Introduction
Radiotherapy serves an important role in cancer
treatment. Radiation-induced gastrointestinal disorder is the most
common complication of toxicity due to the treatment for
malignancies in the abdomen and pelvis, which often reduces the
quality of life (QOL) for patients and occasionally limits the
therapeutic dose that can be used for treating abdominal and pelvic
tumors with radiotherapy. Modern radiotherapeutic modalities,
including intensity-modulated radiation therapy and proton therapy,
can significantly decrease the toxicities of radiotherapy and can
benefit the outcomes (1). Amifostine
has been recommended for the prevention of severe radiation-induced
toxicities and is the only radioprotectant that has been approved
by the US Food and Drug Administration (2). However, the daily use of amifostine has
been limited due to its hematological and gastrointestinal
toxicity.
Polaprezinc (PZ) is an antiulcer drug and a
chelating compound consisting of a zinc ion, L-carnosine, a
β-alanine dipeptide and L-histidine. It was previously reported
that PZ exerts antioxidant effects and scavenges free radicals
(3–5).
Our previous study reported the efficacy of PZ for acute radiation
proctitis in an animal model and demonstrated that PZ has an
anti-inflammatory effect following exposure to radiation (6). However, the radioprotective effects of
PZ have been reported in only a limited number of reports and
remain unclear (6–9).
The purpose of the present study was to examine the
optimal timing to administer PZ and the pattern of apoptosis in the
normal intestine. It is important that premedication of the
clinical medicine enables the prevention of radiation injuries.
Materials and methods
Animal models and reagents
Male C57BL/6J mice (8-weeks-old; weight, ~20 g) were
used in the present study. The mice were purchased from Charles
River Laboratories Japan, Inc. (Kanagawa, Japan) and were
acclimated for 7 days. They were housed 4–5/cage and fed a
laboratory rodent pellet formula and tap water ad libitum.
Mice were maintained at a constant temperature of 22°C±0.5°C, a
humidity of 50%±5% and were exposed to 12 h light/dark cycles. The
Hyogo College of Medicine Institutional Animal Care and Use
Committee approved all animal procedures prior to the initiation of
the project (nos. 13–047 and 13–070). The mice orally received 100
mg/kg body weight PZ in the drinking water. The mice were
irradiated at a dose rate of ~200 cGy/min using a 150 kVp X-ray
unit (Hitachi MBR-1520; Hitachi, Tokyo, Japan; 20 mA; 150 kV). For
dosimetry, a probe connected to an electrometer system was placed
close to the target site.
Radioprotection of PZ on normal
intestine
Prior to the experiments, the present study
confirmed that the agents were retained in the whole intestine
using an imaging examination with sodium amidotrizoate (100 mg/ml)
2 h after the oral administration of PZ (Zeria Pharmaceutical Co.,
Ltd., Tokyo, Japan; data not shown). To examine the optimal timing
of the administration of PZ for the protective effect on the normal
intestine, the present study used an established animal model that
measured the intestinal stem cell survival (as the number of
crypts/cross section) after ionizing radiation (IR).
The detailed procedure for the intestinal crypt cell
survival analysis following irradiation has been described
previously (10). Briefly, the mice
were divided into the following three groups: Group A, mice that
received IR without PZ; group B, mice that received PZ 2 h before
IR; group C, mice that received PZ 2 h after IR (n=3/group). The
mice were irradiated with a total body irradiation (TBI) of 15 Gy
in a single fraction. Three days after radiation, the mice were
sacrificed by injection of pentobarbital (200 mg/kg) in the
abdominal cavity, and the duodenum, jejunum and ileum were removed,
fixed and stained with hematoxylin and eosin (H&E). Surviving
crypts with ≥10 cells for each cross-section were counted using
microscopy.
In the subsequent studies, based on the results, the
mice were divided into two groups: Mice that received oral
administration of PZ 2 h prior to IR [PZ (+) group] and mice that
were treated with drinking water without PZ [PZ (−) group]. The
samples were harvested for a pathological evaluation and were
immediately fixed in 10% neutral buffered formalin solution. The
intestine was divided into the duodenum, jejunum and ileum, and was
subsequently submitted for a histological analysis. All slides were
stained with H&E and examined using light microscopy. The
radioprotective effects of PZ in these two murine groups were
evaluated.
