Introduction
Prostate cancer (PCa) is the one of the most common
malignant tumors among men in developed countries. Thus far, it has
been suggested that diet and other environmental factors may affect
the incidence of PCa, as this incidence differs between countries
and ethnic populations (1). In fact,
migration studies showed an increased incidence of PCa in
first-generation immigrants to the United States from Japan
(2). These observations suggest that
diet may play an important role in the incidence of PCa (3). In addition, several articles have
reported various endogenous and exogenous factors that may
contribute to PCa (4).
Obesity, which is generally measured by body mass
index (BMI), is associated with increased mortality for all cancers
combined (5). Obesity has also been
suggested to be a risk factor in prostate cancer as well as breast
and colon cancer (6,7). However, the association of higher BMI
with increased PCa incidence remains controversial (8). Previous studies presented evidence that
obesity was associated with an increased risk of diagnosis of
larger tumors, more aggressive disease and PCa-related mortality
(9,10), whereas other studies reported that
obesity was not associated with aggressive pathological
characteristics (11,12). As regards biochemical recurrence, it
has been reported that obese men are at increased risk of
biochemical recurrence (13–17). Recently, obesity has become more
prevalent among Asian countries, including Japan. Although obesity
in Asian countries is less severe compared with that in western
countries, certain studies have suggested an association between
BMI and PCa, including pathological characteristics (18,19).
However, the effect of obesity on PCa-related mortality has been
controversial, and it remains unclear whether obesity contributes
to the aggressiveness of PCa in Asian patients (20,21). The
aim of the present study was to investigate the association between
BMI and the clinicopathological characteristics of PCa, and
determine whether obesity increases the risk of biochemical
recurrence after radical prostatectomy (RP) in Japanese
patients.
Patients and methods
Patients
The subjects included 2003 Japanese patients with
PCa who were treated with RP between 2005 and 2014 at Hiroshima
University Hospital and affiliated hospitals. None of the patients
had a history of preoperative hormonal or radiation therapy.
Resection was considered to be curative in all patients based on
node-negative pathology and a decrease in the serum level of
prostate-specific antigen (PSA) postoperatively. The clinical
records of these patients were retrospectively reviewed to
investigate clinical information including age, serum PSA level,
BMI and pathological characteristics. Staging was based on the 2005
TNM classification (https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf).
Gleason score (GS) was assessed according to the International
Society of Urological Pathology modified Gleason grading system
(22). BMI was calculated as body
weight divided by the square of the height (kg/m2) and
was used to categorize patients into two groups according to the
classification of obesity of the Japan Society for the Study of
Obesity (http://www.jasso.or.jp/data/office/pdf/guideline.pdf).
Patients with BMI <25 kg/m2 were considered as the
normoweight group, whereas those with BMI ≥25 kg/m2 were
considered as the overweight group. The associations between the
two BMI groups and clinicopathological characteristics were
examined. For the evaluation of prognosis, the serum PSA level was
measured every 3 months after RP and biochemical recurrence was
defined as an increase in the serum PSA level of >0.2 ng/ml over
two subsequent measurements.
Statistical analysis
Associations between BMI and clinicopathological
characteristics were analyzed using the Chi-squared test. Age (≥70
vs. <70 years) and serum PSA level (≥10 vs. <10 ng/ml) were
treated as categorical variables for all analyses. Logistic
regression models were used to predict the risk for high-grade (GS
≥8) tumors at RP. Kaplan-Meier survival curves were constructed for
the normoweight and overweight groups. Biochemical recurrence-free
survival (bRFS) was compared between the normoweight and overweight
groups and evaluated for statistical significance using a log-rank
test. Univariate and multivariate Cox regression analyses were used
to evaluate the associations between clinical covariates and bRFS.
