Introduction
Tuberculoma is considered a benign disease, and
accounts for 5–24% of all solitary pulmonary nodules (SPNs)
(1). Of all cases of tuberculoma,
77.3% are asymptomatic and only detected during routine health
checkups. Tuberculoma assumes the form of SPN in 77–85% of all
cases, and two or more nodules or satellite lesions are present in
15–22% of total cases. The nodules are commonly located in the
upper lobes of the lungs and frequently measure 2–5 cm in diameter
(2,3).
The likelihood of a malignant in the SPN is known to
increase with the size of it. For SPNs measuring <5 mm in
diameter, the probability of malignancy is 0–1%, whereas with an
increase in the diameter to 11–12 mm, the probability of the tumor
becoming malignant increases to 33–64%. With a further increase in
the diameter to 20 mm, the likelihood of malignancy increases to
64–82% (4). The efficacy of positron
emission tomography (PET) in the management of cancer has been
reported previously (4,5). However, a distinction between
tuberculoma and lung cancer is extremely difficult to make, even
with the use of PET (5).
Desensitization therapy for the treatment of
tuberculoma has rarely been reported previously. The present study
reports a case of desensitization therapy in the treatment of a
patient with solitary pulmonary tuberculoma, and provides reference
to the current literature. The solitary pulmonary tuberculoma was
diagnosed by video-assisted thoracoscopic surgery (VATS) and
treatment with antituberculous drugs resulted in drug-induced
hepatotoxicity (DIH). Subsequently, desensitization therapy with
quinolones and other therapeutic agents proved effective in
treating the patient's tuberculoma.
Case report
The patient was a non-smoking 31-year-old female who
does not consume alcohol and had no history of health problems.
However, during a routine health checkup on November 26th 2013, an
intramural nodule (2.5 cm in diameter) in the S10 area of the left
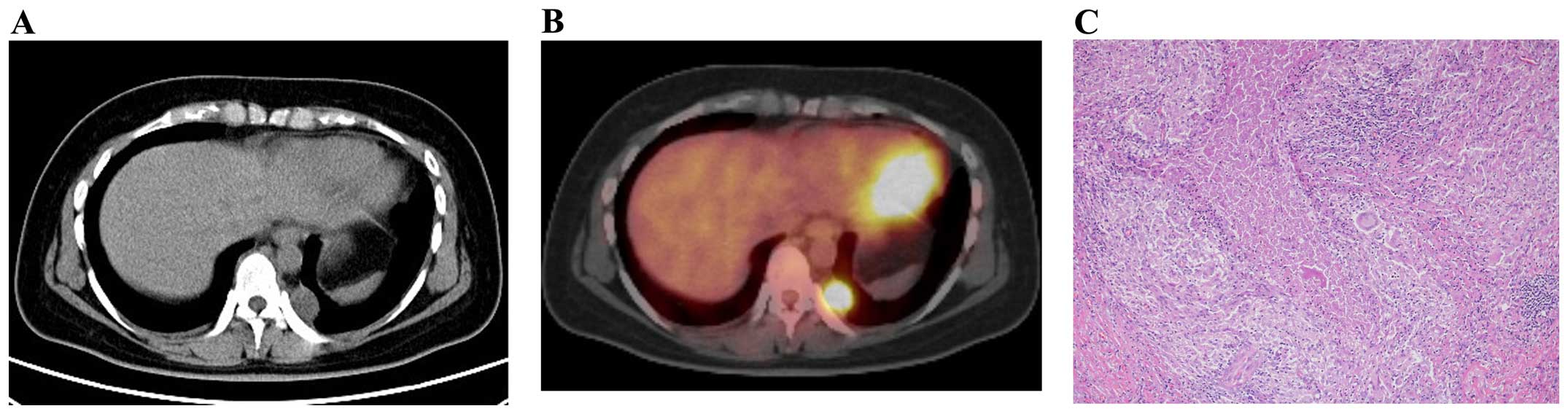
lung was identified. The patient was asymptomatic and PET-computed
tomography (CT) revealed accumulation (standardized uptake value of
4.5) in the lesion (Figs. 1A and 1B).
