Introduction
Malignant primary brain tumors are a fairly uncommon
malignancy, representing 2–3% of all adult tumors in Saudi Arabia.
Glioblastoma multiforme (GBM) is the most common and most malignant
primary tumor of the brain. It arises from astrocytes, and is
characterized by rapid growth and short time to progression.
Consequently, it is associated with one of the worst 5 year
survival rates amongst all human cancer types (1–4).
The standard of care in patients with GBM includes
maximal surgical resection, followed by radiotherapy (60 Gy in 30
fractions) with concomitant and adjuvant temozolomide (TMZ). The
addition of TMZ to radiation therapy has increased both the median
survival (12.1 to 14.6 months) and the 2 year survival duration (10
to 26%) (5). Despite recent advances
in the understanding of the molecular mechanism of tumorigenesis,
the outcome of patients with cancer remains poor and therefore
there is an urgent requirement for more effective initial
treatments for this intractable disease (6–10). Recent
therapies under investigation include immunotherapy, chemotherapy,
targeted molecular therapy, antiangiogenic therapy, gene therapy,
radiation-enhancement and drugs for overcoming resistance (11).
Although prognosis is extremely poor, a limited
number of patients with GBM do survive past 36 months. However, a
limited understanding of the predictors for survival amongst
patients with GBM exists. Additionally, no studies have as yet
examined the outcome of GBM in Saudi Arabia. Therefore the aim of
the present study was to assess the real world outcome of patients
with GBM in this region and to determine the important clinical,
pathological and molecular prognostic factors correlated with
patient outcomes in this population.
Patients and methods
Study design
The present single-center retrospective cohort study
was performed in the Comprehensive Cancer Center at King Fahad
Medical city (Riyadh, Saudi Arabia). The present study was approved
by the local Ethics Committee of the Comprehensive Cancer Center
(no. 14-163).
Patients
All adult patients (>18-years-old), who were
diagnosed with histologically proven GBM between January 2008 and
December 2013 were included in the present study.
Intervention
Patients underwent either a biopsy or a resection,
following which they received standard or hypo-fractionated
radiotherapy (XRT), with or without concurrent temozolmide (TMZ),
as the current standard of care. Following completion of XRT, a
proportion of patients received further cycles of TMZ.
Data collection
The data were obtained from electronic medical
records, where a data collection form was developed to collect
patient demographics, pathology, XRT and chemotherapy details, and
progression and survival outcomes. The patient and tumor
characteristics are categorized, as shown in Tables I and II.
 | Table I.Patient characteristics (n=90). |
Table I.
Patient characteristics (n=90).
| Characteristic | No. patients, n
(%) |
|---|
| Age, years
(range) |
|
|
Median | 49 (18–81) |
| ≥65 | 18 (20) |
| Gender |
|
| Male | 67 (74) |
|
Female | 23 (26) |
| Eastern Cooperative
Oncology Group |
|
| ≤2 | 54 (60) |
|
>2 | 36 (40) |
| Co-morbidities |
|
| Yes | 36 (40) |
| No | 54 (60) |
| Year of
diagnosis |
|
| 2008 | 14 (15) |
| 2009 | 17 (19) |
| 2010 | 10 (11) |
| 2011 | 16 (18) |
| 2012 | 15 (17) |
| 2013 | 18 (20) |
 | Table II.Tumor characteristics (n=90). |
Table II.
Tumor characteristics (n=90).
| Characteristic | No. patients, n
(%) |
|---|
| Tumor site |
|
|
Frontal | 22 (24) |
|
Temporal | 21 (23) |
|
Parietal | 13 (14) |
|
Other | 34 (38) |
| Hemisphere |
|
|
Right | 44 (49) |
|
Left | 32 (36) |
|
Bilateral | 14 (16) |
| Pathology |
|
|
GBM | 78 (87) |
| GBM
variant | 12 (13) |
|
Oligodendroglioma | 7 (58) |
|
Primitive neuroectodermal
tumor | 2 (17) |
|
Gliosarcoma | 2 (17) |
| Giant
cell | 1 (8) |
| MGMT status |
|
|
Methylated | 2 (2) |
|
Unmethylated | 5 (6) |
|
Unknown | 83 (92) |
Statistical analysis
The OS and progression-free survival (PFS) were
estimated using Kaplan-Meier methodology. Univariate analyses were
performed using the log-rank test and multivariate analyses using
the Cox proportional hazards model. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed using SPSS version 22 (IBM SPSS, Armonk, NY, USA).