To examine the suppressive effect of PZ on the
apoptosis caused by IR in the normal intestine, the mice received
TBI of 2 Gy in a single fraction and were subsequently sacrificed
at 0, 4 and 8 h following IR. The group for 0 h used two mice for
the PZ (−) group and two for the PZ (+) group, and the group for 4
and 8 h used four mice for the PZ (−) group and four for the PZ (+)
group. The duodenum, jejunum, ileum and rectum were harvested for
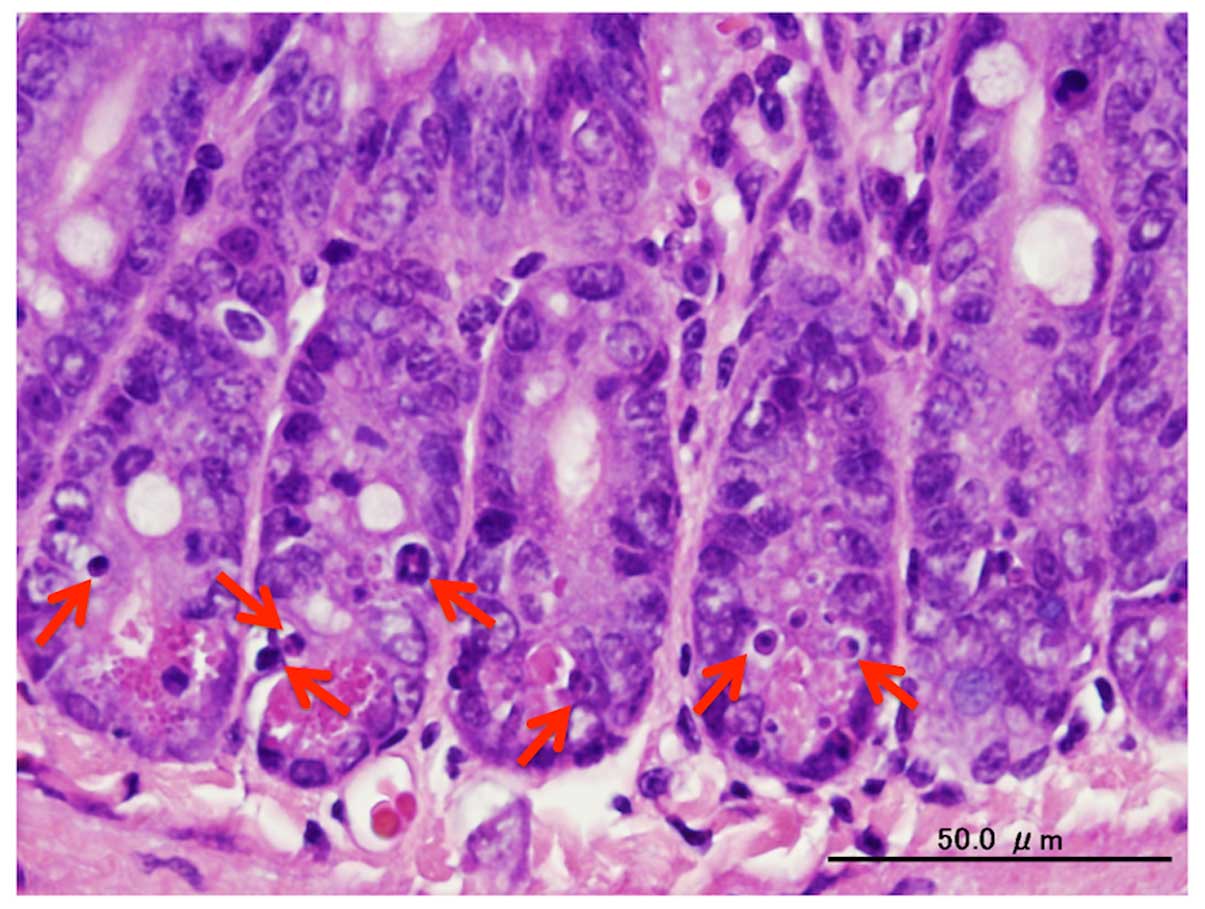
pathological examination. The present study defined apoptotic cells
as epithelial cells with apoptotic fragments in the H&E stained
tissue sections. In addition, the proportion of apoptotic cells in
each crypt was analyzed to determine an apoptotic index. The
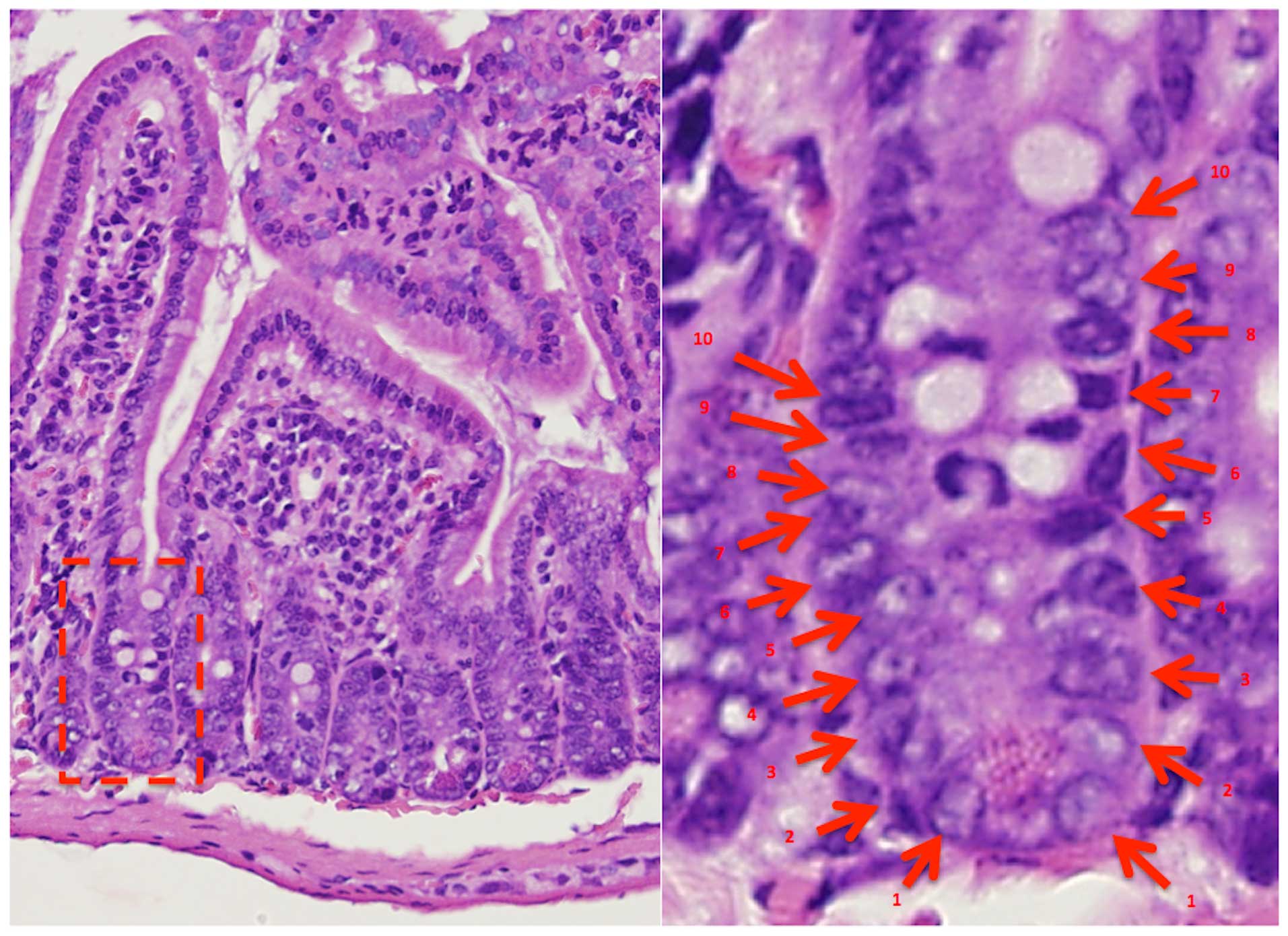
position of apoptotic cells were determined by the numbering from
the bottom of the crypt cells.
