Introduction
The incidence of hepatocellular carcinoma (HCC) has
increased significantly over previous decades, and globally it
represents the sixth most common cancer type and the third highest
cause of cancer mortality among the general population (1). Hepatitis B and C and alcohol abuse are
major risk factors for HCC. Although local ablation techniques and
multikinase inhibitors, such as radiofrequency ablation and
sorafenib, are increasingly prevalent, transcatheter arterial
chemoembolization (TACE) remains one of the standard treatments for
patients with intermediate- and advanced-stage HCC (1–3). Several
randomized controlled studies have revealed that TACE improves
survival and controls the symptoms of HCC (4–8).
Despite its significant anti-tumor effects, repeat
TACE is frequently required in patients with HCC and can cause more
local complications than conservative management. To minimize the
risk associated with TACE, it is important to understand the major
complications of this procedure. The most common and severe
complication is TACE-associated hepatic and biliary damage, which
primarily consists of hepatic insufficiency, liver abscess and
intrahepatic biloma formation among others (9).
Once intrahepatic biloma has developed, repeat TACE
becomes more difficult and riskier in patients with HCC (9). Therefore the methods for effective
prevention and treatment of intrahepatic biloma after TACE must be
determined. The purpose of this study was to clarify the clinical
course, incidence, risk factors, interventional management and
outcome of intrahepatic biloma following TACE.
Patients and methods
Patients
A total of 4,695 TACE procedures were performed for
the 1,923 patients with HCC in the third affiliated hospital
(between January 2007 and October 2012) and in the sixth affiliated
hospital (between November 2012 and July 2015) of Sun Yat-sen
University (Guangzhou, China). Of these patients, 20 consecutive
cases of intrahepatic biloma following TACE confirmed by clinical
history and computed tomography (CT) and/or magnetic resonance
imaging (MRI) were analyzed retrospectively. The study protocol was
approved by the Institutional Ethics Review Board of our hospitals.
Written informed consent was obtained from each patient.
The diagnosis of HCC was established by dynamic
radiological finding and clinical data. The conventional
triple-phase dynamic radiological behavior of HCC included early
enhancement on the arterial phase and fast washout on the portal
venous and delayed venous phases (10). Chronic hepatitis B, chronic hepatitis
C, liver cirrhosis and tumor markers (such as α-fetoprotein) were
also considered as other supporting evidence for the presence of
HCC.
According to previous reports (9,11), the
diagnosis of intrahepatic biloma following TACE was based on i)
round, solitary or multiple cystic lesions located in nontumoral
parenchyma with or without segmental bile duct dilatation or ii) a
branched hypoattenuating area along the Glisson's sheath similar to
dilatation of the intrahepatic bile duct without enhancement at any
of the vascular phases on follow-up CT scans. On MRI scans, these
lesions demonstrated hypointensity and hyperintensity on T1- and
T2-weighted images, respectively, without evidence of
enhancement.
Procedure
TACE was typically performed using the following
steps. A right transfemoral approach was used for artery access,
and following insertion of a 4- to 5-French catheter into the
hepatic artery, the feeding arteries were evaluated using hepatic
angiography. For the fine and tortuous feeding artery, a 2.2–2.8
French microcatheter was used to minimize the embolized area.
Subsequent to microcatheter insertion into the proper branch,
chemoembolization was performed through the injection of a 2–50 ml
mixture of anticancer drugs and iodized oil (Lipiodol; Andre
Guerbet, Aulnaysous-Bois, France) with or without gelfoam or
polyvinyl alcohol particles. The total amount of mixture in a
single procedure was determined based on tumor size and blood
supply. No more than three of the following anticancer drugs were
used for each procedure: Doxorubicin hydrochloride (10–30 mg),
epirubicin hydrochloride (10–30 mg), mitomycin C (6–10 mg),
nedaplatin (40–100 mg), lobaplatin (50–100 mg), cisplatin (25–100
mg), oxaliplatin (50–200 mg) and 5-fluorouracil (250–1250 mg).