Hazard ratio and 95% confidence intervals were estimated with Cox
proportional hazard models. All statistical tests were two-sided
and a P-value of <0.05 was considered to indicate statistically
significant differences. All statistical analyses were performed
using JMP v10.0 software (SAS Institute, Cary, NC, USA) and the
Kaplan-Meier survival curves were drawn using GraphPad Prizm v6.0
software (GraphPad Software Inc., San Diego, CA, USA).
Results
BMI is associated with higher GS
The median age of the patients was 68 years (range,
45–83 years), the PSA level was 7.50 ng/ml (range, 1–120 ng/ml) and
the BMI was 23.50 kg/m2 (range, 15.9–38.0
kg/m2). The pathological GS was ≥8 in 537 patients
(26.8%), with extraprostatic extension (EPE) and a positive
resection margin (RM) observed in 432 (21.6%) and 554 (27.6%)
patients, respectively. Based on the BMI distribution, 569 patients
(28.4%) comprised the overweight group (BMI ≥25 kg/m2),
and 1,434 patients (71.6%) comprised the normoweight group (BMI
<25 kg/m2). The BMI exhibited a normal distribution.
Only 33 patients (1.6%) had a BMI >30 kg/m2. When
comparing the clinicopathological characteristics between the
normoweight and overweight groups (Table
I), no significant differences were observed in age (≥70
years), PSA (≥10 ng/ml), pathological T stage (≥T3), EPE and
RM.
 | Table I.Associations between BMI and
clinicopathological characteristics of prostate cancer. |
Table I.
Associations between BMI and
clinicopathological characteristics of prostate cancer.
|
| BMI
(kg/m2) |
|---|
|
|
|
|---|
| Parameters | <25, n (%) | ≥25, n (%) | P-value |
|---|
| Operative
approach |
|
| 0.2705 |
| RRP | 533 (73.6) | 191 (26.4) |
|
| RPP | 95 (70.9) | 39 (29.1) |
|
| LRP | 587 (69.4) | 259 (30.6) |
|
| RALP | 219 (73.2) | 80 (26.8) |
|
| Age (years) |
|
| 0.1379 |
|
<70 | 836 (72.9) | 311 (27.1) |
|
| ≥70 | 598 (69.8) | 258 (30.1) |
|
| PSA (ng/ml) |
|
| 0.4603 |
|
<10 | 943 (71.1) | 384 (28.9) |
|
| ≥10 | 491 (72.6) | 185 (27.4) |
|
| pT stage |
|
| 0.2943 |
| T2 | 1,120 (72.6) | 432 (27.8) |
|
| T3 | 314 (69.6) | 137 (30.4) |
|
| GS |
|
| 0.0308 |
| ≤7 | 1,069 (72.9) | 397 (27.1) |
|
| ≥8 | 365 (68.0) | 172 (32.0) |
|
| EPE |
|
| 0.2182 |
|
EPE0 | 1,135 (72.3) | 436 (27.8) |
|
|
EPE1 | 299 (69.2) | 133 (30.8) |
|
| RM |
|
| 0.1999 |
|
RM0 | 1,049 (72.4) | 400 (27.6) |
|
|
RM1 | 385 (69.5) | 169 (30.5) |
|
However, the number of patients with pathological GS
≥8 was higher in the overweight group (P=0.0308, Chi-squared test).
Logistic regression analysis was next performed to evaluate whether
BMI may be a predictor for PCa with higher GS (Table II). The univariate analysis revealed
that age (≥70 years), PSA (≥10 ng/ml), GS at biopsy (≥4+3) and BMI
(≥25 kg/m2) were significantly associated with GS ≥8 at
RP. In addition, a multivariate analysis including age, PSA, GS at
biopsy and BMI also revealed that PSA, GS at biopsy and BMI were
independent indicators for GS ≥8 at RP. These results suggest that
obesity may be associated with adverse pathological findings of
PCa.
 | Table II.Univariate and multivariate logistic
regression models to predict tumors with GS ≥8. |
Table II.
