Introduction
The prediction of survival is crucial for
determining the indications for radiotherapy (RT) and the dose
fractionation schedule (1,2). Although various patient and tumor
characteristics have been investigated for their prognostic value
in patients receiving palliative RT (3–5),
predicting prognosis remains a challenge (1,6).
Cancer-related inflammation affects the
proliferation and survival of malignant cells, angiogenesis, tumor
metastasis, the subversion of adaptive immunity and the tumor
response to chemotherapeutic drugs and hormones (7,8).
Parameters derived from white blood cell (WBC) and differential
counts have been reported to be powerful prognostic predictors in
patients with malignant and non-malignant diseases (9–11). The
total WBC count (TWBC) (12),
absolute lymphocyte count (ALC) (13–15),
relative lymphocyte count (RLC) (12,13,16),
absolute neutrophil count (ANC) (13,17,18),
relative neutrophil count (RNC) (13,19), the
neutrophil-to-lymphocyte ratio (N/L ratio) (13,20–22) and
the lymphocyte-to-monocyte ratio (L/M ratio) (22–24) have
been identified as significant prognostic factors in patients with
malignant diseases. As the majority of these studies only evaluated
one or a few of these parameters (12,14–24), their
comparative assessment in patients undergoing palliative RT is
required.
To identify WBC parameters with high prognostic
value for the survival of patients receiving palliative RT,
parameters derived from WBC and differential counts were
retrospectively compared and subgroup analysis was performed to
investigate the prognostic value of these parameters in patients on
medication affecting their WBC count.
Patients and methods
Patients
This retrospective study was approved by the
Kumamoto University Hospital Institutional Review Board (no. 986),
and patient informed consent was waived due to the retrospective
nature of the study. The inclusion criteria for this study were as
follows: i) Patients treated with palliative RT between October,
2010 and June, 2013; ii) acquisition of laboratory data, including
WBC and differential counts and albumin and lactate dehydrogenase
(LDH) levels, within 2 weeks prior to the initiation of RT; and
iii) availability of chemotherapy data obtained within 1 month
prior to blood sampling and of data on medications administered at
the time of blood sampling. When more than one blood sample was
obtained within 2 weeks prior to the initiation of RT, the latest
sample was used for analysis. Patient, tumor and treatment data
were collected from medical charts.
Prognostic factors of survival
Seven parameters, namely TWBC, ALC, RLC, ANC, RNC,
N/L ratio and L/M ratio, were analyzed. The TWBC, RLC, and RNC were
available from the patients' medical charts; the other 4 parameters
were calculated based on these counts.
Statistical analysis
Data were summarized using descriptive statistics
(frequency, percentage, median and range). Overall survival,
calculated from the initiation of RT, was estimated using the
Kaplan-Meier method; differences were assessed using the log-rank
test. For this test, continuous variables were dichotomized based
on our laboratory's reference values. Univariate Cox regression
analysis was performed, first to assess the effect of the WBC
parameters on the overall survival of all patients, and then on
patients receiving medications that affect WBC and differential
counts: The patients who had received chemotherapy within 1 month
prior to blood sampling (subgroup A) and those who received
steroids at the time of blood sampling (subgroup B). Finally, the
parameters that were statistically significant predictors of
survival in all patients and in the two subgroups, were subjected
to multivariate analysis. Multivariate Cox regression analysis was
performed to adjust for gender, age, disease type, previous
chemotherapy, previous RT and albumin and LDH levels. For
univariate and multivariate Cox regression analysis, the seven WBC
parameters, age, and albumin and LDH levels, were used as
continuous variables. Receiver operating characteristic (ROC)
analysis was performed in patients who were followed up for >5
months or until death. The prognostic value of the WBC parameters
for death within 5 months was evaluated using the area under the
curve (AUC) of the ROC curves. Differences of P<0.05 were
considered to indicate statistically significant differences. All
statistical analyses were performed using SPSS software, version 22
(IBM SPSS, Armonk, NY, USA).