Statistical analysis
The data are expressed as the means, with the range
in parentheses, unless otherwise indicated. A statistical analysis
was performed to compare the differences between the two or three
groups. For the two groups, the parametric data were analyzed using
an unpaired two-tailed F test and the two-tailed Student's t-test.
For the three groups, the parametric data were analyzed using the
Bartlett test and by a one-way analysis of variance.
When significant differences in the three groups
were observed, a multiple comparison using the Tukey-Kramer method
was performed. In addition, a risk of 5% was defined as a
significant difference between the two groups. The StatMate IV
software program (ATMS Co., Ltd., Tokyo, Japan) was used to perform
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
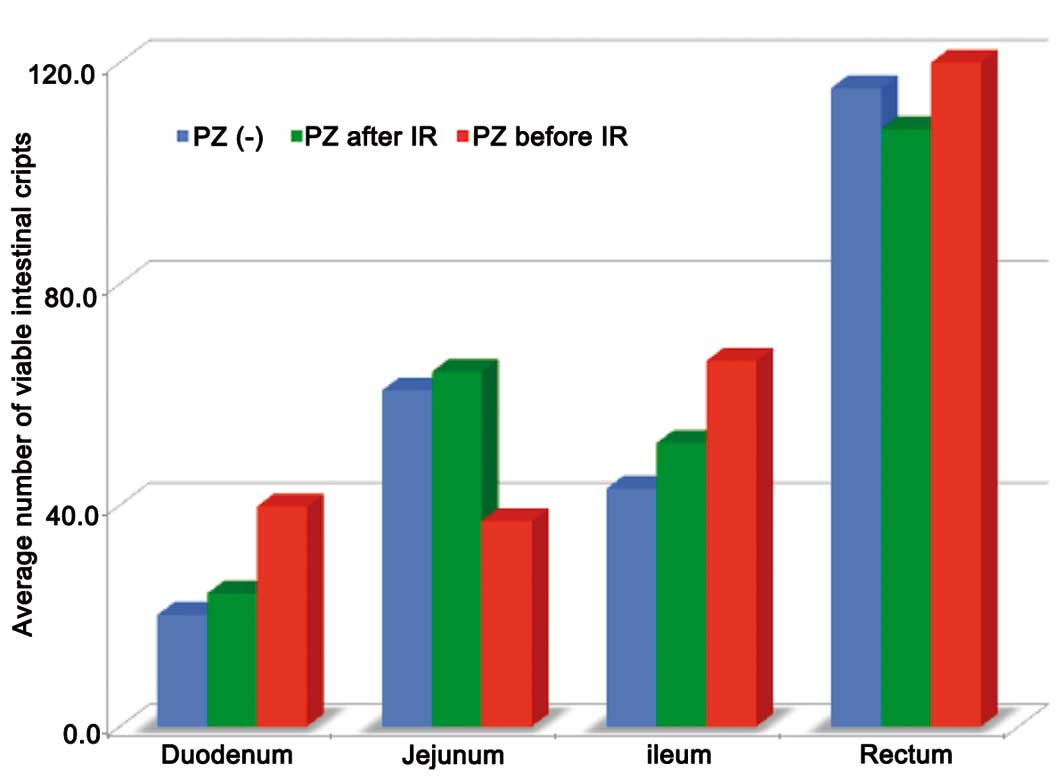
The present study performed experiments to study the
effect of PZ on the intestinal crypt survival following 15 Gy TBI.
The results demonstrated that the administration of PZ decreased
the radiation-induced cell death of crypt stem cells in the
duodenum, jejunum and ileum. In addition, PZ treatment protected
the crypt cells against TBI and increased the number of viable
crypt cells in the mice that received PZ prior to IR compared with
those that received PZ after IR (Fig.
1).
The average number of viable intestinal crypts in
groups A, B and C was 20.2, 24.1 and 39.8 in the duodenum (P=0.04),
and 43, 51.3 and 66.1 in the ileum (P=0.02), respectively (Fig. 1).
In addition, according to the multiple comparison
test method, significant differences existed between groups A and C
in both the duodenum and ileum. Mice that received premedication of
PZ exhibited significantly more viable intestinal stem cells in the
crypts, compared with those administered PZ after IR (Fig. 1).
