Introduction
In the South West Oncology Group (SWOG9916) (n=770)
and TAX 327 (n=1,006) studies, the median overall survival (OS)
rate of patients treated with docetaxel (DOC)-based chemotherapy
for castration-resistant prostate cancer (CRPC) was 2–3 months
longer compared with the median OS of patients treated with
mitoxantrone and prednisone (MP therapy). Specifically, in the
SWOG9916 study, the OS with DOC-based chemotherapy was 17.5 months
vs. 15.6 months with MP therapy (P=0.02); and in the TAX 327 study,
the OS was 18.9 months with DOC every 3 weeks vs. 17.4 months with
weekly DOC-based chemotherapy vs. 16.5 months with mitoxantrone
(P=0.009) (1,2). Based on these reports, DOC-based
chemotherapy was considered as the standard first-line treatment
for patients with CRPC. Recently, molecular-targeted therapy for
CRPC, including abiraterone (3) and
enzalutamide (4), has been used for
CRPC prior to DOC-based chemotherapy. However, some patients may
have developed CRPC or show no response to new agents within a
short period (5,6). Thus, it may be meaningful to identify
such patients and introduce DOC-based chemotherapy in the early
phase of the disease.
Patients with CRPC may present with pain and
anorexia, anxiety, constipation, diarrhea, sleep disturbances,
mucositis, nausea, peripheral sensory neuropathy, rash, vomiting,
urinary symptoms and fatigue (7,8). DOC is
associated with the risk of worsening the patients' physical
status. Although standard-dose 3-weekly DOC (70–75
mg/m2) has been widely used for CRPC, it has been
associated with significant side effects. In previous studies,
hematological toxicity (grade ≥3 neutropenia) was found to be a
major side effect of standard-dose 3-weekly DOC in 32–93% of the
cases (2,9). Therefore, patients treated with this
regimen may have to withdraw from chemotherapy due to hematological
toxicity. In the TAX 327 study, low-grade non-hematological
toxicities occurred in at least 10% of the patients on
standard-dose 3-weekly DOC, including fatigue, nausea and/or
vomiting, alopecia and diarrhea. In the SWOG9916 study,
non-hematological toxicities occurred in the patients treated with
standard-dose 3-weekly DOC, compared with those treated with
mitoxantrone, including cardiovascular events, nausea and vomiting,
metabolic disturbances and neurological events. However, in
previous studies, low-dose weekly DOC displayed comparable
oncological effectiveness, with a lower rate of adverse events
(AEs) compared with standard-dose 3-weekly DOC (10,11).
Therefore, low-dose weekly DOC may be of value for the treatment of
patients with CRPC. The aim of the present study was to evaluate
the usefulness of low-dose weekly DOC for CRPC patients in terms of
oncological outcome, side effects and tolerability.
Patients and methods
Population
Patients who were treated with low-dose DOC between
September, 2007 and April, 2014 were included in the present study.
The eligibility criteria included histologically diagnosed
adenocarcinoma of the prostate and confirmed failure of prior
hormonal therapy. All the patients had an Eastern Cooperative Group
performance status of ≤2 prior to treatment.
Protocol of low-dose weekly DOC
treatment
Treatment was repeated every 28 days on an
outpatient basis. DOC 20 mg/m2 was administered on days
1, 8 and 15. Estramustine phosphate 280 mg and prednisolone 5 mg
were taken orally every day.
DOC chemotherapy on day 1 was administered in the
hospital for observation of AEs; after the second chemotherapy, it
was administered during outpatient visits.
Analysis of oncological outcome
The primary endpoint was prostate-specific antigen
(PSA) level decrease. The extent of decrease in the PSA levels of
the patients who received DOC chemotherapy was evaluated in the
present study. A PSA response was defined as a decrease from the
pretreatment serum concentration by 50%. PSA progression was
defined as an increase in the serum PSA from the pretreatment
level. To avoid early treatment discontinuation due to an increase
in PSA level caused by tumor flare during the first weeks of DOC
treatment, the first PSA assessment was performed 2 months after
treatment initiation.
Analysis of AEs and tolerability
AEs were classified according to the Common
Terminology Criteria for Adverse Events, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/About.html).
Treatment was discontinued due to disease progression or occurrence
of severe AEs (grade ≥4). Tolerability was evaluated using AEs and
laboratory tests. The patients underwent a laboratory
investigation, including a complete blood count and blood chemistry
tests, including PSA, at least every 4 weeks.