Results
Patients
A total of 220 patients with a median survival of
4.7 months (95% confidence interval: 3.7–5.7 months) were
identified. The median follow-up period from the initiation of RT
was 3.5 months (range, 0–53.5 months) for all patients. The patient
characteristics and laboratory data are summarized in Table I. In 189 patients (86%) with solid
tumors, the primary site was the lung (n=59), digestive tract
(n=40), liver (n=10) and other sites (n=80). A total of 31 patients
(14%) had hematological tumors; 16 presented with multiple myeloma,
9 with malignant lymphoma and 6 with other diseases. The median
total radiation dose was 30 Gy (range, 3–60 Gy) and the median
number of fractions was 10 (range, 1–30).
 | Table I.Patient characteristics and laboratory
data (n=220). |
Table I.
Patient characteristics and laboratory
data (n=220).
| Characteristics | No. of patients |
| % |
|---|
| Male gender | 139 |
| 63 |
| Age (years) |
|
|
|
|
Median |
| 67 |
|
|
Range |
| 20–86 |
|
| Type of
malignancy |
|
|
|
|
Solid | 189 |
| 86 |
|
Hematological | 31 |
| 14 |
| Previous
chemotherapy | 150 |
| 68 |
| Previous
radiotherapy | 82 |
| 37 |
| Albumin (g/dl) |
|
|
|
|
Median |
| 3.4 |
|
|
Range |
| 1.6–4.8 |
|
| LDH (U/l) |
|
|
|
|
Median |
| 249.5 |
|
|
Range |
| 79–6,500 |
|
| TWBC
(×109/l) |
|
|
|
|
Median |
| 6.520 |
|
|
Range |
| 1.700–70.500 |
|
| ALC
(×109/l) |
|
|
|
|
Median |
| 1.066 |
|
|
Range |
| 0.110–3.969 |
|
| RLC (%) |
|
|
|
|
Median |
| 16.0 |
|
|
Range |
| 1.3–55.9 |
|
| ANC
(×109/l) |
|
|
|
|
Median |
| 4.693 |
|
|
Range |
| 0.656–68.174 |
|
| RNC (%) |
|
|
|
|
Median |
| 74.4 |
|
|
Range |
| 29.4–96.7 |
|
| N/L ratio |
|
|
|
|
Median |
| 4.68 |
|
|
Range |
| 0.57–74.35 |
|
| L/M ratio |
|
|
|
|
Median |
| 2.54 |
|
|
Range |
| 0.35–30.93 |
|
Prognostic factors for survival
In all 220 patients, univariate Cox regression
analysis revealed that all seven WBC parameters were statistically
significant predictors of survival (Table II). A low RLC and high RNC were
consistent predictors of poor survival in the two subgroups
(Table II). Multivariate Cox
regression analysis revealed that RLC and RNC were independent
predictors of survival in all 220 patients (P<0.05, Table III).
 | Table II.Univariate Cox regression analyses
for overall survival. |
Table II.
Univariate Cox regression analyses
for overall survival.
|
| All patients
(n=220) | Subgroup
Aa (n=68) | Subgroup
Bb (n=49) |
|---|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| TWBC (per increase
of 1×109/l) | 1.05 | 1.03–1.07 | <0.001 | 1.05 | 0.97–1.14 | 0.217 | 1.02 | 0.99–1.05 | 0.114 |
| ALC (per increase
of 1×109/l) | 0.55 | 0.40–0.74 | <0.001 | 0.68 | 0.44–1.06 | 0.090 | 0.68 | 0.42–1.10 | 0.120 |
| RLC (per 1%
increase) | 0.95 | 0.93–0.96 | <0.001 | 0.97 | 0.95–0.99 | 0.005 | 0.95 | 0.91–0.99 | 0.007 |
| ANC (per increase
of 1×109/l) | 1.05 | 1.03–1.07 | <0.001 | 1.06 | 0.99–1.15 | 0.116 | 1.02 | 0.99–1.05 | 0.085 |
| RNC (per 1%
increase) | 1.04 | 1.03–1.05 | <0.001 | 1.02 | 1.00–1.03 | 0.021 | 1.04 | 1.01–1.07 | 0.010 |
| N/L ratio (per
increase of 1) | 1.02 | 1.01–1.03 | 0.002 | 0.99 | 0.98–1.02 | 0.944 | 1.00 | 0.99–1.02 | 0.707 |
| L/M ratio (per
increase of 1) | 0.84 | 0.77–0.91 | <0.001 | 0.96 | 0.89–1.04 | 0.350 | 0.99 | 0.92–1.06 | 0.715 |
 | Table III.Multivariate Cox regression analyses
for overall survival in all patients (n=220). |
Table III.
