Introduction
The prognosis of multiple myeloma (MM) has been
significantly improved by the recent introduction of several novel
agents, such as thalidomide (THAL), lenalidomide (LEN) and
bortezomib (BOR) (1). The use of
these agents, particularly in younger patients, prior to or
following autologous peripheral stem cell transplantation
(auto-PBSCT), may result in improved transplant outcomes, with a
higher very good partial response (VGPR) or complete response (CR)
rate, which may eventually translate into prolonged
progression-free survival (PFS) and/or overall survival (OS)
(2,3).
Regarding maintenance therapy, the results of several recent
studies have suggested use of these agents as a promising option
for preventing relapse or disease progression after auto-PBSCT
(4–7).
However, THAL may not be suitable as a long-term maintenance agent,
mainly due to its neurotoxic side effect profile (4,5).
Conversely, LEN is less toxic and may be more
feasible for use in a maintenance setting, as the results from
several studies have demonstrated its effects in terms of improved
outcomes and prolonged PFS after auto-PBSCT.
The results of a recent randomized, prospective
trial support the rationale for the use of LEN after auto-PBSCT
(7). Based on the aforementioned
results, more patients are currently undergoing LEN maintenance
therapy following auto-PBSCT; however, the recommended dose of LEN
for maintenance has yet to be firmly established. Currently, LEN is
administered at 5–15 mg/day for maintenance. We herein report the
results of a multicenter clinical study for determining the
feasible initial dose (FID) of LEN for maintenance therapy in
patients with MM following auto-PBSCT.
Patients and methods
Study participants
Patients with MM, aged 15–70 years, who achieved
VGPR or a better response after auto-PBSCT, were enrolled in this
trial. The main inclusion criteria were a good performance status
(0–2), an absence of renal dysfunction as assessed by creatinine
clearance >60 ml/min, an absence of deep vein thrombosis or
pulmonary thromboembolism, and recovery from myelosuppression
following auto-PBSCT. Female patients were either postmenopausal,
surgically sterilized, or willing to use an acceptable method of
birth control for the entire duration of the study. Likewise, male
patients consented to using an acceptable method of contraception
for the entire duration of the study. The main exclusion criteria
included grade ≥2 peripheral neuropathy, active infection, positive
serology for human immunodeficiency virus or hepatitis B or C
viruses, pregnancy and breast-feeding. This trial was approved by
the institutional ethics committee at each participating
institution and registered as UMIN000012700. Written informed
consent was obtained from all the patients prior to registration in
accordance with the Declaration of Helsinki.
Treatment schedule
The patients required LEN maintenance therapy within
6 months of undergoing auto-PBSCT. The three selected dose levels
of LEN (level 0, 5 mg; level 1, 10 mg; and level 2, 15 mg) were
determined based on previously published data (6–8). The
assigned dose of LEN was administered orally once per day for 12
weeks, without any drug holidays. The patients were able to
continue receiving LEN during the scheduled period, unless a
dose-limiting toxicity (DLT) occurred, obvious evidence of disease
progression appeared, or the patient refused to continue
treatment.
Assessment of treatment toxicity and
efficacy
According to the National Cancer Institute Common
Toxicity Criteria version 4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40),
any hematological or non-hematological adverse event (AE) of grade
≥3, or discontinuation of drug administration due to any grade of
AEs directly attributable to LEN, were considered as DLTs. The
assessment of disease response was based on the International
Uniform Response Criteria (9).
Study design and statistical
analysis
The FID was estimated using the continual
reassessment method (CRM) (10,11). Dose
escalations or de-escalations for consecutive patient cohorts and
the size of each cohort were based on the dose-finding CRM
algorithm and clinical judgment. Jumping from level 0 to 2 was not
permissible in the CRM algorithm. The target completion rate of
maintenance therapy was set at 70%. The FID of this trial was
determined as the dose that was closest to the level at which 70%
of patients would complete 12 weeks of maintenance therapy without
experiencing a severe AE. We determined the starting dose in this
trial to be level 1, and the first 3 patients were treated at this
level. According to the pre-specified dose de-escalation rule, if
DLT was observed in 2 of the first 3 patients, another 3 patients
allocated to the second cohort were treated at level 0. This phase
I study was expected to require a project sample-size of ≤15
patients.