Statistical analysis
All the statistical analyses were performed using
IBM SPSS statistical software, version 19 (IBM SPSS, Armonk, NY,
USA). The Kaplan-Meier product-limit method with log-rank
comparisons was used to estimate survival distribution. The Cox's
proportional hazards model was used to assess the prognostic
significance of factors in the univariate and multivariate
analyses. P-values of <0.05 were considered to indicate
statistically significant differences.
Results
Patient characteristics
Between September, 2007 and April, 2014, 39 CRPC
patients were treated with low-dose weekly DOC chemotherapy in our
hospital. All the patients had developed antiandrogen syndrome and
received alternative antiandrogen therapy, and 29 patients (74%)
received ethinylestradiol. The patient characteristics are
summarized in Table I. The median age
at initiation of DOC was 71 years (range, 55–83 years) and the
median serum PSA level at initial diagnosis was 187 ng/ml (range,
2.0–1,711 ng/ml). The median number of DOC cycles was 7 (range,
1–45 cycles). The median Gleason score was unknown (n=4), 3+3
(n=3), 3+4 (n=6), 4+3 (n=4), 4+4 (n=5), 4+5 (n=9), 5+4 (n=5) or 5+5
(n=3). The median PSA level at the initiation of DOC treatment was
50.1 ng/ml (range, 0.01–1,710 ng/ml). There was no association
between the PSA levels at initiation of DOC treatment and PSA
response.
 | Table I.Patient characteristics (n=39). |
Table I.
Patient characteristics (n=39).
| Characteristics | Values, median
(range) |
|---|
| Age (years) | 71 (55–83) |
| PSA at diagnosis
(ng/ml) | 87 (2.0–1,711) |
| Gleason score at
diagnosis | 8 (5–10) |
| DOC cycles (n) | 7 (1–45) |
| Survival after DOC
(months) | 16.7 (1–45) |
Of the 39 patients, 13 (33%) responded to treatment