Therefore, the patient was suspected to have lung cancer and VATS
was performed on December 10th 2013. Histopathological examination
of the resected specimen revealed the presence of epithelioid
granulomas, accompanied by caseous necrosis in the lesion (Fig. 1C). The tissue specimen was negative on
Ziehl-Neelsen staining, however, culture for Mycobacterium
tuberculosis was positive (M. tuberculosis
antigen-positive), which led to a final diagnosis of tuberculoma.
Anti-M. tuberculosis treatment [isoniazid (INH) + rifampicin
(RFP) + ethambutol (EB) + pyrazinamide (PZA)] commenced on January
13th 2014; however, the patient developed hepatic dysfunction
[aspartate transaminase/alanine transaminase (AST/ALT): 506/867] on
February 1st 2014 (Table I), which
necessitated the suspension of the treatment. The medications were
resumed (EB + PZA) following improvement of the hepatic function;
however, the hepatic dysfunction (AST/ALT: 455/928) relapsed on
March 12th 2014 (two weeks following the resumption) and the
treatment was terminated. During this period, tests for hepatitis
virus markers and human immunodeficiency virus were negative. Based
on the hypothesis that the patient may have an allergy to
antituberculous drugs, the drug-induced lymphocyte stimulation test
(DLST) was performed for the four antituberculous drugs; however,
the data were within the criterion range for each of the drugs. Two
months elapsed before the patient's hepatic function was recovered.
Although the patient tested negative for DLST, a diagnosis of
drug-induced hepatotoxicity (DIH) due to the antituberculous drugs
was made on the basis of the clinical course. Standard treatment
with antituberculous drugs was considered to be associated with
risks; therefore, reductive-sensitizing therapy with
antituberculous drugs was performed. On May 12th 2014, the
treatment with EB + PZA was changed to levofloxacin (LVFX) at the
initial dose of 250 mg/day. This dose level was increased to the
maintenance dose level of 500 mg/day following confirmation of the
absence of deteriorating hepatic function one week later.
Subsequently, on May 19th 2014, desensitization therapy was
initiated in accordance with the protocol of the Japanese Society
for Tuberculosis (JST) (6). Firstly,
RFP (25 mg/day) was used and its dose level was gradually increased
at intervals of 3 days to the maintenance dose level (450 mg/day).
Following confirmation of a lack of deteriorating hepatic function,
INH was added on June 16th 2014 at 25 mg/day, and the dose level
increased at intervals of 3 days to the maintenance dose level (300
mg/day). Subsequent to the dose level of each drug reaching the
maintenance dose level (LVFX, 500 mg/day; RFP, 450 mg/day; and INH,
300 mg/day), the therapy was continued for an additional 6 months
and completed on January 7th 2015. During this period, there were
no cases of hepatic function deterioration or any other adverse
events. During the subsequent follow-up, no signs of relapse were
detected.
 | Table I.Laboratory findings of the liver
functions in the clinical course. |
Table I.
Laboratory findings of the liver
functions in the clinical course.