Results
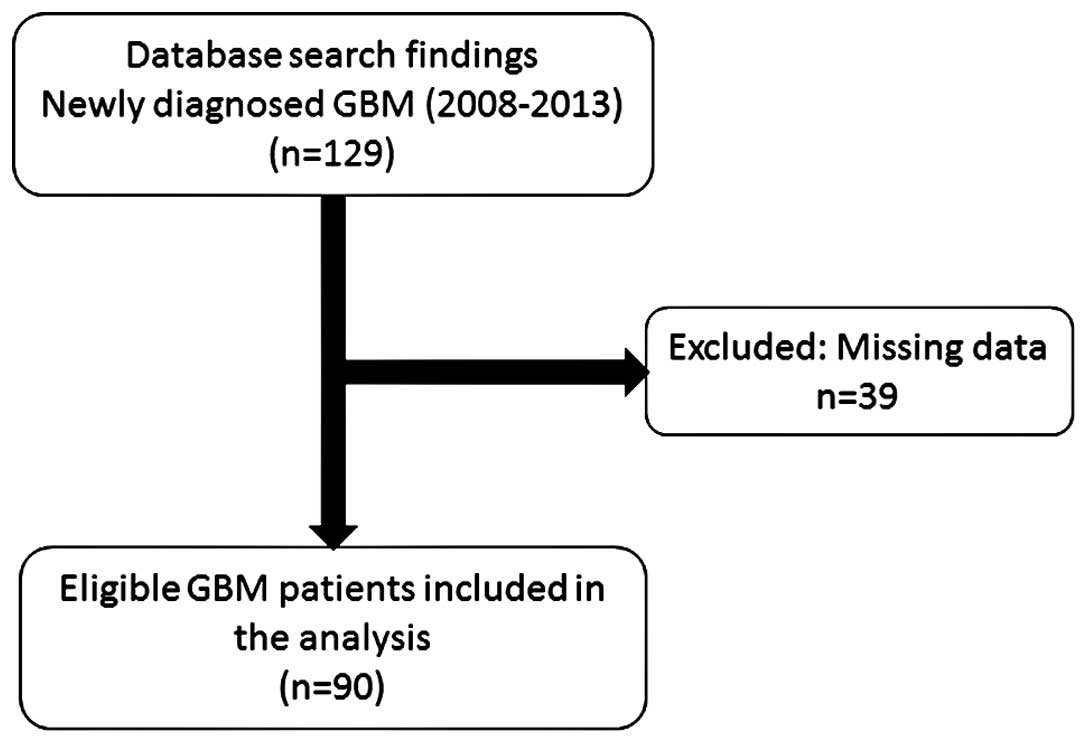
A total of 129 patients with newly diagnosed GBM
were identified for inclusion in the present study (Fig. 1). Of these patients, 39 had missing
data and were excluded from the analysis. Therefore, the data from
90 patients was included for analysis in the final study
population: 67 (74.4%) males and 23 (25.6%) females, with a median
age of 49.0 (Table I).
Tumor characteristics were described in Table II. The majority of patients (87%)
were diagnosed with a GBM, whilst the remaining 13% were diagnosed
with GBM variants, most commonly oligodendroglioma (n=7/12, 58%).
Frontal (24%) and temporal (23%) tumors were the most common tumor
sites, with almost half of the tumors (49%) located in the right
hemisphere. In general, the methylation status of the tumor could
not be determined.