with a decrease to <50% of the PSA level. The median evaluation
period of the PSA response was 3 months (range, 1–13 months). There
was no response to chemotherapy in 9 patients (27%).
AEs
The grade ≥3 AEs are summarized in Table II. There was no significant
hematological toxity. Grade 3 hepatic enzyme increase, limb edema
and nausea were observed in 1 (3%), 2 (5%) and 2 (5%) patients,
respectively. One patient succumbed to interstitial pneumonia.
 | Table II.Adverse events of grade ≥3 in the 39
CRPC patients treated with weekly low-dose docetaxel
chemotherapy. |
Table II.
Adverse events of grade ≥3 in the 39
CRPC patients treated with weekly low-dose docetaxel
chemotherapy.
| Adverse
eventsa | Patients, n (%) |
|---|
| Hepatic enzyme
increase | 1 (3) |
| Interstitial
pneumonia | 1 (3) |
| Limb edema | 2 (5) |
| Nausea | 2 (5) |
Survival analysis
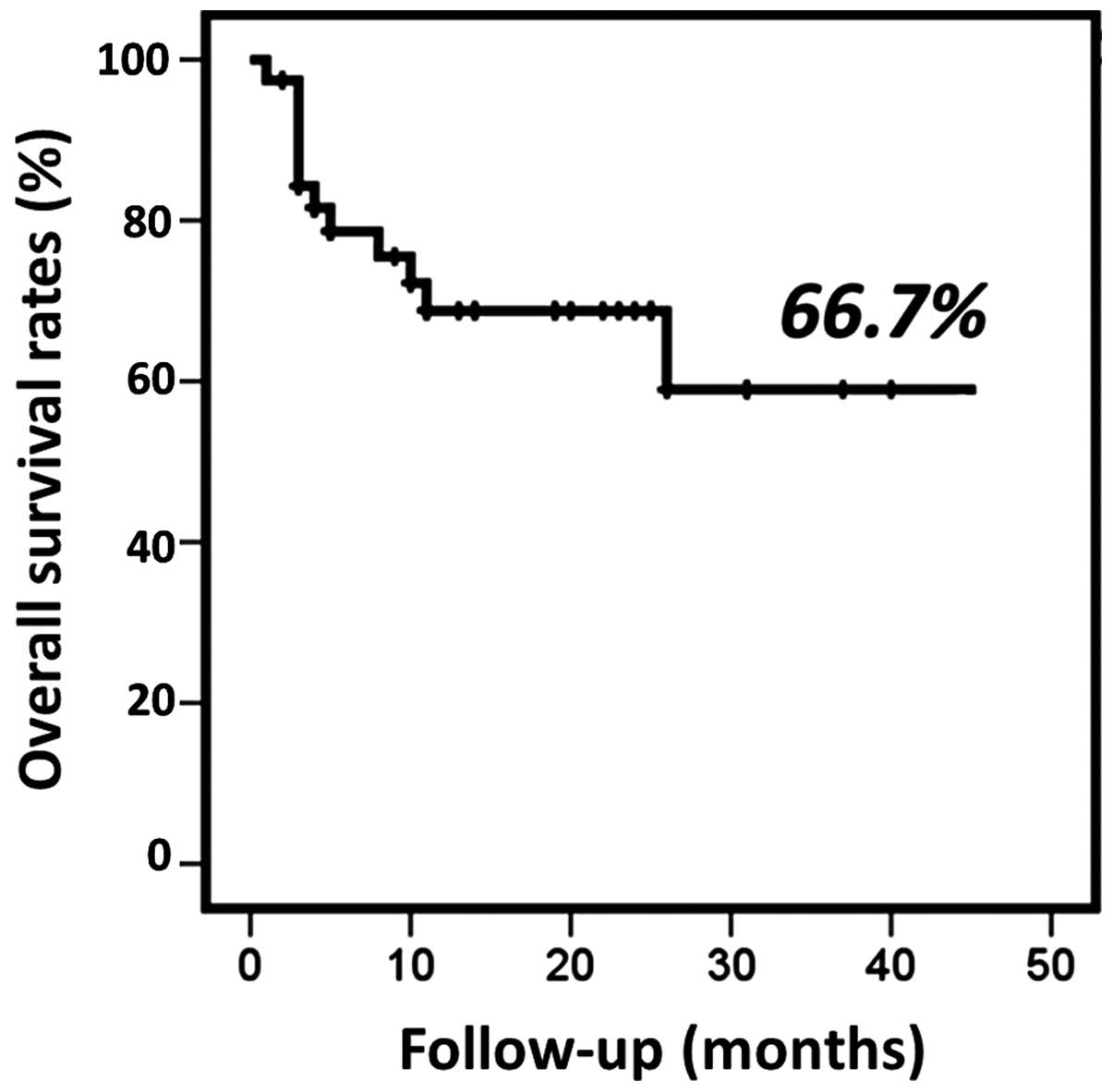
The OS after treatment ranged from 2 to 54 months
(median, 16.7 months) (Fig. 1). The
results of the uni- and multivariate analysis of predictors of OS
in the patient cohort are presented in Table III. In the univariate (P=0.019) as
well as the multivariate analysis (hazard ratio = 6.913; 95%
confidence interval: 1.147–41.669; P=0.035), the decrease of the
PSA level to <50% was a statistically significant factor
predictive of survival.
 | Table III.Univariate and multivariate analyses
of predictors of overall survival in CRPC patients treated with
low-dose DOC. |
Table III.
Univariate and multivariate analyses
of predictors of overall survival in CRPC patients treated with
low-dose DOC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | P-value | P-value | Hazard ratio (95%
confidence interval) |
|---|
| Clinical stage (I/II
vs. III/IV) | 0.9 | 0.7 |
|
| PSA level (≤20 vs.