Follow-up and data analysis
Dynamic enhanced CT or MRI was performed every 1–2
months after TACE to check for iodized oil distribution and tumor
recurrence. If local recurrence or new lesions were confirmed, an
additional TACE procedure and/or other treatment (e.g.
radiofrequency or microwave ablation, percutaneous ethanol
injection, radioactive 125I seed implantation or
systemic chemotherapy) were performed. Once the signs of
intrahepatic biloma formation were detected, these patients were
monitored closely. Percutaneous puncture into the biloma cavity
directly and aspiration or drainage were performed for infectious
lesions and jaundice.
Clinical data were analyzed with respect to age,
gender, Child-Pugh classification (11), main tumor size, TACE procedures,
volume of ipiodol administered, treatment and outcome of biloma,
which were collected from the original hospital charts, operation
notes and outpatient medical records via telephone questionnaires.
The end-point of follow-up was the time of patient mortality and
liver transplantation. Data analysis was performed using SPSS
version 19.0 (IBM SPSS, Armonk, NY, USA) to generate Kaplan-Meier
curves.
Results
Baseline characteristics
As listed in Table I,
a total of 20 patients were included for analysis. There were 19
males and 1 female (mean age, 51 years; range, 20–76 years). The
incidence of intrahepatic biloma following TACE was 1.04% in this
series. Prior to intrahepatic biloma formation, the 20 patients
underwent 55 TACE procedures (mean per patient, 2.75; range, 1–6)
and the mean HCC diameter was 7.97 cm (range, 1.3–13 cm). The rate