Results
Patient characteristics
Between August, 2011 and April, 2014, a total of 11
patients (8 in CR and 3 in VGPR) from five transplant centers in
Japan were enrolled in this study, with a median of 99 days (range,
60–168 days) after auto-PBSCT. Using the CRM algorithm, 6 patients
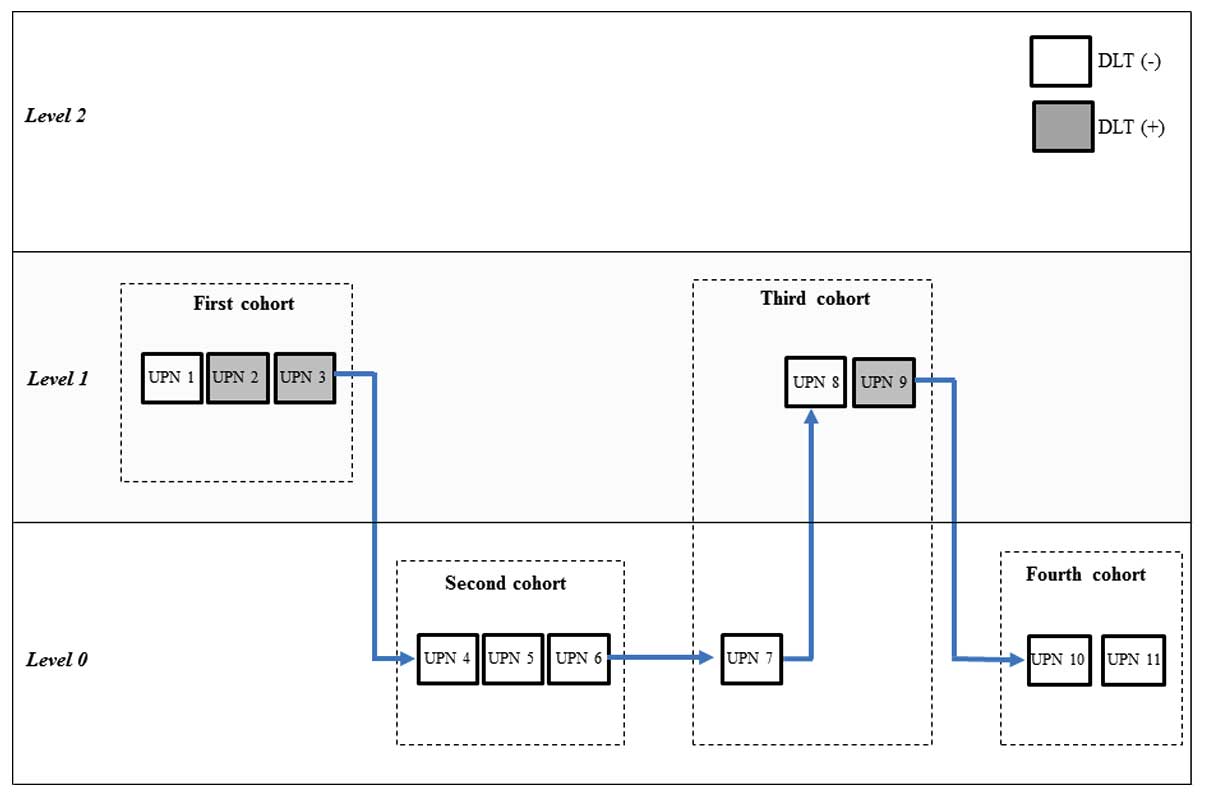
were assigned to level 0 and 5 were assigned to level 1 (Fig. 1). the characteristics of these 11
patients are summarized in Table I.