Multivariate Cox regression analyses
for overall survival in all patients (n=220).
| A, RLC |
|---|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| Male vs. female
gender | 0.86 | 0.62–1.21 | 0.387 |
| Age (per 1 year
increase) | 1.02 | 1.00–1.03 | 0.028 |
| Hematological vs.
solid tumors | 0.64 | 0.37–1.10 | 0.104 |
| Previous
chemotherapy (yes vs. no) | 1.58 | 1.07–2.34 | 0.021 |
| Previous
radiotherapy (yes vs. no) | 1.08 | 0.76–1.53 | 0.667 |
| Albumin (per 1 g/dl
increase) | 0.70 | 0.55–0.90 | 0.006 |
| LDH (per 1 U/l
increase) | 1.00 | 1.00–1.00 | 0.001 |
| RLC (per 1%
increase) | 0.96 | 0.94–0.98 | <0.001 |
|
| B, RNC |
|
| Variables | HR | 95% CI | P-value |
|
| Male vs. female
gender | 0.87 | 0.63–1.22 | 0.427 |
| Age (per 1 year
increase) | 1.01 | 1.00–1.03 | 0.053 |
| Hematological vs.
solid tumors | 0.62 | 0.36–1.07 | 0.086 |
| Previous
chemotherapy (yes vs. no) | 1.60 | 1.08–2.36 | 0.019 |
| Previous
radiotherapy (yes vs. no) | 1.14 | 0.80–1.61 | 0.473 |
| Albumin (per 1 g/dl
increase) | 0.67 | 0.52–0.86 | 0.001 |
| LDH (per 1 U/l
increase) | 1.00 | 1.00–1.00 | 0.001 |
| RNC (per 1%
increase) | 1.02 | 1.01–1.04 | 0.001 |
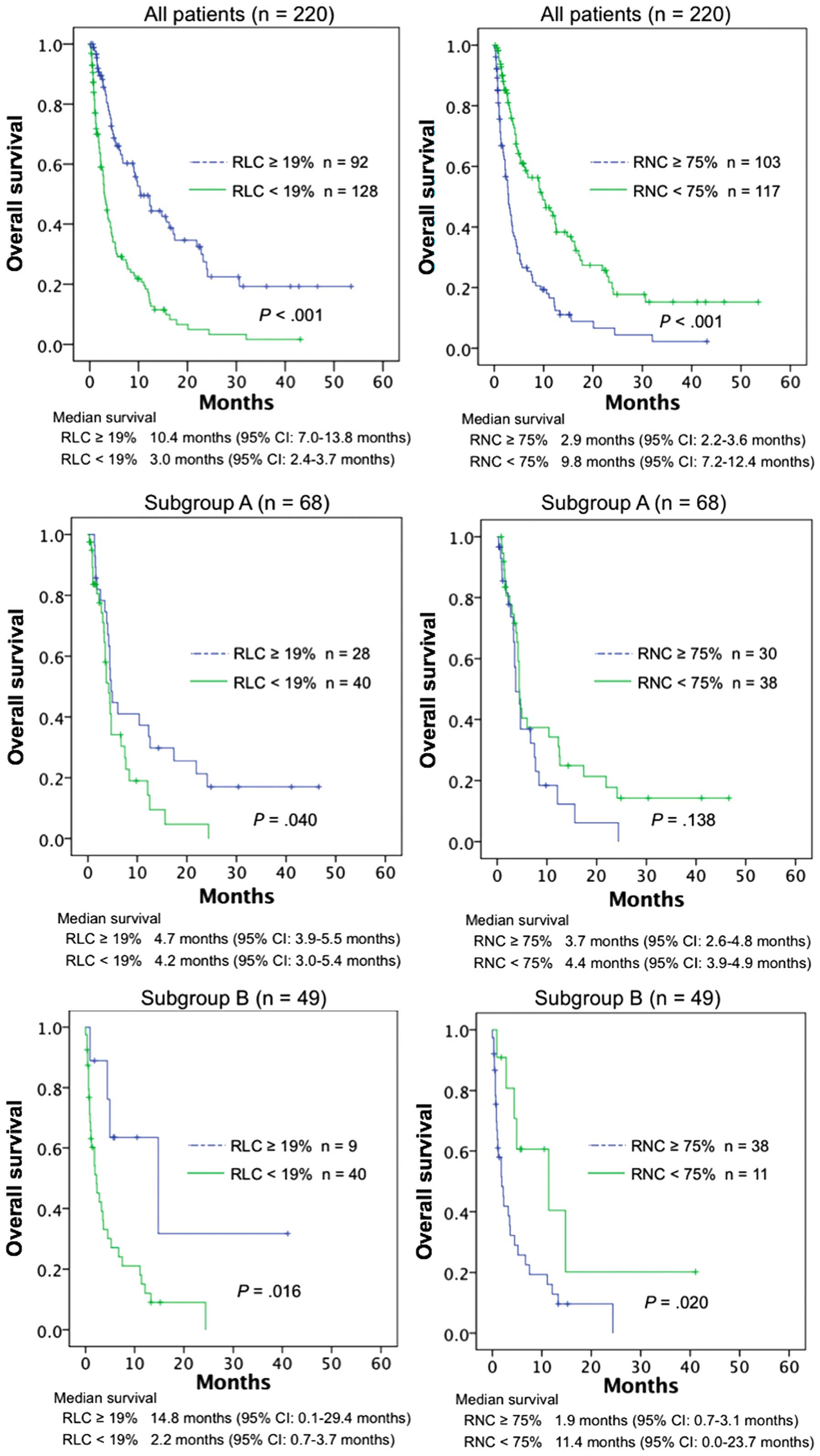
Survival curves were compared using the log-rank
test (Fig. 1). The continuous
variables were dichotomized according to the reference values in
our laboratory. The lower limit of RLC and the upper limit of RNC
were 19 and 75%, respectively. Among all patients, those with an
RLC of ≥19% had significantly better overall survival compared with
those with an RLC of <19% (P<0.001); this was also true for