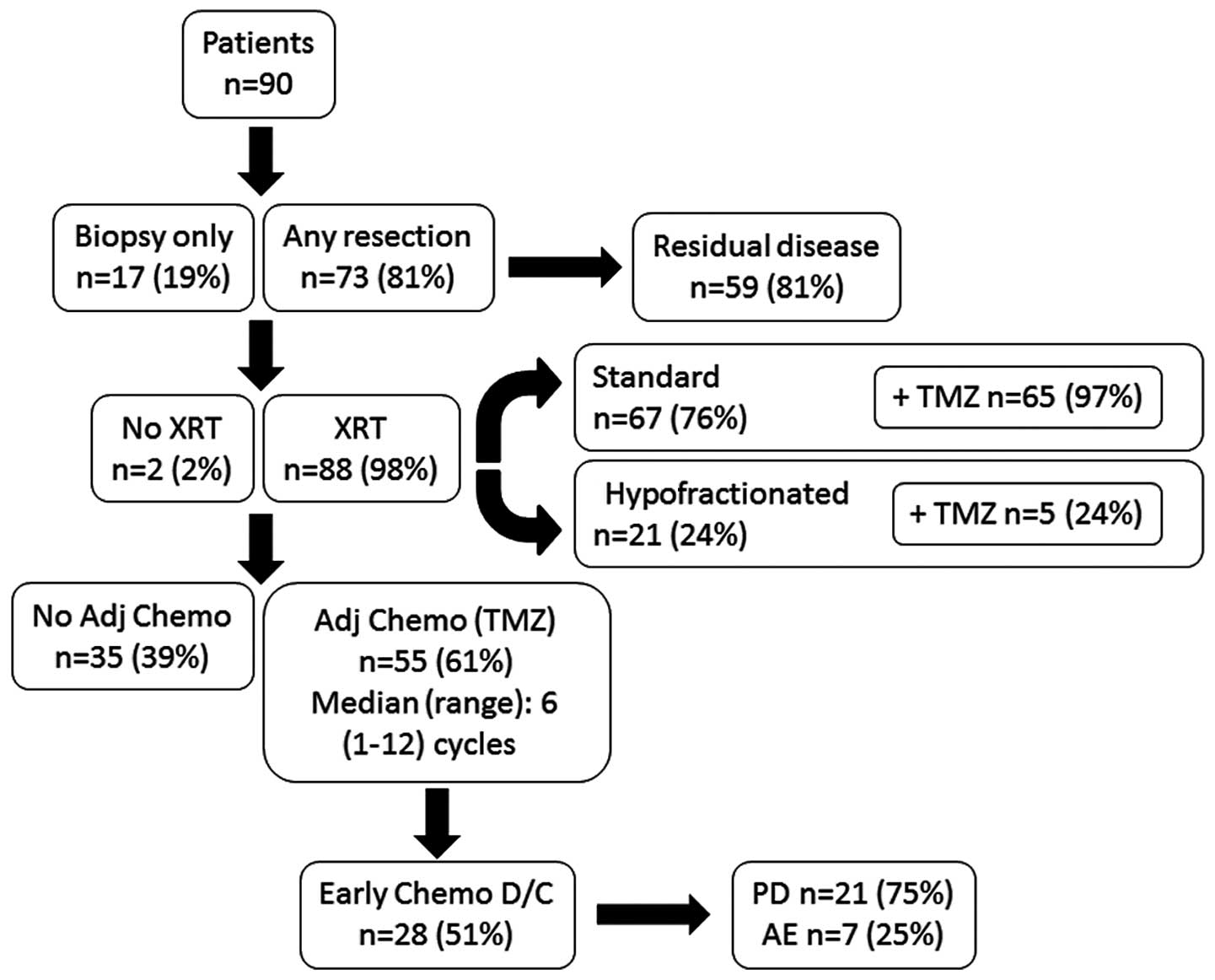
In the study cohort, 81% of patients underwent
resection, whilst the remaining 19% underwent biopsy only (Fig. 2). Of those patients who had their
tumors resected, the majority (81%) were deemed to have residual
disease following surgery. The majority (98%) of patients received
XRT; most received standard XRT (n=67/88, 76%) whilst the remaining
24% received a hypo-fractionated dosage (Fig. 2). The median dose of radiotherapy was
59.4 Gy. Concurrent TMZ was received by 72% of patients. Notably,
whilst nearly all patients (n=65/67, 97%) receiving standard XRT
also received TMZ, only a quarter (n=5/21, 24%) of those receiving
hypo-fractionated dose also received TMZ. Following completion of
XRT, the majority of patients (n=55/90, 61%) received further
cycles of TMZ, although half were unable to complete their
chemotherapy regimen.