The median age was 58 years (range, 41–65 years), and 7 patients
were men (64%). The subtypes of monoclonal immunoglobulin (Ig)
detected in the 11 patients were IgG (n=4), IgA (n=4), IgD (n=1)
and light-chain-only disease (n=2). All the patients received a
BOR-containing induction regimen prior to auto-PBSCT; however, no
patients received prior THAL.
 | Table I.Characteristics of the patients who
received maintenance therapy with two dose levels of
lenalidomide. |
Table I.
Characteristics of the patients who
received maintenance therapy with two dose levels of
lenalidomide.
| Characteristics | Level 0 (5 mg)
n=6 | Level 1 (10 mg)
n=5 |
|---|
| Gender
(male/female) | 5/1 | 2/3 |
| Median age, years
(range) | 59 (41–65) | 57 (46–63) |
| Performance
status |
|
|
| 0–1 | 3 | 5 |
| 2 | 3 | 0 |
| Types of
immunoglobulin |
|
|
| IgG | 3 | 1 |
| IgA | 2 | 2 |
| IgD | 0 | 1 |
| BJP | 1 | 1 |
| International staging
system |
|
|
| Stage
I | 2 | 0 |
| Stage
II | 3 | 3 |
| Stage
III | 1 | 2 |
| Durie-salmon staging
system |
|
|
| Stage
I | 1 | 0 |
| Stage
II | 1 | 1 |
| Stage
III | 4 | 4 |
|
Durie-Salmon
sub-classifications (A/B) | 0/6 | 4/1 |
| Symptoms related to
myeloma |
|
|
|
Hypercalcemia | 2 | 4 |
| Renal
insufficiency | 0 | 2 |
|
Anemia | 5 | 4 |
| Bone
disease | 4 | 4 |
| Initial therapy |
|
|
|
VAD-BD | 2 | 2 |
| BD | 2 | 3 |
| VCD | 2 | 0 |
| Best response after
auto-PBSCT |
|
|
|
CRa | 4 | 4 |
|
VGPRb | 2 | 1 |
| Median duration from
auto-PBSCT to maintenance therapy (range) | 95 days (60–121) | 99 days (68–168) |
Toxicity
The time-dependent changes in hematological and
non-hematological AEs observed during maintenance therapy with LEN
are detailed in Table II. Of the 6
patients assigned to level 0, 3 (50%) experienced neutropenia
(grade ≤2) at approximately 8 weeks after initiating LEN
maintenance therapy; however, all the patients spontaneously
recovered from neutropenia despite continuous administration of
LEN. As for the remaining patients, no serious AEs (SAEs) were
observed at level 0. As shown in Table
II, more patients experienced AEs at level 1. One patient
[unique patient number (UPN) 3]developed pneumonia as a consequence
of neutropenia in week 10 of treatment; therefore, LEN
administration was discontinued at 11 weeks. Another patient (UPN
2) developed both neutropenia and thrombocytopenia in addition to
peripheral neuropathy in week 4 of treatment; although this
patient's dose was reduced to 5 mg (level 0), thrombocytopenia
progressed and LEN administration was discontinued soon after.
Another patient (UPN 9) developed liver dysfunction in week 2 of
the treatment, which had progressed to grade 2 by week 6.
Administration of LEN was discontinued for this patient as well.
Grade 3/4 toxicities were not observed in any of the study
patients.
 | Table II.Time-dependent changes of adverse
events observed during lenalidomide maintenance therapy. |
Table II.
Time-dependent changes of adverse
events observed during lenalidomide maintenance therapy.