subgroups A (P=0.040) and B (P=0.016). Overall survival for all
patients and subgroup B was significantly better for RNC<75%
compared with RNC≥75% (P<0.001 and P=0.020, respectively); the
difference was not statistically significant in subgroup A
(P=0.138).
ROC analysis was performed in184 patients (84%) who
were followed up for >5 months or until death. Table IV shows the AUC for the ROC curves
for each WBC parameter. RLC and the N/L and L/M ratios were
slightly better at predicting death within 5 months compared with
the remaining 4 parameters.
 | Table IV.Receiver operating characteristic
curve analysis to predict death within 5 months (n=184). |
Table IV.
Receiver operating characteristic
curve analysis to predict death within 5 months (n=184).
| Variables | AUC | 95% CI | P-value |
|---|
| TWBC | 0.625 | 0.532–0.717 |
0.027 |
| ALC | 0.697 | 0.594–0.800 | <0.001 |
| RLC | 0.777 | 0.694–0.861 | <0.001 |
| ANC | 0.671 | 0.581–0.761 |
0.002 |
| RNC | 0.727 | 0.635–0.819 | <0.001 |
| N/L ratio | 0.766 | 0.680–0.851 | <0.001 |
| L/M ratio | 0.774 | 0.691–0.858 | <0.001 |
Discussion
Of the seven WBC parameters investigated, RLC and
RNC were of high prognostic value for the survival of patients
receiving palliative RT. Overall, patients with low RLC and those
with high RNC had poor overall survival; this was also true for
patients receiving steroids or chemotherapy. The RLC value was a
slightly better predictor, as its prognostic value was consistently
significant in our subgroup analyses using the log-rank test, and
its AUC value in the ROC curve analysis was higher.