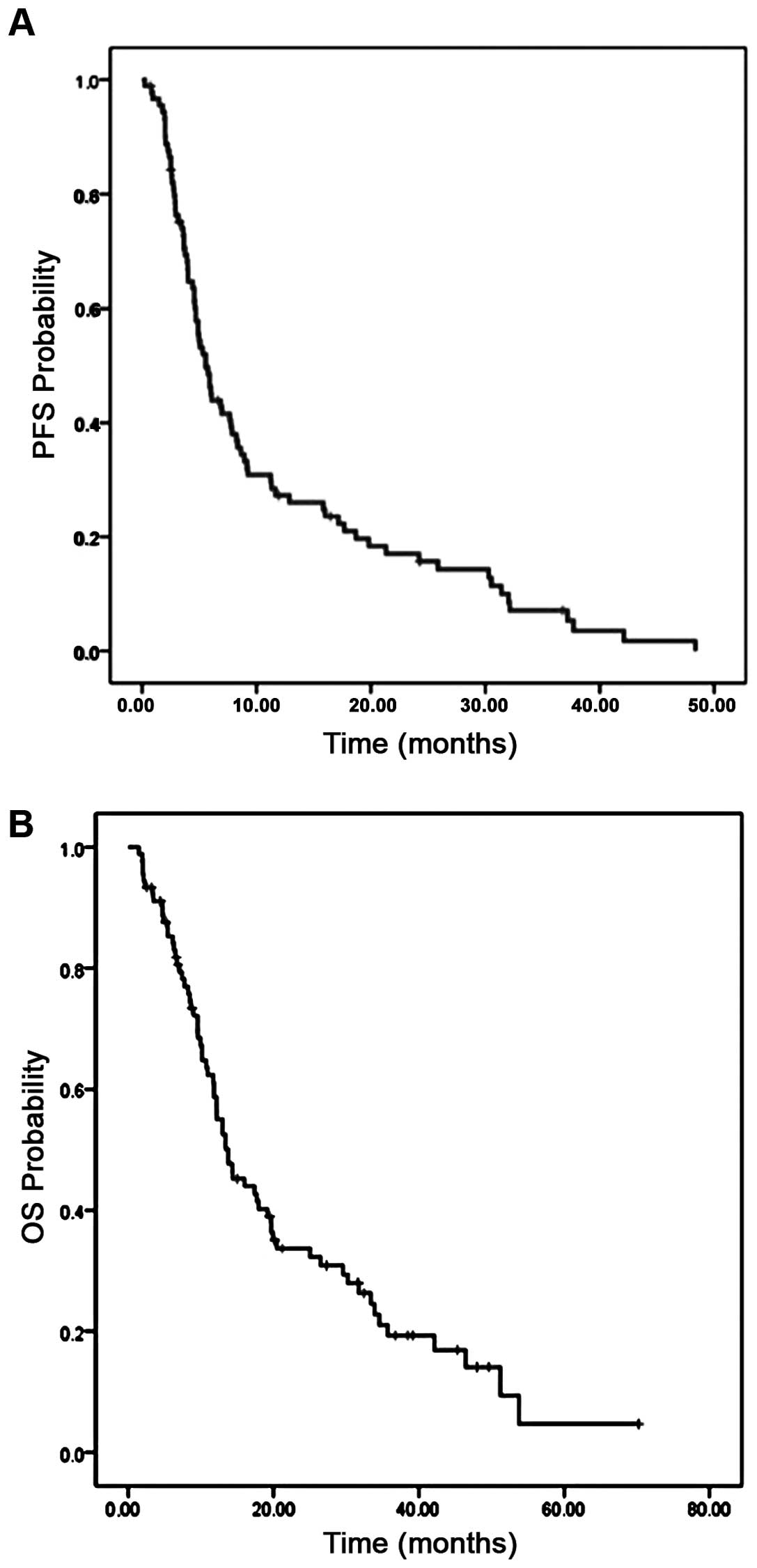
The median PFS was 5.3 months [95% confidence
intervals (CI), 4.47–6.62], with 43% PFS at 6 months (Fig. 3A). The median OS was 13.7 months (95%
CI, 10.1–17.5), with 53% OS at 1 year and median follow-up 12.5
months (1.5–70.2 months; Fig. 3B).