Univariate and multivariate logistic
regression models to predict tumors with GS ≥8.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
<70 | 1 (Reference) |
|
| 1 (Reference) |
|
|
≥70 | 1.301 | 1.067–1.588 |
0.0095 | 1.208 | 0.970–1.506 |
0.0913 |
| PSA (ng/ml) |
|
|
|
|
|
|
|
<10 | 1 (Reference) |
|
| 1 (Reference) |
|
|
≥10 | 2.698 | 2.200–3.311 | <0.0001 | 2.180 | 1.747–2.722 | <0.0001 |
| GS (at biopsy) |
|
|
|
|
|
|
|
≤3+4 | 1 (Reference) |
|
| 1 (Reference) |
|
|
≥4+3 | 6.784 | 5.418–8.544 | <0.0001 | 6.113 | 4.865–7.724 | <0.0001 |
| BMI
(kg/m2) |
|
|
<25 | 1 (Reference) |
|
| 1 (Reference) |
|
|
≥25 | 1.269 | 1.022–1.571 |
0.0308 | 1.291 | 1.016–1.638 |
0.0364 |
BMI is a predictor of the prognosis of
PCa at lower PSA levels
The median follow-up period of this study was 34
months (range, 0–108 months). Of the 2003 patients, 396 (19.8%)
experienced biochemical recurrence, including 283 of the 1,434
(19.7%) in the normoweight group and 113 of the 569 (19.9%) in the
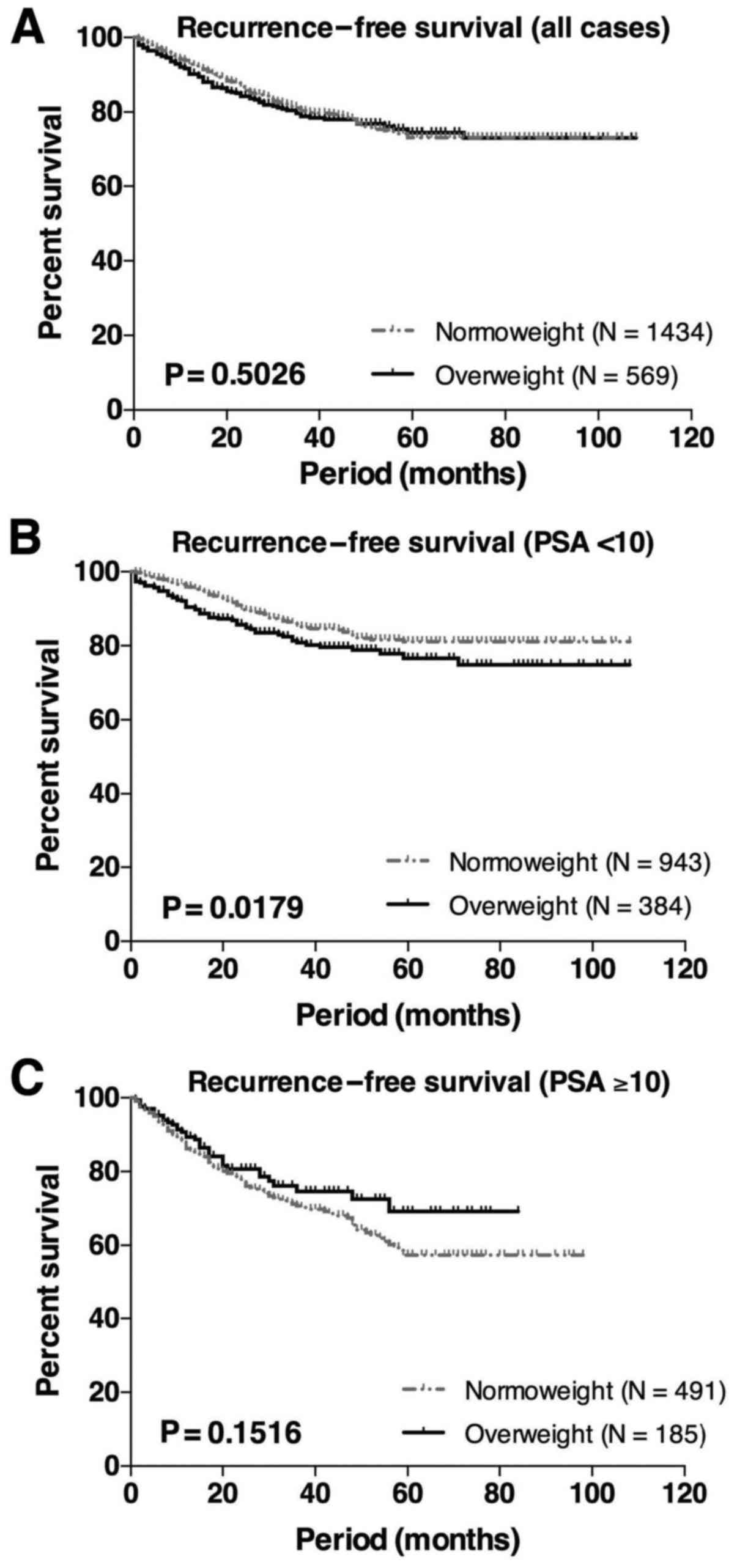
overweight group. Kaplan-Meier analysis was used to evaluate the
association of BMI with biochemical recurrence. When evaluated in
all 2003 cases, there was no significant difference between bRFS in
the normoweight vs. the overweight group (Fig. 1A). In patients with lower PSA levels
(<10 ng/ml), the overweight group exhibited a significantly
worse prognosis compared with the normoweight group (P=0.0179,
log-rank test, Fig. 1B). However, no
significant difference was observed in patients with higher PSA
levels (>10 ng/ml, Fig. 1C).
Univariate and multivariate Cox proportional hazards
analyses were next performed to evaluate the role of BMI as a
predictor of bRFS in patients with PSA<10 ng/ml (Table III). The univariate analysis
indicated that pathological stage T3, GS ≥8, EPE1, RM1 and BMI