>20 ng/ml) | 0.8 | 0.7 |
|
| Gleason score (6/7
vs. 8–10) | 0.9 | 0.7 |
|
| Change in PSA level
(<50 vs. ≥50%) |
0.019 |
0.035 | 6.913
(1.147–41.669) |
| PSA change within 3
months of DOC therapy (increase vs. decrease) | 0.2 | 0.8 |
|
| Time to initiation of
DOC therapy after PSA failure (≤12 vs. >12 months) | 0.4 | 0.3 |
|
Discussion
As of 2010, five new agents targeting CRPC,
including abiraterone (3),
enzalutamide (4), cabazitaxel,
sipuleucel-T and radium-223, have been approved by the US Food and
Drug Administration and the European Medicines Agency. These agents
may be preferable to chemotherapy in patients with asymptomatic
disease and in those with factors predisposing to poor torelance of
chemotherapy. However, for patients with rapidly progressing
disease or visceral metastases, or those with a poor response to
initial androgen-deprivation therapy (ADT), the use of chemotherapy
may be preferred. In the 2014 version of the European Association
of Urology guidelines, DOC may be the treatment of choice for all
patients with metastatic CRPC, excluding patients with with a
performance status >2 (12). In
the 2014 version of the American Society of Clinical Oncology
guidelines, Basch et al suggested DOC and prednisone should
be offered, but this type of therapy should be discussed with
patients at the time of decision-making in relation to the
apparently lower risk associated with other options and to the
patient's individual circumstances (13). Pre-DOC therapy against CRPC with
abiraterone acetate (14) and
enzalutamide (15) demonstrated
survival and quality-of-life benefits. DOC may exert its
pharmacological effect without androgen receptor (AR). If patients
with CRPC have developed early resistance to ADT, DOC should be
used early during the course of treatment. Houédé et al
(16) reported that patients with a
high Gleason score (8–10) tended to respond poorly to abiraterone.
Similarly, it was reported that patients with poorly differentiated
cancers (Gleason score 8–10) benefit the most from DOC use
(17). Therefore, DOC may be more
suitable for patients with tumors of high Gleason score,
particularly those with a durable response to initial ADT, who may
benefit more from androgen-targeted therapies. In the present
study, the percentage of patients with a Gleason score >8 was
63%.
Since the approval of DOC in 2007, DOC-based
chemotherapy has been widely administered to patients with CRPC.
However, the patients were often forced to decrease the dose or
suspend DOC due to AEs, particularly Asian patients (9). Kamiya et al (18) reported that the average body weight of
Asian individuals is relatively low, resulting in a higher
incidence of AEs. Only a limited number of studies have
investigated the efficacy and tolerability of low-dose DOC in
Asians (18–22). Nakai et al (20) reported that there was no significant
difference in median time-to-progression between the standard-dose
(60–75 mg/m2) and low-dose (20–30 mg/m2)
groups (10.0 vs. 7.1 months, respectively; P=0.09), whereas there
were significantly fewer grade 3–4 hematological toxicities in the
low-dose group compared with those in the standard-dose group (82.7
vs. 11.8%, respectively). Shimabukuro et al (21) compared low- (30–49 mg/m2),
medium- (50–69 mg/m2), and standard-dose (≥70
mg/m2) DOC and found no significant differences in
survival among the DOC dose groups (P=0.3018); however, the
incidence rate of grade 3 or 4 AEs associated with low-, medium-
and standard-dose DOC treatment was 21.9, 35.7 and 90.7%,
respectively. Low-dose DOC may be a viable treatment option for
CRPC patients who suffered from severe hematological AEs. In the
TAX 327 study, the frequency of AEs was compared between 3-weekly
and weekly DOC (1). Of note, the dose
intensity of DOC for both regimens was the same, but there were
significant differences in grade 3–4 neutropenia (32% with 3-weekly
vs. 2% with weekly DOC). This result suggests that dividing the
dosage of DOC into a weekly schedule may help reduce hematological
events. As mentioned above, hematological AEs were more frequent in
Asian CRPC patients treated with standard-dose DOC (9,22–24). The incidences of grade 3–4 neutropenia
and febrile neutropenia were significantly higher compared with
those in the TAX 327 study (93.0% with standard vs. 32% with
weekly; and 16.3% with standard vs. 3% with weekly, respectively)
(1,10). Furtheremore, several reports
demonstrated that a dose reduction due to hematological toxity was
required in 29.1–85.6% of Asian CRPC patients (9,22–24), suggesting a higher incidence of severe
hematological AEs in Asian populations treated with standard-dose
DOC.
We observed no grade 3–4 hematological AEs in our
cohort; however, 1 patient succumbed to interstitial pneumonia. The
causative association between DOC and interstitial pneumonia has
not been clearly explained. However, a sensitivity reaction to DOC
is considered to be the cause, as the interstitial pneumonia often
occurs after the second administration of DOC. Heidenreich et
al reported symptomatic and extensive metastasis and rapid
tolerance to first ADT as predictors of response to DOC in CRPC
patients (12). Several poor
prognostic factors have been described, such as visceral
metastases, pain, anemia (hemoglobin concentration <13 g/dl),
bone scan progression, and estramustine administration prior to
DOC. Patients were categorized into three risk groups and the
median OS from the introduction of DOC to patient death was 25.7
months for the low-risk (0–1 factors), 18.7 months for the
intermediate-risk (2 factors) and 12.8 months for the high-risk
(3–4 factors) groups (25).