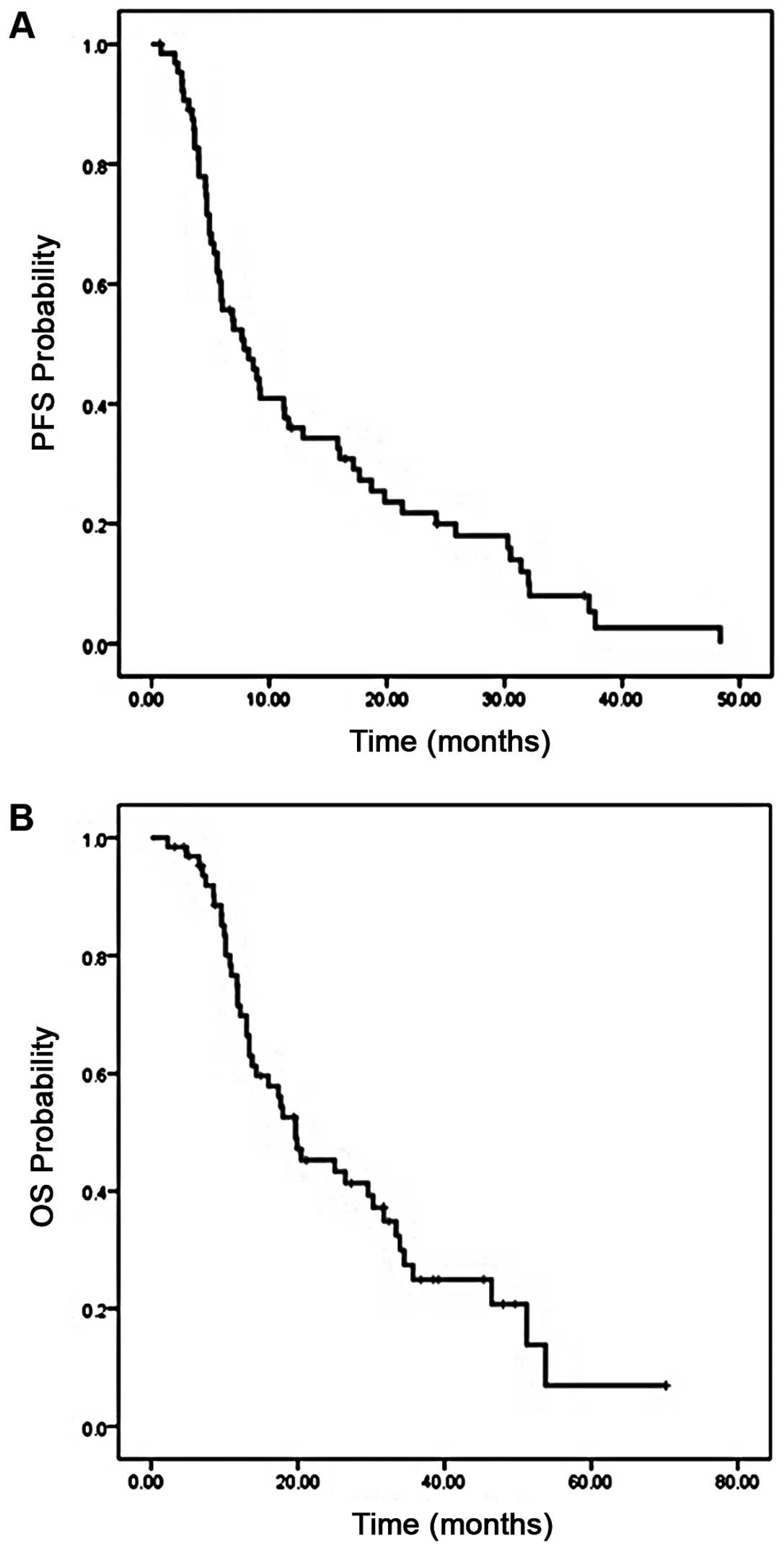
The PFS and OS were also examined in the subset of patients who
received standard XRT with concurrent TMZ (n=65). In these
patients, the median PFS was 6.7 months (95% CI, 4.7–11), with a 6
month PFS of 55% (Fig. 4A). The
median OS was 19.7 months (95% CI, 11.9–27.4), with a 1 year OS of
65% (Fig. 4B).
To determine the factors, which may be associated
with PFS and OS, univariate and multivariate analyses were
performed. In univariate analysis, age, Eastern Cooperative
Oncology Group (ECOG) performance scale, surgery type, presence of
residual disease, type of radiotherapy and receipt of chemotherapy
were all predictors of both PFS and OS (Tables III and IV). However, in multivariate analysis, only
ECOG ≤2 [odds ratio (OR), 0.3; P<0.001], absence of residual
disease (OR, 0.3; P=0.02) and receipt of adjuvant TMZ (OR, 0.5;
P=0.05) were significant predictors of survival (Tables III and IV).
 | Table III.Univariate and multivariate analysis
for progression-free survival. |
Table III.