|
|
|
| Time-dependent
changes, grade (CTCAE version 4.0) |
|---|
|
|
|
|
|
|---|
| Dose level | UPN | Adverse events | Before | 2 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|
| Level 0 (5 mg) | 4 | Liver
dysfunction | 1 | 1 | 1 | 1 | 1 |
|
|
| Thrombocytopenia | 1 | 1 | 1 | 1 | 1 |
|
|
| Hypokalemia | 1 | 1 | 1 | 1 | 1 |
|
|
| Hyperglycemia | – | – | – | – | 1 |
|
| 5 | Neutropenia | – | – | 1 | 2 | – |
|
| 6 | Thrombocytopenia | – | – | – | – | 1 |
|
| 7 | Constipation | – | 1 | – | – | – |
|
|
| Muscle
weakness | – | – | 1 | – | – |
|
|
| Neutropenia | – | – | – | 2 | – |
|
|
| URTI | – | – | – | 2 | – |
|
| 10 | No adverse
events | – | – | – | – | – |
|
| 11 |
Thrombocytopenia | 1 | – | – | – | – |
|
|
| Skin rash | – | 1 | 1 | 1 | 1 |
|
|
| Neutropenia | – | – | – | 2 | – |
| Level 1 (10
mg) | 1 | Diarrhea | – | – | – | 1 | 1 |
|
|
| Hypothyroidism | – | – | – | – | 1 |
|
|
| URTI | – | – | – | – | 1 |
|
|
| Peripheral
neuropathy | – | – | – | – | 1 |
|
| 2a |
Thrombocytopenia | 1 | 1 | 1 | 2 (at 6 weeks) | N/A |
|
|
| Peripheral
neuropathy | – | 1 | 1 | 1 (at 6 weeks) | N/A |
|
|
| Neutropenia | – | – | 2 | 2 (at 6 weeks) | N/A |
|
| 3b | Neutropenia | – | – | – | – | 2 (at 10
weeks) |
|
|
| Pneumonia | – | – | – | – | 2 (at 10
weeks) |
|
| 8 | Neutropenia | – | – | – | – | 1 |
|
|
|
Thrombocytopenia | – | – | – | – | 1 |
|
| 9c | Liver
dysfunction | – | 1 | 1 | 2 (at 6 weeks) | N/A |
Dose escalation and de-escalation
The dose-finding CRM algorithm and clinical judgment
used in this study are shown in Fig.
1. of the first 3 patients assigned to level 1, 2 patients (UPN
2 and 3) encountered DLT [discontinuation of LEN with the attending
physician's judgment based on progressive grade 2 hematological
(n=1) and non-hematological (n=1) AEs]. According to the
pre-specified dose de-escalation rules for this trial, the dose
level for the second patient cohort was de-escalated to level 0.
However, none of the 3 patients (UPN 4, 5 and 6) at level 0 in the
second cohort experienced any DLTs. In the third cohort, 1 patient
(UPN 7) remained assigned to level 0, but did not experience any
DLTs. Therefore, the next 2 patients (UPN 8 and 9) were assigned to
level 1 in the third cohort, although 1 (UPN 9) of the 2 patients
again experienced a DLT (discontinuation of LEN administration
occurred with the attending physician's judgment based on a
progressive grade 2 non-hematological AE). Consequently, the
estimated level 1 completion rate was considerably lower compared
with the target level, and the next patient cohort was treated at
level 0. Of the subsequent 2 patients (UPN 10 and 11) treated at
level 0, as the fourth cohort, neither experienced DLTs. Based on
the results of the sensitivity analysis and the observed toxicities
at each dose level, we concluded that LEN maintenance therapy at
level 0 was acceptable in terms of safety.
Response to LEN maintenance
The disease status prior to and following LEN
maintenance therapy is indicated in Table III. The results from the 8
assessable patients who completed 12 weeks of LEN maintenance
therapy revealed no disease progression or obvious response. The 2
patients with VGPR maintained that status and did not achieve CR.
The 6 patients with CR also maintained stable disease conditions
during LEN maintenance therapy.
 | Table III.Disease status of myeloma before and
after lenalidomide maintenance therapy. |
Table III.
Disease status of myeloma before and
after lenalidomide maintenance therapy.