Data on the prognostic value of parameters derived
from WBC and differential counts in patients receiving palliative
RT are limited. Gripp et al (25) found that an elevated TWBC was
associated with a poor prognosis in 216 patients recently referred
for palliative RT. Others reported that decreased ALC values were a
prognostic factor for poor survival in 104 patients with brain
metastases who received whole-brain RT (26) and in 130 patients with brain
metastases from breast cancer who received whole-brain RT (27). Survival prediction is critical for
determining the dose fractionation schedule in patients receiving
palliative RT (1,2). Our findings may contribute to the
identification of appropriate parameters for survival prediction in
these patients.
Our subgroup analyses demonstrated that RLC and RNC
were useful predictors of survival, even in patients receiving
medications that affect WBC and differential counts. Certain
patients receiving palliative RT are also treated with chemotherapy
or steroids. Earlier studies on the prognostic value of WBC
parameters excluded patients receiving steroids or chemotherapy
(14,24,28); our
subgroup analysis renders our findings applicable to such
patients.
WBC parameters have been shown to be of prognostic
value in patients with various malignancies (9,12–24). Palliative RT is delivered to
heterogeneous patient populations, i.e., patients with solid tumors
from different primary sites and patients with hematological
tumors. In such patients, disease-specific factors, such as tumor
stage (20,29–31), tumor
size (31,32), or tumor-specific markers (33–35) do not
appear to be useful for the prediction of prognosis. However, RLC
and RNC may represent useful prognostic factors in heterogeneous
patients receiving palliative RT.
Our study had certain limitations. First, as it was
retrospective, potential confounders, such as performance status,
could not be included in the multivariate analysis. Furthermore,
the size of the subgroups was limited and parameters other than RLC
or RNC may be found to be of prognostic value in larger patient
populations.
In summary, the prognostic value of seven WBC
parameters was compared and low RLC and high RNC levels were found
to predict poor survival in patients receiving palliative RT. Our
subgroup analyses demonstrated that these were significant
prognostic factors, even in patients treated with medications
affecting WBC and differential counts. As the investigated
parameters are derived from complete blood counts, they may be used
in daily clinical practice. Our findings may contribute to the
selection of appropriate treatment schedules for patients receiving
palliative RT.
References
|
1
|
Gripp S, Mjartan S, Boelke E and Willers
R: Palliative radiotherapy tailored to life expectancy in end-stage
cancer patients: Reality or myth? Cancer. 116:3251–3256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nieder C, Angelo K, Dalhaug A, Pawinski A,
Haukland E and Norum J: Palliative radiotherapy during the last
month of life: Predictability for referring physicians and
radiation oncologists. Oncol Lett. 10:3043–3049. 2015.PubMed/NCBI
|
|
3
|
Chow E, Abdolell M, Panzarella T, Harris
K, Bezjak A, Warde P and Tannock I: Validation of a predictive
model for survival in metastatic cancer patients attending an
outpatient palliative radiotherapy clinic. Int J Radiat Oncol Biol
Phys. 73:280–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krishnan MS, Epstein-Peterson Z, Chen YH,
Tseng YD, Wright AA, Temel JS, Catalano P and Balboni TA:
Predicting life expectancy in patients with metastatic cancer
receiving palliative radiotherapy: The TEACHH model. Cancer.
120:134–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizumoto M, Harada H, Asakura H, Hashimoto
T, Furutani K, Hashii H, Takagi T, Katagiri H, Takahashi M and
Nishimura T: Prognostic factors and a scoring system for survival
after radiotherapy for metastases to the spinal column: A review of
544 patients at Shizuoka cancer center hospital. Cancer.