Univariate and multivariate analysis
for progression-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | OR | CI | P-value | OR | CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≥65
(n=18) |
|
|
|
|
|
|
| <65
(n=72) | 0.5 | 0.3–0.8 | 0.007 | 1.0 | 0.5–1.9 | 0.7 |
| Eastern Cooperative
Oncology Group |
|
|
|
|
|
|
| >2
(n=36) |
|
|
|
|
|
|
| ≤2
(n=54) | 0.2 | 0.1–0.3 | <0.001 | 0.3 | 0.2–0.5 | <0.001 |
| Surgery |
|
|
|
|
|
|
| Biopsy
(n=17) |
|
|
|
|
|
|
| Any
resection (n=73) | 0.5 | 0.3–0.8 | 0.006 | 0.9 | 0.5–1.7 | 0.8 |
| Residual |
|
|
|
|
|
|
| Yes
(n=76) |
|
|
|
|
|
| No
(n=14) | 0.3 | 0.1–0.5 | <0.001 | 0.4 | 0.1–0.8 | 0.01 |
| Radiotherapy |
|
|
|
|
|
|
|
Hypo-fractionated (n=21) |
|
|
|
|
|
|
|
Standard (n=67) | 0.2 | 0.1–0.3 | <0.001 | 0.7 | 0.3–1.5 | 0.3 |
| Chemotherapy |
|
|
|
|
|
|
| No
(n=35) |
|
|
|
|
|
|
| Yes
(n=55) | 0.3 | 0.2–0.4 | <0.001 | 0.4 | 0.2–0.7 | 0.004 |
 | Table IV.Univariate and multivariate analysis
for overall survival. |
Table IV.
Univariate and multivariate analysis
for overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | OR | CI | P-value | OR | CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≥65
(n=18) |
|
|
|
|
|
|
| <65
(n=72) | 0.5 | 0.3–0.8 | 0.009 | 0.6 | 0.3–1.2 | 0.7 |
| Eastern Cooperative
Oncology Group |
|
|
|
|
|
|
| >2
(n=36) |
|
|
|
|
|
|
| ≤2
(n=54) | 0.2 | 0.1–0.3 | <0.001 | 0.3 | 0.1–0.5 | <0.001 |
| Surgery |
|
|
|
|
|
|
| Biopsy
(n=17) |
|
|
|
|
|
|
| Any
resection (n=73) | 0.4 | 0.2–0.8 | 0.004 | 0.9 | 0.5–1.7 | 0.8 |
| Residual |
|
|
|
|
|
|
| Yes
(n=76) |
|
|
|
|
|
| No
(n=14) | 0.2 | 0.1–0.5 | <0.001 | 0.3 | 0.1–0.8 | 0.02 |
| Radiotherapy |
|
|
|
|
|
|
|
Hypo-fractionated (n=21) |
|
|
|
|
|
|
|
Standard (n=67) | 0.2 | 0.1–0.4 | <0.001 | 0.7 | 0.3–1.5 | 0.3 |
| Chemotherapy |
|
|
|
|
|
|
| No
(n=35) |
|
|
|
|
|
|
| Yes
(n=55) | 0.2 | 0.1–0.4 | <0.001 | 0.5 | 0.2–1.0 | 0.05 |
Discussion
The present retrospective analysis is the first
study, to the best of our knowledge, to examine the outcome of
patients with malignant GBM in Saudi Arabia, and to determine the
important clinical and pathological prognostic factors that are
correlated with the outcome in this region.
A median PFS of 5.3 months and a median OS of 13.7
months was demonstrated. These results are consistent with previous
studies in patients with GBM (5,12,13). A 6 month PFS of 43% and a 1 year OS of
53% was observed, which was also in line with previous studies.
Notably, the PFS and OS were improved in the subgroup analysis of
patients receiving combined modality treatment, reflecting recent
studies which demonstrated that addition of concomitant
chemotherapy to XRT resulted in improved outcomes (14,15).
In the present multivariate analysis, only ECOG,
residual disease and receipt of chemotherapy were significant
predictors of survival. Previous studies have reported that age,
tumor grade (anaplastic glioma vs. GBM), Karnofsky performance
status, the number of molecular alterations and the extent of
initial surgical resection are all prognostic factors for outcome
in patients with GBM. Therefore in general, the present results are
in line with those reported elsewhere. Although no randomized
controlled trials have been performed to establish the benefit of
maximal surgical resection over a more limited resection, numerous
previous studies have suggested that maximal resection,
particularly gross total resection, does improve survival (16–24).
However, evidence remains conflicting, with further studies failing
to show a benefit with more extensive surgical resection (either
subtotal resection vs. biopsy, or complete vs. subtotal resection)
(25–27). The association of chemotherapy with
survival is supportive of recent trials demonstrating superior
outcomes for those patients treated with combined modality therapy
compared with radiotherapy alone (5).