PZ was administered 2 h prior to IR, according to
the above mentioned data. In the pathological examination,
apoptotic cells were observed (Fig.
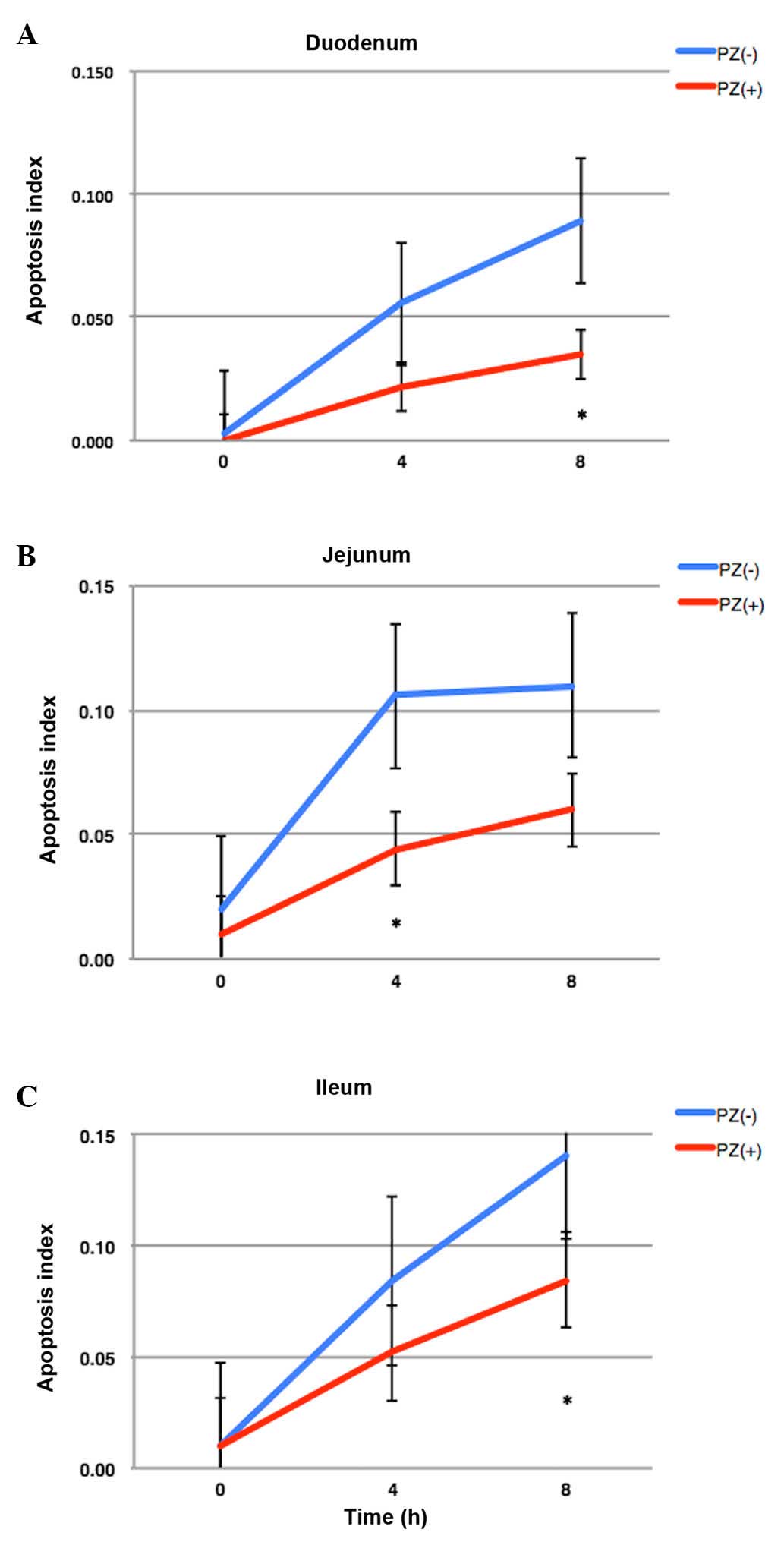
2). The number of apoptotic cells in the small intestine peaked
at 8 h following IR and the oral administration of PZ protected the
normal intestinal cells from apoptosis caused by IR (Fig. 3). The apoptotic index in the PZ (−)
group was 0.056, 0.106 and 0.084 in the duodenum, jejunum and
ileum, respectively. The apoptotic index in the PZ (+) group was
0.022, 0.044 and 0.052 in the duodenum, jejunum and ileum,
respectively. PZ tended to reduce the apoptosis in the duodenum and
jejunum (Fig. 3), however not the
ilium. The P-values between the PZ (+) and PZ (−) groups were 0.07,
0.01 and 0.12 in the duodenum, jejunum, and ileum,
respectively.