>25 kg/m2 were significantly associated with bRFS,
whereas age was not. The multivariate model, which included pT
stage, GS, EPE, RM and BMI, revealed that BMI was not an
independent predictor of bRFS, whereas pT3, GS ≥8 and RM1 were.
 | Table III.Univariate and multivariate Cox
proportional hazard models for biochemical recurrence after
prostatectomy in patients with PSA <10 ng/ml. |
Table III.
Univariate and multivariate Cox
proportional hazard models for biochemical recurrence after
prostatectomy in patients with PSA <10 ng/ml.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
|
|
0.1715 |
|
|
0.1768 |
|
<70 | 1 (Reference) |
|
| 1 (Reference) |
|
|
≥70 | 1.224 | 0.916–1.631 |
| 1.222 | 0.913–1.631 |
| pT stage |
|
| <0.0001 |
|
|
0.0330 |
| T2 | 1 (Reference) |
|
| 1 (Reference) |
|
| T3 | 3.866 | 2.872–5.174 |
| 2.273 | 1.070–4.528 |
| GS |
|
| <0.0001 |
|
| <0.0001 |
| ≤7 | 1 (Reference) |
|
| 1 (Reference) |
|
| ≥8 | 3.461 | 2.585–4.617 |
| 2.433 | 1.791–3.291 |
| EPE |
|
| <0.0001 |
|
|
0.9371 |
|
EPE0 | 1 (Reference) |
|
| 1 (Reference) |
|
|
EPE1 | 3.433 | 2.541–4.603 |
| 0.971 | 0.489–2.049 |
| RM |
|
| <0.0001 |
|
| <0.0001 |
|
RM0 | 1 (Reference) |
|
| 1 (Reference) |
|
|
RM1 | 3.926 | 2.946–5.235 |
| 2.712 | 1.992–3.693 |
| BMI
(kg/m2) |
|
|
0.0309 |
|
|
0.4604 |
|
<25 | 1 (Reference) |
|
| 1 (Reference) |
|
|
≥25 | 1.400 | 1.032–1.882 |
| 1.122 | 0.824–1.515 |
Discussion
The association between BMI and the
clinicopathological characteristics was investigated in 2003
Japanese patients with PCa who underwent RP. First, it was
demonstrated that high BMI was associated with adverse pathological
findings. These results, supported those of previous studies
showing that obese men in the United States who underwent RP had
higher-grade and larger tumors (8,10,13–17).