Age was not predictive of XRT, which is in contrast to previous
studies reporting that age is a reliable predictor for outcome and
treatment response in elderly patients with glioma (28–31).
Another previous study revealed that age was only prognostic in
patients undergoing biopsy, however, not in those undergoing
resection (32). As a result of the
small number of biopsy patients within the present study, age was
not determined as a prognostic for biopsy patients within the
present population.
Certain limitations to the present study exist. Due
to the retrospective nature of the present study, certain data was
missing and therefore a third of the original population were
excluded from the analysis due to this. Although the present study
attempted to control for potential confounders through multivariate
analyses, a randomized controlled trial may provide a more robust
environment to control for factors which may influence the outcome.
Additionally, other factors which could not be collected, including
tumor size and extent of resection, may have impacted on patient
outcome. Another limitation was that treatment conditions for
patients in the present study were heterogeneous since
administration of adjuvant chemotherapy was based on personal
preferences and experience. In addition, since the present study
was a single centre study, it is possible that results are not
fully generalizable across Saudi Arabia. Furthermore, evaluation of
pseudo-progression may not have been systematic as the common
response criteria were unclear. Molecular profiling data for
O6-methylguanin-DNA-methyltransferase-status was not
part of routine assessment during the majority of the study
time-frame and is therefore not complete for the majority of
patients. Previous studies have suggested that symptom type and
histological factors, including necrosis, endothelial abnormalities
and degree of anaplasia are important prognostic factors and these
were not included in the present analysis (33–35).
In conclusion, the present study found that outcomes
for patients in Saudi Arabia with GBM are in line with those
reported in previous studies worldwide. Good performance status at
diagnosis, absence of residual disease and chemotherapy were
independently associated with an improved outcome. Future studies
must examine these factors further, whilst clinicians must focus on
optimizing these components within their treatment of patients with
GBM, in order to improve outcomes.
References
|
1
|
Ohgaki H, Dessen P, Jourde B, Horstmann S,
Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM,
Maiorka PC, et al: Genetic pathways to glioblastoma: A
population-based study. Cancer Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bauchet L, Mathieu-Daudé H, Fabbro-Peray
P, Rigau V, Fabbro M, Chinot O, Pallusseau L, Carnin C, Lainé K,
Schlama A, et al: Oncological patterns of care and outcome for 952
patients with newly diagnosed glioblastoma in 2004. Neuro Oncol.
12:725–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bokstein F, Shpigel S and Blumenthal DT:
Treatment with bevacizumab and irinotecan for recurrent high-grade
glial tumors. Cancer. 112:2267–2273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reardon DA and Wen PY: Therapeutic
advances in the treatment of glioblastoma: Rationale and potential
role of targeted agents. Oncologist. 11:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S,
Gururangan S, Sampson J, et al: Bevacizumab plus irinotecan in
recurrent glioblastoma multiforme. J Clin Oncol. 25:4722–4729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanu OO, Mehta A, Di C, Lin N, Bortoff K,
Bigner DD, Yan H and Adamson DC: Glioblastoma multiforme: A review
of therapeutic targets. Expert Opin Ther Targets. 13:701–718. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smrdel U, Kovac V, Popovic M and Zwitter
M: Glioblastoma patients in Slovenia from 1997 to 2008. Radiol
Oncol. 48:72–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenthal MA, Drummond KJ, Dally M, Murphy
M, Cher L, Ashley D, Thursfield V and Giles GG: Management of
glioma in Victoria (1998–2000): Retrospective cohort study. Med J
Aust. 184:270–273. 2006.PubMed/NCBI
|
|
14
|
Gutenberg A, Bock HC, Reifenberger G,
Brück W and Giese A: Toxicity and survival in primary glioblastoma
patients treated with concomitant plus adjuvant temozolomide versus
adjuvant temozolomide: Results of a single-institution,
retrospective, matched-pair analysis. Acta Neurochir (Wien).
155:429–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stummer W, Meinel T, Ewelt C, Martus P,
Jakobs O, Felsberg J and Reifenberger G: Prospective cohort study
of radiotherapy with concomitant and adjuvant temozolomide
chemotherapy for glioblastoma patients with no or minimal residual
enhancing tumor load after surgery. J Neurooncol. 108:89–97. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bucci MK, Maity A, Janss AJ, Belasco JB,
Fisher MJ, Tochner ZA, Rorke L, Sutton LN, Phillips PC and Shu HK:
Near complete surgical resection predicts a favorable outcome in
pediatric patients with nonbrainstem, malignant gliomas: Results
from a single center in the magnetic resonance imaging era. Cancer.