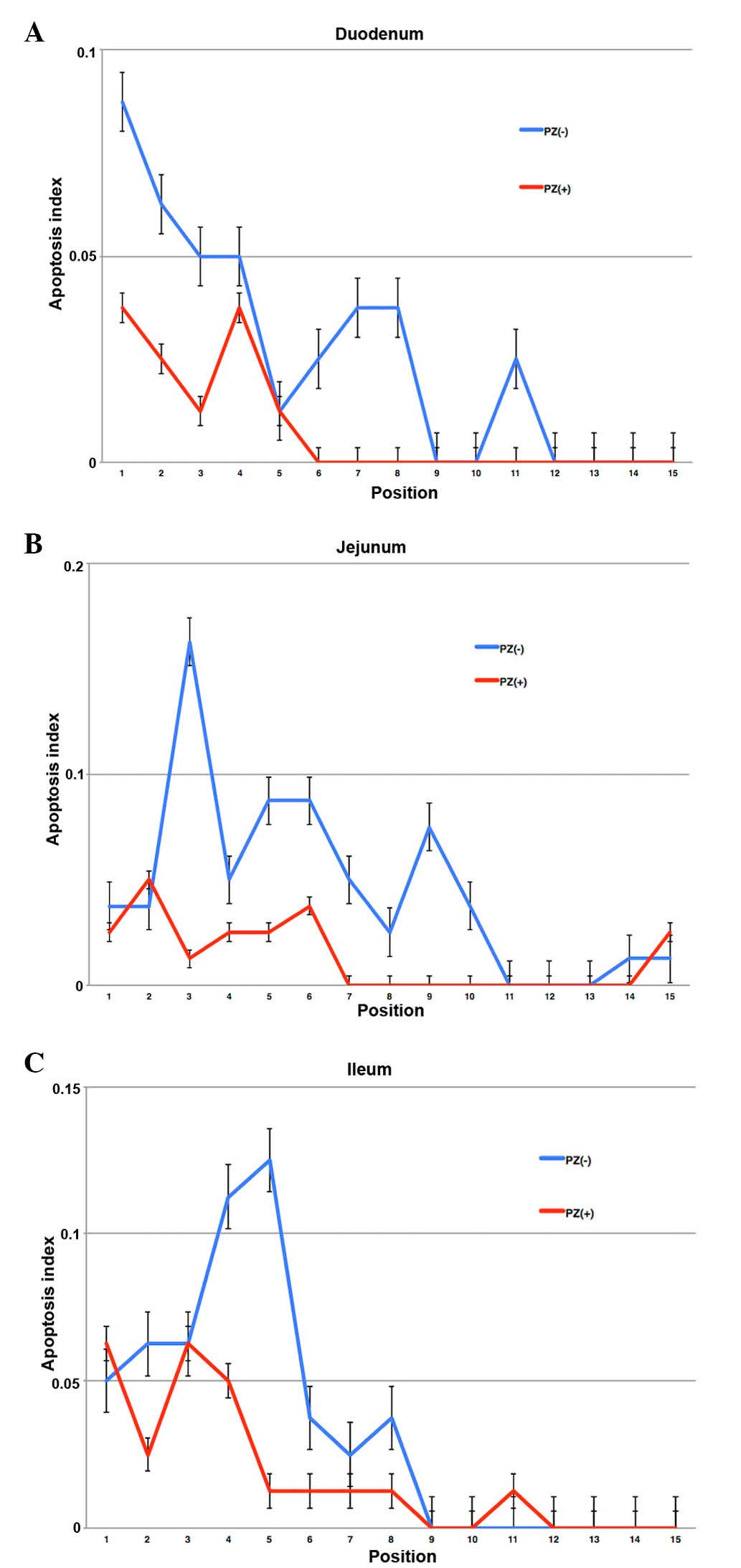
The positions of high apoptotic frequency were
observed in lower positions of each crypt in the intestine. The
mice that received PZ exhibited less apoptotic cells in each
position of crypts (Figs. 4 and
5).