of portal vein invasion was 50% (10/20). The mean interval time
between intrahepatic biloma formation and most recent TACE
procedure was 69.1 days (range, 19–155 days).
 | Table I.Clinical characteristics of 20
patients prior to intrahepatic biloma formation. |
Table I.
Clinical characteristics of 20
patients prior to intrahepatic biloma formation.
| Case no. | Sex | Age, years | CPC | Location | Largest tumor
dimension, cm | PV invasion | Embolized
branches | No. of TACE
procedures; embolic agents(iodized oil, ml)a | Interval time,
daysb |
|---|
| 1 | M | 76 | A | RL | 12 | No | Branches of right
HA | 2; (20 + gelfoam +
PVA)/(20 + PVA) | 19 |
| 2 | M | 55 | A | RL | 4 | Right PV and A-V
fistulas | Branches of right HA
and SMA | 1; (10) | 90 |
| 3 | M | 52 | A | LL | 1.3 | No | Branches of left
HA | 1; (10) | 49 |
| 4 | M | 38 | A | RL | 9 | Right anterior
branch | Branches of right
HA | 3; (30 + PVA)/(20 +
PVA)/(20 + PVA) | 43 |
| 5 | M | 52 | A | RL | 4.2 | Right posterior | Branches of right
HA | 3; (10)/(10)/(4) | 55 |
| 6 | M | 57 | A | LL | 1.9 | No | Branches of left HA
and RIPA | 2; (5)/(5) | 93 |
| 7 | M | 37 | A | RL | 10 | Right anterior
branch | Branches of left and
right HA and SMA | 3; (10 +
PVA)/(6)/(10) | 56 |
| 8 | M | 71 | A | RL | 9.2 | No | Branches of right
HA | 2; (10)/(10) | 33 |
| 9 | M | 50 | B | RL | 10 | No | Branches of right
HA | 3; (10)/(7)/(5) | 34 |
| 10 | M | 48 | A | RL | 12.5 | Right branch | Branches of right
HA | 5;
(15)/(10)/(8)/(6)/(7) | 23 |
| 11 | M | 62 | A | RL | 6.4 | No | Branches of right
HA | 2; (25 + PVA)/(6 +
PVA) | 155 |