101:817–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Devaux BC, O'Fallon JR and Kelly PJ:
Resection, biopsy and survival in malignant glial neoplasms. A
retrospective study of clinical parameters, therapy and outcome. J
Neurosurg. 78:767–775. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kreth FW, Thon N, Simon M, Westphal M,
Schackert G, Nikkhah G, Hentschel B, Reifenberger G, Pietsch T,
Weller M, et al: Gross total but not incomplete resection of
glioblastoma prolongs survival in the era of radiochemotherapy. Ann
Oncol. 24:3117–3123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laws ER, Parney IF, Huang W, Anderson F,
Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, et
al: Survival following surgery and prognostic factors for recently
diagnosed malignant glioma: Data from the glioma outcomes project.
J Neurosurg. 99:467–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pichlmeier U, Bink A, Schackert G and
Stummer W: ALA Glioma Study Group: Resection and survival in
glioblastoma multiforme: An RTOG recursive partitioning analysis of
ALA study patients. Neuro Oncol. 10:1025–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simpson JR, Horton J, Scott C, Curran WJ,
Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO and Nelson
JS: Influence of location and extent of surgical resection on
survival of patients with glioblastoma multiforme: Results of three
consecutive radiation therapy oncology group (RTOG) clinical
trials. Int J Radiat Oncol Biol Phys. 26:239–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ: ALA-Glioma Study Group:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wood JR, Green SB and Shapiro WR: The
prognostic importance of tumor size in malignant gliomas: A
computed tomographic scan study by the brain tumor cooperative
group. J Clin Oncol. 6:338–343. 1988.PubMed/NCBI
|
|
25
|
Coffey RJ, Lunsford LD and Taylor FH:
Survival after stereotactic biopsy of malignant gliomas.
Neurosurgery. 22:465–473. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duncan GG, Goodman GB, Ludgate CM and
Rheaume DE: The treatment of adult supratentorial high grade
astrocytomas. J Neurooncol. 13:63–72. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quigley MR and Maroon JC: The relationship
between survival and the extent of the resection in patients with
supratentorial malignant gliomas. Neurosurgery. 29:385–388. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barker CA, Chang M, Chou JF, Zhang Z, Beal
K, Gutin PH and Iwamoto FM: Radiotherapy and concomitant
temozolomide may improve survival of elderly patients with
glioblastoma. J Neurooncol. 109:391–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ewelt C, Goeppert M, Rapp M, Steiger HJ,
Stummer W and Sabel M: Glioblastoma multiforme of the elderly: The
prognostic effect of resection on survival. J Neurooncol.
103:611–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwamoto FM, Cooper AR, Reiner AS, Nayak L
and Abrey LE: Glioblastoma in the elderly: The memorial
sloan-kettering cancer center experience (1997–2007). Cancer.
115:3758–3766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaichana KL, Chaichana KK, Olivi A,
Weingart JD, Bennett R, Brem H and Quiñones-Hinojosa A: Surgical
outcomes for older patients with glioblastoma multiforme:
Preoperative factors associated with decreased survival. Clinical
article. J Neurosurg. 114:587–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oszvald A, Güresir E, Setzer M, Vatter H,
Senft C, Seifert V and Franz K: Glioblastoma therapy in the elderly
and the importance of the extent of resection regardless of age. J
Neurosurg. 116:357–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Curran WJ Jr, Scott CB, Horton J, Nelson
JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO and
Krisch RE: Recursive partitioning analysis of prognostic factors in
three radiation therapy oncology group malignant glioma trials. J
Natl Cancer Inst. 85:704–710. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Intergroup Radiation Therapy Oncology
Group Trial 9402. Cairncross G, Berkey B, Shaw E, Jenkins R,
Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre
N, et al: Phase III trial of chemotherapy plus radiotherapy
compared with radiotherapy alone for pure and mixed anaplastic
oligodendroglioma: Intergroup radiation therapy oncology group
trial 9402. J Clin Oncol. 24:2707–2714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van den Bent MJ, Carpentier AF, Brandes
AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC,
Grisold W, Sipos L, et al: Adjuvant procarbazine, lomustine and
vincristine improves progression-free survival but not overall
survival in newly diagnosed anaplastic oligodendrogliomas and
oligoastrocytomas: A randomized European organisation for research
and treatment of cancer phase III trial. J Clin Oncol.
24:2715–2722. 2006. View Article : Google Scholar : PubMed/NCBI
|