Discussion
Radiotherapy is a significant treatment option for
malignancy and can improve patient survival. Gastrointestinal
toxicity commonly occurs as a complication of radiotherapy for
malignancy in the abdomen and pelvis, which significantly decreases
a patient's QOL (2,11). In addition, no standardized treatment
options are currently available to prevent gastrointestinal
toxicity (2).
PZ, which is a chelating compound consisting of a
zinc ion, L-carnosine and L-histidine, has been reported to possess
superoxide scavenging activity (3).
It has also been previously reported that PZ exerts its effects via
a variety of cytoprotective mechanisms, including the suppression
of lipid peroxidation, reduction of the levels of various
cytokines, inhibition of superoxide generation and promotion of the
restoration of the gastric mucosa (3–5).
Therefore, the efficacy of PZ for radiation-induced mucositis
appears promising. Our previous study reported the efficacy of PZ
for acute radiation proctitis in a rat model and demonstrated the
anti-inflammatory effect of PZ on radiation-induced mucosal damage
(6). In addition, the clinical use of
PZ has been reported to be feasible and can increase a patient's
QOL during radiotherapy (8,9).
In the present study, the pretreatment of PZ
revealed improved protective effects in the mice that received PZ
prior to radiation exposure compared with those that received PZ
after radiation exposure and those that were irradiated without PZ.
The present study therefore administered PZ 2 h prior to IR. This
is the first report, to the best of our knowledge, that
demonstrated the optimal timing for PZ treatment as a
radioprotectant. Radiotherapy is generally performed in a
fractionated schedule with the aim of maximizing the anti-tumor
effect and minimizing the toxicity of normal organs in the clinical
practice. However, the present study used a single fraction
schedule to examine the efficacy of PZ. Therefore, the optimization
of the administration of PZ and IR must be evaluated in future
studies.
Matsuu-Matsuyama et al (7) have reported that PZ can protect the
normal intestine from radiation-induced apoptosis via the
suppression of p53, p21 and B-cell associated X protein genes.
However, the mechanism of the radioprotective effect of PZ
treatment for radiation-induced mucosal damages remains to be
investigated in greater detail. The present study examined the
potential differences in the sensitivity of PZ treatment in various
positions of the crypts in the intestine since intestinal stem
cells, which are critical drivers of epithelial homeostasis and
regeneration, have been reported to localize to the lower areas in
each crypt (12). It was revealed
that radiation induced apoptosis in the low positions from the base
of the crypt axis, which was reduced following PZ treatment. In
addition, the present study also demonstrated the radioprotective
effect of PZ in the established-intestinal crypt stem cell assay.
According to these results, PZ protects the intestinal stem cells
from cell death caused by IR and can reduce gastrointestinal
toxicity associated with radiotherapy.
Cytoprotection of radioprotectants can include joint
binding between the tumor cells and normal cells, since reactive
oxygen species are believed to serve a significant role in
radiotherapy delivered by photons. There is, however, little data
of the negative effects of PZ use for tumor control and the
survival outcomes of the patients who received PZ as a supportive
treatment have been poorly understood. However, no direct evidence
has suggested that the use of PZ can lead to inferior tumor
outcomes in patients with malignancies (8,9). Watanabe
et al (8) reported that the
initial responses of tumors to radiotherapy were not affected by
the PZ intake in a randomized clinical trial. In addition, our
previous study recently reported that patients who received a
PZ-containing rinse showed no inferior survival outcomes compared
with those that did not received PZ in a retrospective study
(9). However, further investigations
are required prior to a large clinical trial.
The present study administered PZ 2 h prior to IR in
order to distribute PZ to whole intestine, according to the
findings from our preliminary data. In addition, Myagmarjalbuu
et al (13) have reported the
gastrointestinal transit time was 2 h in mice. It has been
previously reported that PZ causes its pharmacological effect by
adhesion to damaged mucosa (4,6). However,
the time between the administration of PZ and IR can increase the
serum zinc concentration (12).