|
|
| Disease status |
|---|
|
|
|
|
|---|
| Dose level | UPN | Before starting
maintenance | At 12 weeks of
maintenance |
|---|
| Level 0 (5 mg) | 4 | CRa | CR |
|
| 5 | VGPRb | VGPR |
|
| 6 | CR | CR |
|
| 7 | CR | CR |
|
| 10 | CR | CR |
|
| 11 | VGPR | VGPR |
| Level 1 (10
mg) | 1 | CR | CR |
|
| 2 | CR | N/A because of
discontinuation at 6 weeks |
|
| 3 | VGPR | N/A because of
discontinuation at 10 weeks |
|
| 8 | CR | CR |
|
| 9 | CR | N/A because of
discontinuation at 6 weeks |
Discussion
Accumulated clinical experience has provided
convincing evidence that novel agents, such as THAL, LEN and BOR,
alone or in combination, are efficacious in all stages of MM
(12). Moreover, monotherapy with
these agents in a maintenance setting may be a promising option for
preventing relapse or disease progression after auto-PBSCT
(13). However, THAL maintenance has
not received general acceptance due to serious concerns regarding
its neurotoxic side effects (4,5,14,15). Based
on the results from recent reports, the incidence of grade 3–4
peripheral neuropathy was ≤27%, which was the main reason for
discontinuing THAL maintenance (4,5,14,15). It
should be noted that lower doses of BOR may be used with greater
tolerability and therapeutic benefits in a maintenance setting. In
the HOVON-65/GMM-HD4 study, BOR maintenance achieved improved PFS
as compared with THAL; however, it appeared to be less beneficial
for patients who achieved at least VGPR after auto-PBSCT (16). As maintenance therapy, LEN appeared to
be the most promising of the three aforementioned agents. Based on
the results from two recent randomized trials (LEN maintenance vs.
no maintenance), LEN appeared to be associated with improved
time-to-disease progression in both studies (6,7). Moreover,
the beneficial effect of LEN maintenance was relevant even in
patients who achieved VGPR/CR after auto-PBSCT in the IFM 05–02
trial (7). Although LEN appeared to
be the most promising maintenance agent among the three mentioned,
the dosage or duration of LEN maintenance therapy had not been
formally determined in a phase I dose escalation trial. Attal et
al evaluated LEN for maintenance therapy following auto-PBSCT
(administered at 10 mg per day for the first 3 months and increased
up to 15 mg if tolerated), and found that PFS improved from 35% in
the placebo arm to 68% in the LEN arm. Unfortunately, approximately
half of the patients in the LEN arm experienced grade 3–4
myelosuppression during the entire maintenance phase (7). Palumbo et al also investigated
the use of LEN after auto-PBSCT (17); in their trial, LEN maintenance therapy
was administered at 10 mg for 21 days per 28-day period, and the
incidence of grade 3–4 myelosuppression decreased to 23% (17).
To the best of our knowledge, this is the first
study to evaluate the maintenance dose of LEN following auto-PBSCT.
Although no grade 3–4 SAEs were observed in any cohort in our
series, 3 patients (60%) assigned to the level 1 dose (10 mg of
LEN) experienced progressive grade 2 SAEs and eventually dropped
out. As shown in Table II, all 6
patients assigned to level 0 successfully completed at least 12
weeks of continuous administration of 5 mg of LEN without any SAEs.
Thus, continuous administration of LEN at 5 mg may be acceptable in
terms of safety, although that particular dose may be suboptimal
considering the completion rate in our cohorts (i.e., 100% in the
5-mg cohort and 40% in the 10-mg cohort). Therefore, it may be
hypothesized that continuous administration of 7.5 mg of LEN may be
the next best dose; however, administration of 10 mg of LEN for 21
out of every 28 days may represent a more realistic approach, as a
2.5-mg capsule form is not currently commercially available, and
prolonged administration without drug holidays may raise concerns
regarding the development of unexpected AEs. Although the number of
patients in this study was inadequate for determining the response
to therapy, none of the patients exhibited any evidence of disease
progression or relapse while on LEN maintenance therapy.
In conclusion, our small study demonstrated that 5
mg of LEN was acceptable in terms of safety, although maintenance
therapy with 10 mg of LEN administration for 21 out of every 28
days may be a more practical strategy for patients with MM
following auto-PBSCT.
Acknowledgements
We would like to thank the nursing staff in all the
participating transplant centers for their assistance in the
collection of samples from patients included in this study. We are
also grateful to the staff in all the participating transplant
centers for their excellent patient care.