113:2816–2822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tseng YD, Krishnan MS, Sullivan AJ, Jones
JA, Chow E and Balboni TA: How radiation oncologists evaluate and
incorporate life expectancy estimates into the treatment of
palliative cancer patients: A survey-based study. Int J Radiat
Oncol Biol Phys. 87:471–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagai S, Abouljoud MS, Kazimi M, Brown KA,
Moonka D and Yoshida A: Peritransplant lymphopenia is a novel
prognostic factor in recurrence of hepatocellular carcinoma after
liver transplantation. Transplantation. 97:694–701. 2014.PubMed/NCBI
|
|
10
|
Vaduganathan M, Ambrosy AP, Greene SJ,
Mentz RJ, Subacius HP, Maggioni AP, Swedberg K, Nodari S, Zannad F,
Konstam MA, et al: Predictive value of low relative lymphocyte
count in patients hospitalized for heart failure with reduced
ejection fraction: Insights from the EVEREST trial. Circ Heart
Fail. 5:750–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kovesdy CP, George SM, Anderson JE and
Kalantar-Zadeh K: Outcome predictability of biomarkers of
protein-energy wasting and inflammation in moderate and advanced
chronic kidney disease. Am J Clin Nutr. 90:407–414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hyodo I, Morita T, Adachi I, Shima Y,
Yoshizawa A and Hiraga K: Development of a predicting tool for
survival of terminally ill cancer patients. Jpn J Clin Oncol.
40:442–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin Y, Ye X, He C, Zhang B and Zhang Y:
Pretreatment neutrophil-to-lymphocyte ratio as predictor of
survival for patients with metastatic nasopharyngeal carcinoma.
Head Neck. 37:69–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Giorgi U, Rihawi K, Aieta M, Lo Re G,
Sava T, Masini C, Baldazzi V, De Vincenzo F, Camerini A, Fornarini
G, et al: Lymphopenia and clinical outcome of elderly patients
treated with sunitinib for metastatic renal cell cancer. J Geriatr
Oncol. 5:156–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Scodan R, Massard C, Mouret-Fourme E,
Guinebretierre JM, Cohen-Solal C, De Lalande B, Moisson P,
Breton-Callu C, Gardner M, Goupil A, et al: Brain metastases from
breast carcinoma: Validation of the radiation therapy oncology
group recursive partitioning analysis classification and
proposition of a new prognostic score. Int J Radiat Oncol Biol
Phys. 69:839–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kikuchi N, Ohmori K, Kuriyama S, Shimada
A, Nakaho T, Yamamuro M and Tsuji I: Survival prediction of
patients with advanced cancer: The predictive accuracy of the model
based on biological markers. J Pain Symptom Manage. 34:600–606.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmidt H, Suciu S, Punt CJ, Gore M, Kruit
W, Patel P, Lienard D, von der Maase H, Eggermont AM, Keilholz U,
et al: Pretreatment levels of peripheral neutrophils and leukocytes
as independent predictors of overall survival in patients with
American joint committee on cancer stage IV melanoma: ReSults of
the EORTC 18951 biochemotherapy trial. J Clin Oncol. 25:1562–1569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teramukai S, Kitano T, Kishida Y, Kawahara
M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al:
Pretreatment neutrophil count as an independent prognostic factor
in advanced non-small-cell lung cancer: An analysis of Japan
multinational trial organisation LC00-03. Eur J Cancer.
45:1950–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He JR, Shen GP, Ren ZF, Qin H, Cui C,
Zhang Y, Zeng YX and Jia WH: Pretreatment levels of peripheral
neutrophils and lymphocytes as independent prognostic factors in
patients with nasopharyngeal carcinoma. Head Neck. 34:1769–1776.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Absenger G, Szkandera J, Pichler M, Stotz
M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H,
Stojakovic T and Gerger A: A derived neutrophil to lymphocyte ratio
predicts clinical outcome in stage II and III colon cancer
patients. Br J Cancer. 109:395–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Templeton AJ, Pezaro C, Omlin A, McNamara
MG, Leibowitz-Amit R, Vera-Badillo FE, Attard G, de Bono JS,
Tannock IF and Amir E: Simple prognostic score for metastatic
castration-resistant prostate cancer with incorporation of
