Cancer Research Group: Clinical efficacy of
alternative antiandrogen therapy in Japanese men with relapsed
prostate cancer after first-line hormonal therapy. Int J Urol.
14:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okegawa T, Nutahara K and Higashihara E:
Alternative antiandrogen therapy in patients with
castration-resistant prostate cancer: A single-center experience.
Int J Urol. 17:950–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kassouf W, Tanguay S and Aprikian AG:
Nilutamide as second line hormone therapy for prostate cancer after
androgen ablation fails. J Urol. 169:1742–1744. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beltran H, Beer TM, Carducci MA, de Bono
J, Gleave M, Hussain M, Kelly WK, Saad F, Sternberg C, Tagawa ST
and Tannock IF: New therapies for castration-resistant prostate
cancer: Efficacy and safety. Eur Urol. 60:279–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzman DL and Antonarakis ES:
Castration-resistant prostate cancer: Latest evidence and
therapeutic implications. Ther Adv Med Oncol. 6:167–179. 2014.
View Article : Google Scholar : PubMed/NCBI
|