|
|
|
| 2014 |
|
|---|
|
|
|
|
|
|
|---|
| Test | Unit | 2013 November
26th | February 1st | February 26th | March 12th | May 12th | 2015 January
28th |
|---|
| T-Bil | mg/dl | 0.6 | 0.6 | 0.5 | 1.3 | 0.9 | 0.5 |
| AST | IU/l | 14.00 | 506.00 | 38.00 | 455.00 | 12.00 | 12.00 |
| ALT | IU/l | 15.00 | 867.00 | 74.00 | 928.00 | 12.00 | 12.00 |
| γ-GTP | IU/l | NP | 253.00 | NP | NP | 30.00 | 25.00 |
| LDH | IU/l | 183.00 | 472.00 | 198.00 | 341.00 | 164.00 | 169.00 |
| ALP | IU/l | 187.00 | 446.00 | NP | NP | 189.00 | 195.00 |
| Cr | mg/dl | 0.51 | 0.57 | 0.46 | 0.53 | 0.53 | 0.46 |
| CRP | mg/dl | NP | 5.34 | 0.06 | 0.26 | 0.07 | 0.05 |
| Plt |
104/µl | 28.8 | 17.8 | 29.6 | 21.1 | 24.5 | 23.8 |
Discussion
The reported incidence of tuberculosis in 2014 was
16.1/100,000 of the population in Japan, 3.1 in USA, 4.7 in Canada,
4.9 in Germany and 5.7 in Australia, with the incidence being
significantly higher in Japan compared with in the Western
countries (7). Tuberculoma is thought
to develop in 6–9% of all patients with post-primary tuberculosis
(2), and when it assumes the form of
an SPN, its distinction from cancer is difficult. Therefore,
VATS-based resection and histological diagnosis are recommended for
the differential diagnosis in such cases (5,8,9). After INH became available for clinical
use in the 1960s, numerous studies on anti-tuberculous drugs and
DIH were performed in that decade. According to reports after 1990,
the year in which the three-drug regimen of INH + RFP + PZA was
established as a standard therapy, DIH (defined as a serum AST
level of ≥100 U/ml and/or serum total bilirubin level of ≥2.5
mg/dl) occurred at an incidence of 2.4% in cases treated with INH +
RFP + PZA for 2 months and INH + RFP for the subsequent 4 months.
The reported incidence following 9 months of treatment with INH +
RFP + EB was 3.6%. The incidence of DIH has been demonstrated not
to vary depending on the presence/absence of PZA in the treatment
regimen (10). According to reports
after 2000, the incidence of DIH following tuberculosis treatment
has become higher, with inter-ethnic differences in the frequency
(11–13). Comparison of the DIH incidence between
two major standard therapies, i.e., the British Thoracic Society
(BTS) and American Thoracic Society (ATS) reintroduction guidelines
for antituberculous therapy, revealed no difference in the
incidence (BTS, 9.8–16%; ATS, 11.1–18%) (14). The possible risk factors for DIH
include age (>35 years old and children), gender (female),
extent of tuberculosis (spread of the disease beyond the lungs),
malnutrition (serum albumin, <3.5 mg/dL), alcohol consumption,
presence of hepatitis, drug dose level and genetic polymorphism
(three major enzymes involved in INH degradation are
N-acetyltransferase 2, cytochrome P450 2E1, and glutathione
S-transferase) (15). However,
certain previous studies have reported the absence of any
association of NIH with the body mass index or serum albumin level
(14,16).
Among the previous reports providing collective data
on the cases of tuberculoma, Lee et al (2) reported the results of treatment in 45
cases of tuberculoma, of which 24 (53.3%) were asymptomatic and
detected during a routine health checkup. Of the 45 patients, 7
(15.6%) had a previous history of tuberculosis and 13 (28.9%) had a
history of diabetes mellitus. The treatment consisted of INH, RFP,
EB and PZA in 38 patients, INH, RFP and EB in six patients, and EB,
streptomycin, cycloserine and LVFX in one patient. In six (13.3%)
patients, the first-line drugs were switched to second-line drugs
due to the appearance of DIH; however, there was no description in
the report of desensitization therapy. According to a report by Hsu
et al (3) of 53 tuberculoma
cases, 41 (77.4%) were asymptomatic and detected during a routine
health check-up, while 12 (22.6%) had respiratory symptoms
(including coughing and sputum). Following VTAS, standard
antituberculous therapy was administered for 6–12 months; however,
there was no description in the report on the occurrence of DIH.
Laisaar et al (17) reported
the results of 43 cases of tuberculoma, in which 37 patients
(86.0%) received treatment with first-line drugs and 5 (11.6%)
received treatment with second-line drugs. During the follow-up
period lasting 9 years, no patient relapsed subsequent to the
postoperative drug therapy (mean duration, 185 days). With regards
to special treatments, Yang et al (18) reported that 54 patients of tuberculoma
were treated by direct injection of 0.1 g INH and 0.2 g amikacin
into the tuberculoma, which resulted in a marked decrease in the
size or disappearance of the tuberculoma in 31 patients (57.4%)
following 10 treatment sessions. The adverse reactions observed
were pneumothorax (5 cases, 9.3%), hemoptysis (4 cases, 7.4%) and
pyrexia (6 cases, 11.1%); however, there was no report of DIH
development.