neutrophil-to-lymphocyte ratio. Cancer. 120:3346–3352. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szkandera J, Gerger A, Liegl-Atzwanger B,
Absenger G, Stotz M, Friesenbichler J, Trajanoski S, Stojakovic T,
Eberhard K, Leithner A and Pichler M: The lymphocyte/monocyte ratio
predicts poor clinical outcome and improves the predictive accuracy
in patients with soft tissue sarcomas. Int J Cancer. 135:362–370.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rambaldi A, Boschini C, Gritti G, Delaini
F, Oldani E, Rossi A, Barbui AM, Caracciolo D, Ladetto M, Gueli A,
et al: The lymphocyte to monocyte ratio improves the IPI-risk
definition of diffuse large B-cell lymphoma when rituximab is added
to chemotherapy. Am J Hematol. 88:1062–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Y and Feng JF: Low preoperative
lymphocyte to monocyte ratio predicts poor cancer-specific survival
in patients with esophageal squamous cell carcinoma. Onco Targets
Ther. 8:137–145. 2015.PubMed/NCBI
|
|
25
|
Gripp S, Moeller S, Bölke E, Schmitt G,
Matuschek C, Asgari S, Asgharzadeh F, Roth S, Budach W, Franz M and
Willers R: Survival prediction in terminally ill cancer patients by
clinical estimates, laboratory tests and self-rated anxiety and
depression. J Clin Oncol. 25:3313–3320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Claude L, Perol D, Ray-Coquard I, Petit T,
Blay JY, Carrie C and Bachelot T: Lymphopenia: A new independent
prognostic factor for survival in patients treated with whole brain
radiotherapy for brain metastases from breast carcinoma. Radiother
Oncol. 76:334–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Scodan R, Massard C, Jouanneau L,
Coussy F, Gutierrez M, Kirova Y, Lerebours F, Labib A and
Mouret-Fourme E: Brain metastases from breast cancer: Proposition
of new prognostic score including molecular subtypes and treatment.
J Neurooncol. 106:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keizman D, Ish-Shalom M, Huang P,
Eisenberger MA, Pili R, Hammers H and Carducci MA: The association
of pre-treatment neutrophil to lymphocyte ratio with response rate,
progression free survival and overall survival of patients treated
with sunitinib for metastatic renal cell carcinoma. Eur J Cancer.
48:202–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loghavi S, Alayed K, Aladily TN, Zuo Z, Ng
SB, Tang G, Hu S, Yin CC, Miranda RN, Medeiros LJ and Khoury JD:
Stage, age, and EBV status impact outcomes of plasmablastic
lymphoma patients: A clinicopathologic analysis of 61 patients. J
Hematol Oncol. 8:652015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kao SC, Vardy J, Chatfield M, Corte P,
Pavlakis N, Clarke C, van Zandwijk N and Clarke S: Validation of
prognostic factors in malignant pleural mesothelioma: A
retrospective analysis of data from patients seeking compensation
from the New South Wales dust diseases board. Clin Lung Cancer.
14:70–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han LH, Jia YB, Song QX, Wang JB, Wang NN
and Cheng YF: Prognostic significance of preoperative
lymphocyte-monocyte ratio in patients with resectable esophageal
squamous cell carcinoma. Asian Pac J Cancer Prev. 16:2245–2250.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng J, Zhang R, Pan Y, Ding X, Cai M, Liu
Y, Liu H, Bao T, Jiao X, Hao X and Liang H: Tumor size as a
recommendable variable for accuracy of the prognostic prediction of
gastric cancer: A retrospective analysis of 1,521 patients. Ann
Surg Oncol. 22:565–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JW, Lim SB, Kim DY, Jung KH, Hong YS,
Chang HJ, Choi HS and Jeong SY: Carcinoembryonic antigen as a
predictor of pathologic response and a prognostic factor in locally
advanced rectal cancer patients treated with preoperative
chemoradiotherapy and surgery. Int J Radiat Oncol Biol Phys.
74:810–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hotta T, Takifuji K, Yokoyama S, Matsuda
K, Oku Y, Nasu T, Ieda J, Yamamoto N, Iwamoto H, Takei Y, et al:
Impact of the post/preoperative serum CEA ratio on the survival of
patients with rectal cancer. Surg Today. 44:2106–2115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Davidson NG, Khanna S, Kirwan PH and
Bircumshaw D: Prechemotherapy serum CA125 level as a predictor of
survival outcome in epithelial carcinoma of the ovary. Clin Oncol
(R Coll Radiol). 3:32–36. 1991. View Article : Google Scholar : PubMed/NCBI
|