In Asian patients, the adverse pathological findings of PCa may be
attributable to obesity according to previous reports (18,19).
However, other reports have not demonstrated any
association of obesity with the clinicopathological characteristics
of PCa (20,21). Such conflicting results may be
explained by the different distribution of BMI among countries. As
the obese (BMI ≥30 kg/m2) population accounted for only
1–2% of the cases in reports from Asian countries, the cutoff for
normal BMI is variably classified as 23.5 or 25.0 kg/m2.
In the present study, a significant association of BMI with GS was
observed, whereas such an association was not observed for serum
PSA level, pT stage, EPE and RM. These results may suggest that a
higher BMI was associated with more aggressive phenotypes of
PCa.
Although there was no significant association
between BMI and serum PSA level (Table
I), the overweight group exhibited a greater risk of
biochemical recurrence in patients with lower PSA levels (Fig. 1B). Indeed, previous studies reported
that BMI is inversely associated with serum PSA levels (23,24), and
a higher BMI is associated with higher plasma volume (25). Thus, the reason why obese men have
lower serum PSA concentrations may be explained by the hemodilution
theory (26,27). Therefore, it is possible that the
serum PSA levels may be modified in the overweight group. However,
in the multivariate analysis, BMI was not an independent predictor
of biochemical recurrence. Recently, PSA mass, which was associated
with visceral adipose tissue, was suggested to be a promising
indicator for determining an absolute PSA level (28).
Previous studies have also demonstrated an
association between obesity and the aggressiveness of PCa through
various molecular mechanisms, including oxidative stress, endocrine
activities, or other cytokine activities (29). It is known that adipose tissue
secretes certain inflammatory cytokines, referred to as
adipocytokines (30). In addition,
we previously reported a positive correlation between the
aggressiveness of PCa and fibroblast growth factor (FGF)-19,
including the endocrine FGF subfamily that circulates in the serum
and acts in an endocrine-like manner (31). Further investigations are required to
elucidate the associations between BMI or obesity and PSA
levels.
The present study had certain limitations. First,
the median follow-up period for establishing biochemical recurrence
after RP was relatively short. Second, this was a retrospective
study that involved patients subjected to RP using different
procedures by several surgeons, although the oncological outcomes
of retropubic, retroperitoneal, laparoscopic and robot-assisted
laparoscopic RP were comparable (32,33).
Third, we investigated whether obesity affects pathological
findings and biological recurrence after RP using only preoperative
BMI, as postoperative BMI values were not available. Although it
remains unclear whether weight loss may help improve outcomes among
patients already diagnosed with PCa, further investigations are
required to elucidate the role of BMI post-RP.
In summary, the results of the present study,
including 2003 Japanese patients who underwent RP for PCa, provide
evidence that a higher BMI may be associated with adverse
pathological findings. Although BMI was not an independent
predictor for bRFS after RP, BMI may be associated with more
aggressive characteristics of PCa.
References
|
1
|
Dunn JE: Cancer epidemiology in
populations of the United States-with emphasis on Hawaii and
California-and Japan. Cancer Res. 35:3240–3245. 1975.PubMed/NCBI
|
|
2
|
Shimizu H, Ross RK, Bernstein L, Yatani R,
Henderson BE and Mack TM: Cancers of the prostate and breast among
Japanese and white immigrants in Los Angeles County. Br J Cancer.