The diagnosis of DIH during antituberculous drug
treatment was based on the finding of elevated serum levels of the
hepatic enzymes and the accompanying clinical symptoms. The
judgment of ‘positive’ in the DLST with drugs is not an absolute
requirement for the diagnosis of DIH, as the reported DLST-positive
rate is only 56% for RFP and 50% for INH (6). Furthermore, a previous study reported
that the onset of DIH bears no correlation with the blood levels of
INH, RFP, EB or PZA (19).
Additionally, in the present case, the DLST was negative for INH,
RFP, EB and PZA; however, hepatic dysfunction developed following
the administration of the second medication, suggesting the strong
possibility that DIH was attributed to EB and PZA in the present
patient.
Desensitization therapy has been used as one of the
methods for the management of DIH, which occurs during
antituberculous treatment. The desensitization protocol used
differs between Japan and Western countries. Kobashi et al
(6) reported the differences as
follows: While the initial dose of IHN or RFP starts at 25 mg/day
and is gradually increased every 3 days over a period of >2
weeks in the desensitization therapy proposed by the JST, in
Western countries the dose starts from 0.1 mg every 45 min and
requires only 2 days to complete the desensitization therapy. The
previous study additionally reported that the protocol for
desensitization therapy used in Western countries for DIH to
antituberculous drugs is based on the penicillin desensitization
therapy. Furthermore, these investigators reported that the
desensitization therapy administered in accordance with the JST
protocol had a success rate of 77% for the case of RFP and 81% for
the case of INH, which is more efficacious compared with the
outcome of the therapy applied in accordance with the Western
protocol. Another previous study also reported that substitute
therapy using levofloxacin or moxifloxacin instead of INH and RFP
was effective (20). In the present
case, LVFX was used initially at half the ordinary dose level when
the medication was resumed, and desensitization therapy with RFP
and INH was applied in accordance with the JST protocol (6), which resulted in a favorable outcome and
no relapse.
In conclusion, the reported incidence of DIH to
antituberculous drugs used in the treatment of tuberculoma was
≤15%. Following the onset of DIH, the majority of patients
continued to receive treatment, although the drugs were frequently
switched to second-line drugs. The literature identified no
previous studies that utilised the application of desensitization
therapy to treat DIH. The present study reports a case of
tuberculoma, which required an initial differentiation from lung
cancer and was diagnosed on the basis of VATS findings. The patient
developed DIH twice following antituberculous drug treatment, and
the DIH in this patient appeared to be attributable to EB and PZA.
Substitute therapy using LVFX and desensitization therapy with INH
and RFP were applied, which yielded a favorable outcome. Therefore,
the present study signifies the importance of DIH management with
regards to antituberculous drugs.
References
|
1.
|
Andreu J, Cáceres J, Pallisa E and
Martinez-Rodriguez M: Radiological manifestations of pulmonary
tuberculosis. Eur J Radiol. 51:139–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lee HS, Oh JY, Lee JH, Yoo CG, Lee CT, Kim
YW, Han SK, Shim YS and Yim JJ: Response of pulmonary tuberculomas
to anti-tuberculous treatment. Eur Respir J. 23:452–455. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hsu KY, Lee HC, Ou CC and Luh SP: Value of
video-assisted thoracoscopic surgery in the diagnosis and treatment
of pulmonary tuberculoma: 53 cases analysis and review of
literature. J Zhejiang Univ Sci B. 10:375–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhan P, Xie H, Xu C, Hao K, Hou Z, et al:
Management strategy of solitary pulmonary nodules. J Thorac Dis.