The PSA decline ≥50% in our study is comparable with
previous studies (1,2). The rate of PSA decline by ≥50% in our
study was 41%; the rate of PSA decline by ≥50% in the TAX 327 and
SWOG9916 studies was 35 and 50%, respectively (1,2). In our
study, there was no association between the PSA levels at the
initiation of DOC treatment and PSA decline. However, the median
duration of survival in our study was inferior (11.0 months in this
study, 18.9 and 17.4 months with standard- and low-dose DOC,
respectively, in TAX 327, and 17.5 months in SWOG9916) (1,2). PSA
values were dichotomized as ≤20 or >20 ng/ml in the univariate
and multivariate analyses. PSA level was shown to be a potential
predictor of CSS (26) and most
prediction models define high-risk prostate carcinoma as a
presenting PSA level of >20 ng/ml (27), with high risk defined as the risk of
prostate cancer causing metastasis.
Our study has several limitations. First, this was a
non-randomized retrospective study; therefore, conclusions should
be interpreted with caution. Second, a sample size of 39 patients
is not sufficiently large, thereby decreasing the stastical power
of our results. Third, in the present study, 280 mg of estramustine
phosphate and 5 mg of prednisolone were used daily. The differences
in dose and duration of the administration of these drugs compared
with those in the previous studies may affect the outcomes.
Furthermore, the dosage of DOC differed between studies. Finally,
we did not perform a comparison with patients treated with 3-weekly
standard-dose DOC.
In conclusion, weekly low-dose DOC chemotherapy is a
viable treatment option for patients with CRPC who are unable to
tolerate the standard-dose regimen. Further studies are required to
evaluate the usefulness of low-dose weekly DOC.
References
|
1
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer. N Engl J
Med. 351:1502–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fizazi K, Scher HI, Molina A, Logothetis
CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F,
et al: Abiraterone acetate for treatment of metastic
castration-resistant prostate cancer: Final overall survival
analysis of the COU-AA-301 randomized, double-blind,
placebo-controlled phase 3 study. Lancet Oncol. 13:983–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harland S, Staffurth J, Molina A, Hao Y,
Gagnon DD, Sternberg CN, Cella D, Fizazi K, Logothetis CJ, Kheoh T,
et al: Effect of abiraterone acetate treatment on the quality of
life of patients with metastatic castration-resistant prostate
cancer after failure of docetaxel chemotherapy. Eur J Cancer.
49:3648–3657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francini E, Petrioli R and Roveiello G: No
clear evidence of a sequential therapy regimen with abiraterone
acetate and enzalutamide. Expert Rev Anticancer Ther. 14:1135–1140.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scher HI, Morris MJ, Basch E and Heller G:
End points and outcomes in castration-resistant prostate cancer:
From clinical trials to clinical practice. J Clin Oncol.
29:3695–3704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sternberg CN, Molina A, North S,
Mainwaring P, Fizazi K, Hao Y, Rothman M, Gagnon DD, Kheoh T, Haqq
CM, et al: Effect of abiraterone acetate on fatigue in patients
with metastatic castration-resistant prostate cancer after
docetaxel chemotherapy. Ann Oncol. 24:1017–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naito S, Tsukamoto T, Koga H, Harabayashi
T, Sumiyoshi Y, Hoshi S and Akaza H: Docetaxel plus prednisolone
for the treatment of metastatic hormone-refractory prostate cancer:
A multicenter phase II trial in Japan. Jpn J Clin Oncol.
38:365–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horgan AM, Seruga B, Pond GR, Alibhai SM,
Amir E, De Wit R, Eisenberger MA and Tannock IF: Tolerability and
efficacy of docetaxel in older men with metastatic
castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J
Geriatr Oncol. 5:119–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Italiano A, Ortholan C, Oudard S, Pouessel
D, Gravis G, Beuzeboc P, Bompas E, Fléchon A, Joly F, Ferrero JM
and Fizazi K: Docetaxel-based chemotherapy in elderly petients (age
75 and older) with castration-resistant prostate cancer. Eur Urol.