63:963–966. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SY, Haiman CA, Cheng I, Park SL,
Wilkens LR, Kolonel LN, Le Marchand L and Henderson BE:
Racial/ethnic differences in lifestyle-related factors and prostate
cancer risk: The multiethnic cohort study. Cancer Causes Control.
26:1507–1515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bostwick DG, Burke HB, Djakiew D, Euling
S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters
DJ and Timms B: Human prostate cancer risk factors. Cancer. 101:(10
Suppl). S2371–S2490. 2004. View Article : Google Scholar
|
|
5
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andersson SO, Wolk A, Bergström R, Adami
HO, Engholm G, Englund A and Nyrén O: Body size and prostate
cancer: A 20-year follow-up study among 135006 Swedish construction
workers. J Natl Cancer Inst. 89:385–389. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giovannucci E, Rimm EB, Liu Y, Leitzmann
M, Wu K, Stampfer MJ and Willett WC: Body mass index and risk of
prostate cancer in U.S. health professionals. J Natl Cancer Inst.
95:1240–1244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Discacciati A, Orsini N and Wolk A: Body
mass index and incidence of localized and advanced prostate
cancer-a dose-response meta-analysis of prospective studies. Ann
Oncol. 23:1665–1671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodriguez C, Patel AV, Calle EE, Jacobs
EJ, Chao A and Thun MJ: Body mass index, height, and prostate
cancer mortality in two large cohorts of adult men in the United
States. Cancer Epidemiol Biomarkers Prev. 10:345–353.
2001.PubMed/NCBI
|
|
10
|
Freedland SJ, Bañez LL, Sun LL, Fitzsimons
NJ and Moul JW: Obese men have higher-grade and larger tumors: An
analysis of the duke prostate center database. Prostate Cancer
Prostatic Dis. 12:259–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chamie K, Oberfoell S, Kwan L, Labo J, Wei
JT and Litwin MS: Body mass index and prostate cancer severity: Do
obese men harbor more aggressive disease on prostate biopsy?
Urology. 81:949–955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomaszewski JJ, Chen YF, Bertolet M,
Ristau BT, Woldemichael E and Nelson JB: Obesity is not associated
with aggressive pathologic features or biochemical recurrence after
radical prostatectomy. Urology. 81:992–996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amling CL, Riffenburgh RH, Sun L, Moul JW,
Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP,
et al: Pathologic variables and recurrence rates as related to
obesity and race in men with prostate cancer undergoing radical
prostatectomy. J Clin Oncol. 22:439–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freedland SJ, Aronson WJ, Kane CJ, Presti
C Jr, Amling CL, Elashoff D and Terris MK: Impact of obesity on
biochemical control after radical prostatectomy for clinically
localized prostate cancer: A report by the Shared Equal Access
Regional Cancer Hospital database study group. J Clin Oncol.
22:446–453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freedland SJ, Terris MK, Presti JC Jr,
Amling CL, Kane CJ, Trock B and Aronson WJ: Search Database Study
Group: Obesity and biochemical outcome following radical
prostatectomy for organ confined disease with negative surgical
margins. J Urol. 172:520–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freedland SJ, Grubb KA, Yiu SK, Humphreys
EB, Nielsen ME, Mangold LA, Isaacs WB and Partin AW: Obesity and
risk of biochemical progression following radical prostatectomy at
a tertiary care referral center. J Urol. 174:919–922. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freedland SJ, Isaacs WB, Mangold LA, Yiu
SK, Grubb KA, Partin AW, Epstein JI, Walsh PC and Platz EA:
Stronger association between obesity and biochemical progression
after radical prostatectomy among men treated in the last 10 years.