5:824–829. 2013.PubMed/NCBI
|
|
5.
|
Sathekge MM, Maes A, Pottel H, Stoltz A
and van de Wiele C: Dual time-point FDG PET-CT for differentiating
benign from malignant solitary pulmonary nodules in a TB endemic
area. S Afr Med J. 100:598–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Japan, Ministry of Health, Labour and
Welfare. http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou03/dl/13Accessed.
June 10–2015
|
|
7.
|
Luh SP and Liu HP: Video-assisted thoracic
surgery-the past, present status and the future. J Zhejiang Univ
Sci B. 7:118–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Totanarungroj K, Chaopotong S and Tongdee
T: Distinguishing small primary lung cancer from pulmonary
tuberculoma using 64-slices multidetector CT. J Med Assoc Thai.
95:574–582. 2012.PubMed/NCBI
|
|
9.
|
Combs DL, O'Brien RJ and Geiter LJ: USPHS
Tuberculosis Short-Course Chemotherapy Trial 21: Effectiveness,
toxicity and acceptability. The report of final results. Ann Intern
Med. 112:397–406. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tostmann A, Boeree MJ, Aarnoutse RE, de
Lange WC, van der Ven AJ and Dekhuijzen R: Antituberculosis
drug-induced hepatotoxicity: Concise up-to-date review. J
Gastroenterol Hepatol. 23:192–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker
S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB,
et al: ATS (American Thoracic Society) Hepatotoxicity of
Antituberculosis Therapy Subcommittee: An official ATS statement:
Hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care
Med. 174:935–952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ohno M, Yamaguchi I, Yamamoto I, Fukuda T,
Yokota S, Maekura R, Ito M, Yamamoto Y, Ogura T, Maeda K, et al:
Slow N-acetyltransferase 2 genotype affects the incidence of
isoniazid and rifampicin-induced hepatotoxicity. Int J Tuberc Lung
Dis. 4:256–261. 2000.PubMed/NCBI
|
|
13.
|
Zuberi BF, Zuberi FF, Bader N, Alvi H and
Salahuddin J: Comparison of British Thoracic Society and American
Thoracic Society reintroduction guidelines for anti-tuberculous
therapy induced liver injury. J Pak Med Assoc. 64:896–899.
2014.PubMed/NCBI
|
|
14.
|
Devarbhavi H: Antituberculous drug-induced
liver injury: Current perspective. Trop Gastroenterol. 32:167–174.
2011.PubMed/NCBI
|
|
15.
|
Abbasi MA, Ahmed N, Suleman A, Zaman H,
Tariq S, Anwar SA and Khan N: Common risk factors for the
development of anti tuberculosis treatment induced hepatotoxicity.
J Ayub Med Coll Abbottabad. 26:384–388. 2014.PubMed/NCBI
|
|
16.
|
Laisaar T, Viiklepp P and Hollo V:
Long-term follow-up after thoracoscopic resection of solitary
pulmonary tuberculoma. Indian J Tuberc. 61:51–56. 2014.PubMed/NCBI
|
|
17.
|
Yang SH, Zhan P, Mao HH, Shi XD and Wang
LL: Perfusing chemotherapy by percutaneous lung puncture ‘holing’
for pulmonary tuberculoma-a ten-year single center experience. J
Thorac Dis. 5:466–471. 2013.PubMed/NCBI
|
|
18.
|
Kobashi Y, Abe T, Shigeto E, Yano S,
Kuraoka T and Oka M: Desensitization therapy for allergic reactions
to antituberculous drugs. Intern Med. 49:2297–2301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jeong I, Park JS, Cho YJ, Yoon HI, Song J,
Lee CT and Lee JH: Drug-induced hepatotoxicity of anti-tuberculosis
drugs and their serum levels. J Korean Med Sci. 30:167–172. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ho CC, Chen YC, Hu FC, Yu CJ, Yang PC and
Luh KT: Safety of fluoroquinolone use in patients with
hepatotoxicity induced by anti-tuberculosis regimens. Clin Infect
Dis. 48:1526–1533. 2009. View
Article : Google Scholar : PubMed/NCBI
|