55:1368–1375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basch E, Loblaw DA, Oliver TK, Carducci M,
Chen RC, Frame JN, Garrels K, Hotte S, Kattan MW, Raghavan D, et
al: Systemic therapy in men with metastatic castration-resistant
prostate cancer: American society of clinical oncology and cancer
care ontario clinical practice guideline. J Clin Oncol.
32:3463–3448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rathkopf DE, Smith MR, de Bono JS,
Logothetis CJ, Shore ND, de Souza P, Fizazi K, Mulders PF,
Mainwaring P, Hainsworth JD, et al: Update interim efficacy
analysis and long-term safety of abiraterone acetate in metastatic
castration-resistant prostate cancer patients without prior
chemotherapy (COU-AA-302). Eur Urol. 66:815–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: PREVAIL Investigators: Enzalutamide in
metastatic prostate cancer before chemotherapy. N Engl J Med.
371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Houédé N, Beuzeboc P, Gourgou S, Tosi D,
Moise L, Gravis G, Delva R, Fléchon A, Latorzeff I, Ferrero JM, et
al: Abiraterone acetate in patients with metastatic
castration-resistant prostate cancer: Long term outcome of the
temporary authorization for use programme in France. BMC Cancer.
15:2222015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Soest RJ, de Morrée ES, Shen L,
Tannock IF, Eisenberger MA and de Wit R: Initial biopsy gleason
score as a predictive marker for survival benefit in patients with
castration-resistant prostate cancer treated with docetaxel: Data
from the TAX327 study. Eur Urol. 66:330–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamiya N, Suzuki H, Ueda T, Sato N,
Nakatsu H, Mikami K, Sato N, Nomura K, Akakura K, Okano T, et al:
Clinical outcomes by relative docetaxel dose and dose intensity as
chemotherapy for Japanese patients with castration-resistant
prostate cancer: A retrospective multi-institutional collaborative
study. Int J Clin Oncol. 19:157–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kita Y, Shimizu Y, Inoue T, Kamba T,
Yoshimura K and Ogawa O: Reduced-dose docetaxel for
castration-resistant prostate cancer has no inferior impact on
overall survival in Japanese patients. Int J Clin Oncol.
18:718–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakai Y, Nishimura K, Nakayama M, Uemura
M, Takayama H, Nonomura N and Tsujimura A: Osaka CRPC Clinical
Study Collaboration: Weekly, low-dose docetaxel combined with
estramustine for Japanese castration-resistant prostate cancer: Its
efficacy and safety profile compared with tri-weekly standard-dose
treatment. Int J Clin Oncol. 19:165–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimabukuro T, Sakano S, Matsuda K,
Kamiryo Y, Yamamoto N, Kaneda Y, Nasu T, Baba Y, Suga A, Yamamoto
M, et al: Can docetaxel therapy improve overall survival from
primary therapy compared with androgen-deprivation therapy alone in
Japanese patients with castration-resistant prostate cancer? A
multi-institutional cooperative study. Int J Clin Oncol. 18:62–67.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyake H, Sakai I, Terakawa T, Harada K
and Fujisawa M: Oncological outcome of docetaxel-based chemotherapy
for Japanese men with metastatic castration-resistant prostate
cancer. Urol Oncol. 31:733–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagami Y, Ohori M, Sakamoto N, Koga S,
Hamada R, Hatano T and Tachibana M: Safety and efficacy of
docetaxel, estramustine phosphate and hydrocortisone in
hormone-refractory prostate cancer patients. Int J Urol.
17:629–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miura N, Numata K, Kusuhara Y, Shirato A,
Hashine K and Sumiyoshi Y: Docetaxel-prednisolone combination
therapy for Japanese patients with hormone-refractory prostate
cancer: A single institution experience. Jpn J Clin Oncol.
40:1092–1098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong AJ, Garrett-Mayer E, de Wit R,
Tannock I and Eisenberger M: Prediction of survival following
first-line chemotherapy in men with castration-resistant metastatic
prostate cancer. Clin Cancer Res. 16:203–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Amico AV, Cote K, Loffredo M, Renshaw AA
and Schultz D: Determinations of prostate cancer-specific survival
after radiation therapy for patients with clinically localized
prostate cancer. J Clin Oncol. 20:4567–4573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crawford ED, Bennett CL, Andriole GL,
Garnick MB and Petrylak DP: The utility of prostate-specific
antigen in the management of advanced prostate cancer. BJU Int.
112:548–560. 2013. View Article : Google Scholar : PubMed/NCBI
|