References
|
1
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Gondos A and Pulte D: Recent
major improvement in long-term survival of younger patients with
multiple myeloma. Blood. 111:2521–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alexanian R, Delasalle K, Wang M, Thomas S
and Weber D: Curability of multiple myeloma. Bone Marrow Res.
2012:9164792012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Attal M, Harousseau JL, Leyvraz S, Doyen
C, Hulin C, Benboubker L, Agha Yakoub I, Bourhis JH, Garderet L,
Pegourie B, et al: Maintenance therapy with thalidomide improves
survival in patients with multiple myeloma. Blood. 108:3289–3294.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgan GJ, Gregory WM, Davies FE, Bell SE,
Szubert AJ, Brown JM, Coy NN, Cook G, Russell NH, Rudin C, et al:
The role of maintenance thalidomide therapy in multiple myeloma:
MRC Myeloma IX results and meta-analysis. Blood. 119:7–15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCarthy PL, Owzar K, Hofmeister CC, Hurd
DD, Hassoun H, Richardson PG, Giralt S, Stadtmauer EA, Weisdorf DJ,
Vij R, et al: Lenalidomide after stem-cell transplantation for
multiple myeloma. N Engl J Med. 366:1770–1781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Attal M, Lauwers-Cances V, Marit G,
Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L,
Garderet L, et al: Lenalidomide maintenance after stem-cell
transplantation for multiple myeloma. N Engl J Med. 366:1782–1791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richardson PG, Schlossman RL, Weller E,
Hideshima T, Mitsiades C, Davies F, LeBlanc R, Catley LP, Doss D,
Kelly K, et al: Immunomodulatory drug CC-5013 overcomes drug
resistance and is well tolerated in patients with relapsed multiple
myeloma. Blood. 100:3063–3067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durie BG, Harousseau JL, Miguel JS, Bladé
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, et al: International uniform response criteria for
multiple myeloma. Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Quigley J, Pepe M and Fisher L:
Continual reassessment method: A practical design for phase 1
clinical trials in cancer. Biometrics. 46:33–48. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishizuka N and Morita S: Practical
implementation of the continual reassessment method. Handbook of
Statistics in Clinical Oncology (2nd). 2006.
|
|
12
|
Palumbo A, Mina R, Cerrato C and Cavallo
F: Role of consolidation/maintenance therapy in multiple myeloma.
Clin Lymphoma Myeloma Leuk. 13(Suppl 2): S349–S354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abidi MH, Gul Z, Abrams J, Ayash L, Deol
A, Ventimiglia M, Lum L, Mellon-Reppen S, Al-Kadhimi Z,
Ratanatharathorn V, et al: Phase I trial of bortezomib during
maintenance phase after high dose melphalan and autologous stem
cell transplantation in patients with multiple myeloma. J
Chemother. 24:167–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spencer A, Prince HM, Roberts AW, Prosser
IW, Bradstock KF, Coyle L, Gill DS, Horvath N, Reynolds J and
Kennedy N: Consolidation therapy with low-dose thalidomide and
prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure.
J Clin Oncol. 27:1788–1793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lokhorst HM, van der Holt B, Zweegman S,
Vellenga E, Croockewit S, van Oers MH, von dem Borne P, Wijermans
P, Schaafsma R, de Weerdt O, et al: A randomized phase 3 study on
the effect of thalidomide combined with adriamycin, dexamethasone,
and high-dose melphalan, followed by thalidomide maintenance in
patients with multiple myeloma. Blood. 115:1113–1120. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sonneveld P, Schmidt-Wolf IG, van der Holt
B, El Jarari L, Bertsch U, Salwender H, Zweegman S, Vellenga E,
Broyl A, Blau IW, et al: Bortezomib induction and maintenance
treatment in patients with newly diagnosed multiple myeloma:
Results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin
Oncol. 30:2946–2955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palumbo A, Cavallo F, Gay F, Di Raimondo
F, Ben Yehuda D, Petrucci MT, Pezzatti S, Caravita T, Cerrato C,
Ribakovsky E, et al: Autologous transplantation and maintenance
therapy in multiple myeloma. N Engl J Med. 371:895–905. 2014.
View Article : Google Scholar : PubMed/NCBI
|