Clin Cancer Res. 11:2883–2888. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SE, Lee WK, Jeong MS, Abdullajanov M,
Kim DS, Park HZ, Jeong SJ, Yoon CY, Byun SS, Choe G and Hong SK: Is
body mass index associated with pathological outcomes after radical
prostatectomy in Korean men? BJU Int. 107:1250–1255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai PD, Hu MB, Xu H, Zhu WH, Hu JM, Yang
T, Jiang HW and Ding Q: Body mass index is associated with higher
Gleason score and biochemical recurrence risk following radical
prostatectomy in Chinese men: A retrospective cohort study and meta
analysis. World J Surg Oncol. 13:3112015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Komaru A, Kamiya N, Suzuki H, Endo T,
Takano M, Yano M, Kawamura K, Imamoto T and Ichikawa T:
Implications of body mass index in Japanese patients with prostate
cancer who had undergone radical prostatectomy. Jpn J Clin Oncol.
40:353–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narita S, Mitsuzuka K, Yoneyama T,
Tsuchiya N, Koie T, Kakoi N, Kawamura S, Kaiho Y, Ohyama C, Tochigi
T, et al: Impact of body mass index on clinicopathological outcome
and biochemical recurrence after radical prostatectomy. Prostate
Cancer Prostatic Dis. 16:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA: Grading Committee: The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI
|
|
23
|
Baillargeon J, Pollock BH, Kristal AR,
Bradshaw P, Hernandez J, Basler J, Higgins B, Lynch S, Rozanski T,
Troyer D and Thompson I: The association of body mass index and
prostate-specific antigen in a population-based study. Cancer.
103:1092–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Werny DM, Thompson T, Saraiya M, Freedman
D, Kottiri BJ, German RR and Wener M: Obesity is negatively
associated with prostate-specific antigen in U.S. men, 2001–2004.
Cancer Epidemiol Biomarkers Prev. 16:70–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bañez LL, Hamilton RJ, Partin AW, Vollmer
RT, Sun L, Rodriguez C, Wang Y, Terris MK, Aronson WJ, Presti JC
Jr, et al: Obesity-related plasma hemodilution and PSA
concentration among men with prostate cancer. JAMA. 298:2275–2280.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rundle A, Richards C and Neugut AI: Body
composition, abdominal fat distribution and prostate-specific
antigen test results. Cancer Epidemiol Biomarkers Prev. 18:331–336.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grubb RL III, Black A, Izmirlian G, Hickey
TP, Pinsky PF, Mabie JE, Riley TL, Ragard LR, Prorok PC, Berg CD,
et al: Serum prostate-specific antigen hemodilution among obese men
undergoing screening in the prostate, lung, colorectal, and ovarian
cancer screening trial. Cancer Epidemiol Biomarkers Prev.
18:748–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SG, Choi HC, Cho B, Kwon YM, Kwon HT
and Park JH: Effect of central obesity on prostate specific antigen
measured by computerized tomography: Related markers and prostate
volume. J Urol. 187:1589–1593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buschemeyer WC III and Freedland SJ:
Obesity and prostate cancer: Epidemiology and clinical
implications. Eur Urol. 52:331–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arisan ED, Arisan S, Atis G, Palavan-Unsal
N and Ergenekon E: Serum adipocytokine levels in prostate cancer
patients. Urol Int. 82:203–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagamatsu H, Teishima J, Goto K, Shikuma
H, Kitano H, Shoji K, Inoue S and Matsubara A: FGF19 promotes
progression of prostate cancer. Prostate. 75:1092–1101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salomon L, Levrel O, de la Taille A,
Anastasiadis AG, Saint F, Zaki S, Vordos D, Cicco A, Olsson LE,
Hoznek A, et al: Radical prostatectomy by the retropubic, perineal
and laparoscopic approach: 12 years of experience in one center.
Eur Urol. 42:104–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim SK, Kim KH, Shin TY and Rha KH:
Current status of robot-assisted laparoscopic radical
prostatectomy: How does it compare with other surgical approaches?
Int J Urol. 20:271–284. 2013. View Article : Google Scholar : PubMed/NCBI
|