Therefore, further studies to determine the mechanism of the
radioprotective effects of PZ are required.
In conclusion, premedication with PZ protected the
normal intestinal tissues from IR-induced apoptosis.
Acknowledgements
The authors would like to thank Ms. Michiko Kakihana
(Department of Pathology, Hyogo College of Medicine, Nishinomiya,
Hyogo, Japan) for her valuable technical assistance and Mr. Daisuke
Nagata (Institute of Experimental Animal Sciences, Hyogo College of
Medicine, Nishinomiya, Hyogo, Japan). The present study was
supported, in part, by funding from the Grants-in-Aid for
Scientific Research (no. 24591854) and unrestricted funding from
Zeria Pharmaceutical Co., Ltd.
References
|
1
|
Zelefsky MJ, Levin EJ, Hunt M, Yamada Y,
Shippy AM, Jackson A and Amols HI: Incidence of late rectal and
urinary toxicities after three-dimensional conformal radiotherapy
and intensity-modulated radiotherapy for localized prostate cancer.
Int J Radiat Oncol Biol Phys. 70:1124–1129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lalla RV, Bowen J, Barasch A, Elting L,
Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O,
Peterson DE, et al: MASCC/ISOO clinical practice guidelines for the
management of mucositis secondary to cancer therapy. Cancer.
120:1453–1461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshikawa T, Naito Y, Tanigawa T, Yoneta T
and Kondo M: The antioxidant properties of a novel zinc-carnosine
chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim
Biophys Acta. 1115:15–22. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshikawa T, Yamaguchi T, Yoshida N,
Yamamoto H, Kitazumi S, Takahashi S, Naito Y and Kondo M: Effect of
Z-103 on TNB-induced colitis in rats. Digestion. 58:464–468. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohkawara T, Nishihira J, Nagashima R,
Takeda H and Asaka M: Polaprezinc protects human colon cells from
oxidative injury induced by hydrogen peroxide: Relevant to
cytoprotective heat shock proteins. World J Gastroenterol.
12:6178–6181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doi H, Kamikonya N, Takada Y, Fujiwara M,
Tsuboi K, Inoue H, Tanooka M, Nakamura T, Shikata T, Tsujimura T
and Hirota S: Efficacy of polaprezinc for acute radiation proctitis
in a rat model. Int J Radiat Oncol Biol Phys. 80:877–884. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuu-Matsuyama M, Shichijo K, Okaichi K,
Nakayama T, Nakashima M, Uemura T, Niino D and Sekine I: Protection
by polaprezinc against radiation-induced apoptosis in rat jejunal
crypt cells. J Radiat Res. 49:341–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe T, Ishihara M, Matsuura K, Mizuta
K and Itoh Y: Polaprezinc prevents oral mucositis associated with
radiochemotherapy in patients with head and neck cancer. Int J
Cancer. 127:1984–1990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doi H, Fujiwara M, Suzuki H, Niwa Y,
Nakayama M, Shikata T, Odawara S, Takada Y, Kimura T, Kamikonya N
and Hirota S: Polaprezinc reduces the severity of radiation-induced
mucositis in head and neck cancer patients. Mol Clin Oncol.
3:381–386. 2015.PubMed/NCBI
|
|
10
|
Withers HR and Elkind MM: Radiosensitivity
and fractionation response of crypt cells of mouse jejunum. Radiat
Res. 38:598–613. 1969. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kavanagh BD, Pan CC, Dawson LA, Das SK, Li
XA, Ten Haken RK and Miften M: Radiation dose-volume effects in the
stomach and small bowel. Int J Radiat Oncol Biol Phys. 76(Suppl 3):
S101–S107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barker N: Adult intestinal stem cells:
Critical drivers of epithelial homeostasis and regeneration. Nat
Rev Mol Cell Biol. 15:19–33. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myagmarjalbuu B, Moon MJ, Heo SH, Jeong
SI, Park JS, Jun JY, Jeong YY and Kang HK: Establishment of a
protocol for determining gastrointestinal transit time in mice
using barium and radiopaque markers. Korean J Radiol. 14:45–50.
2013. View Article : Google Scholar : PubMed/NCBI
|