| 12 | F | 64 | A | RL | 6.1 | Right anterior
branch | Branches of right HA,
SMA and RIPA | 1; (20) | 30 |
| 13 | M | 37 | A | RL | 10 | Right branch | Branches of proper HA
and RIPA | 3; (30 +
PVA)/(30)/(10) | 112 |
| 14 | M | 69 | A | RL | 10.2 | No | Branches of right
HA | 4; (40 + PVA +
gelfoam)/ (20)/(10)/(20) | 153 |
| 15 | M | 20 | A | RL | 13 | Right anterior
branch | Branches of right
and meddle HA | 4;
(30)/(10)/(10)/(12) | 82 |
| 16 | M | 31 | A | RL | 8.5 | Main branches of
PV | Branches of right
HA, LGA and RIPA | 2; (20 + PVA)/(10 +
gelfoam) | 45 |
| 17 | M | 50 | A | RL | 5 | No | Branches of right
HA | 1; (8) | 80 |
| 18 | M | 49 | A | RL | 8 | Right anterior
branch | Branches of right
HA and RIPA | 4; (45 +
gelfoam)/(40 + PVA)/(28)/(30 + gelfoam) | 102 |
| 19 | M | 51 | A | RL | 8.1 | No | Branches of right
HA | 3; (25 +
gelfoam)/(20 + gelfoam)/(13) | 68 |
| 20 | M | 43 | A | RL+LL | 10 | No | Branches of left
and right HA | 6; (30 +
gelfoam)/(15)/(10)/(8)/(8)/(5) | 60 |
As presented in Table
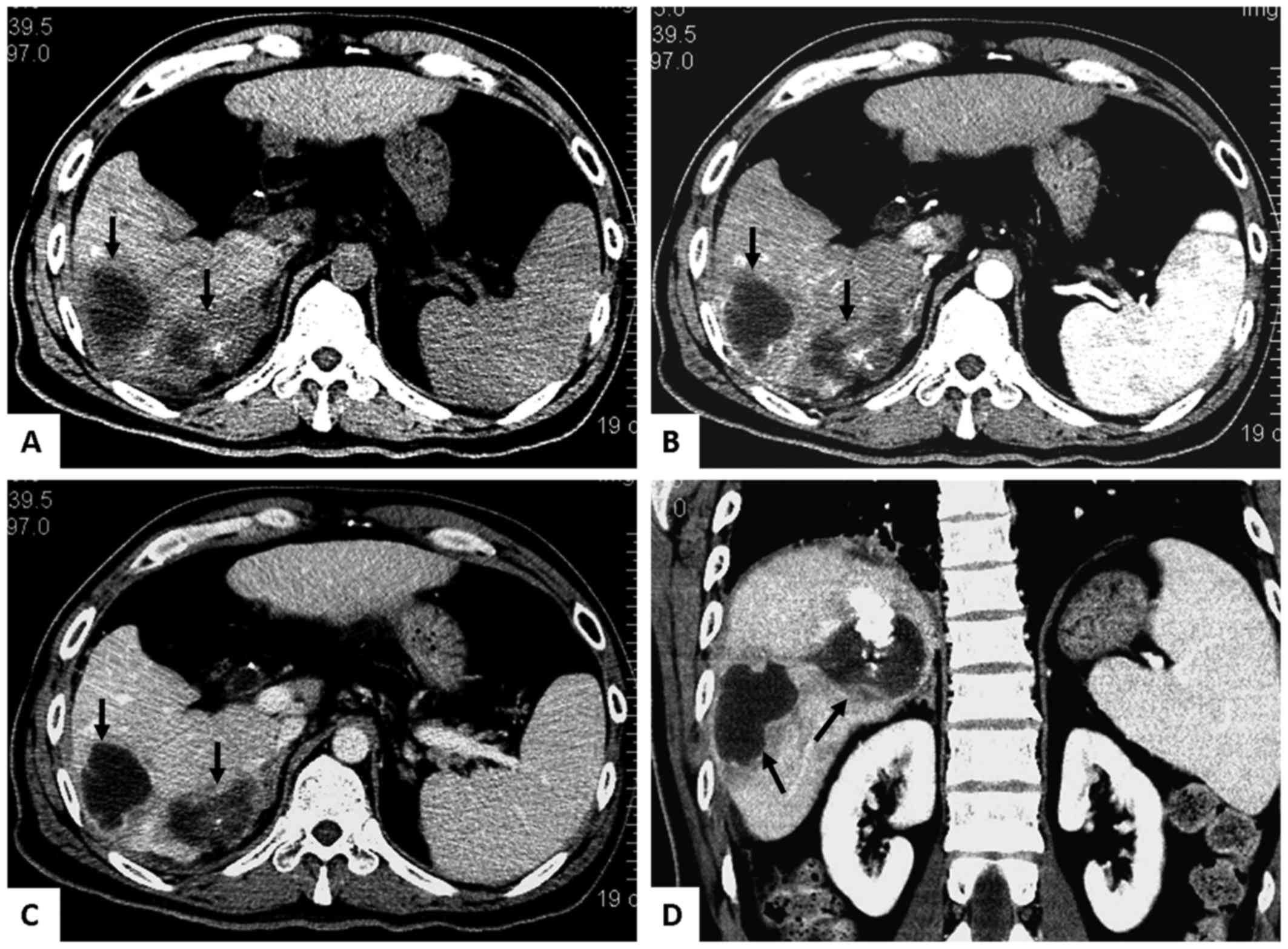
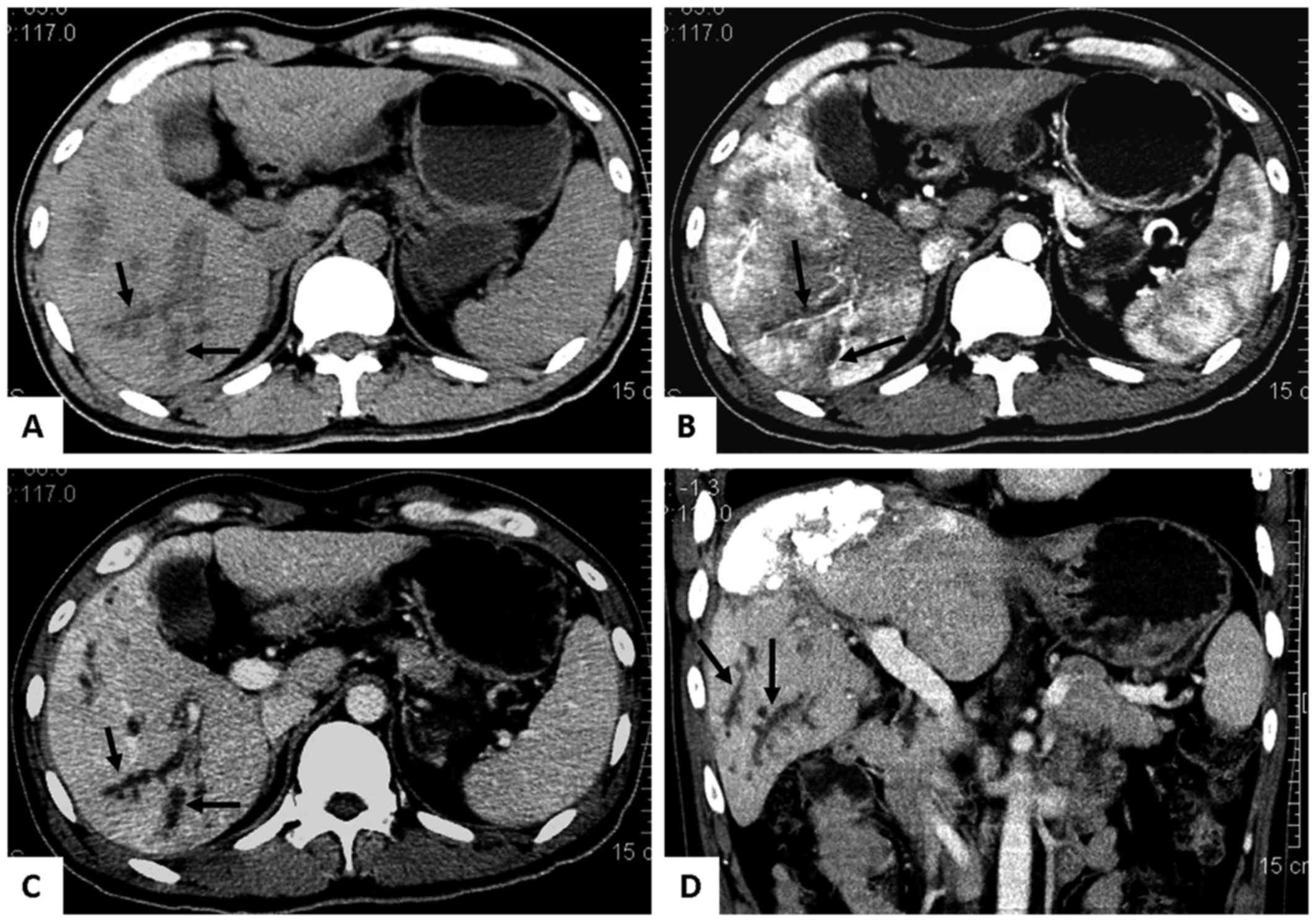
II, eleven patients developed round solitary or multiple cystic
biloma (Fig. 1), six patients
developed branched biloma (Fig. 2)
mimicking diffuse bile duct dilatation and the remaining 3 patients
had cystic and branched biloma.
 | Table II.Follow-up and interventional
treatment of 20 patients with intrahepatic biloma. |
Table II.
Follow-up and interventional
treatment of 20 patients with intrahepatic biloma.
| Case no. | Sex | Age, years | Shape of
Biloma | Treatment | Follow-up,
monthsa | Sequential therapy
of tumor | Biloma Outcome | Clinical
outcome |
|---|
| 1 | M | 76 | Cystic | Percutaneous
drainage | 6 | PEI and
125I seed implantation | Shrank | Survival |
| 2 | M | 55 | Cystic | Conservative | 6.5 | 125I
seed implantation and 1 TACE | Stable | Survival |
| 3 | M | 52 | Multiple
cystic | Conservative | 5 | 2 TACE | Stable | Mortality |
| 4 | M | 38 | Cystic | Percutaneous
drainage | 18 | 2 TACE | Shrank | Survival |
| 5 | M | 52 | Branched | Conservative | 11 | Supportive
therapy | Stable | Survival |
| 6 | M | 57 | Branched and
cystic | Conservative | 12 | 2 TACE and
radiofrequency ablation | Shrank | Survival |
| 7 | M | 37 | Branched | Conservative | 16 | 1 TACE | Stable | Survival |
| 8 | M | 71 | Branched and
cystic | Percutaneous
drainage | 13 | 1 TACE and
microwave ablation | Shrank | Survival |
| 9 | M | 50 | Cystic | Conservative | 15 | 2 TACE | Shrank | Survival |
| 10 | M | 48 | Cystic | Conservative | 15 | 1 TACE | Increased
slightly | Survival |
| 11 | M | 62 | Cystic | PTCD for
jaundice | 19 | 2 TACE | Stable | Survival |
| 12 | F | 64 | Cystic | Conservative | 8 | 1 TACE | Stable | Mortality |
| 13 | M | 37 | Cystic | Conservative | 11 | 2 TACE | Increased
slightly | Mortality |
| 14 | M | 69 | Branched | Conservative | 58 | 1 TACE and partial
hepatectomy | Hepatectomy | Survival |
| 15 | M | 20 | Cystic | Conservative | 62 | Supportive
therapy | Stable | Mortality |
| 16 | M | 31 | Branched | Conservative | 5 | 1 TACE and liver
transplantation | Stable | Liver
transplantation |
| 17 | M | 50 | Branched and
cystic | Conservative | 15 | 3 TACE | Stable | Mortality |
| 18 | M | 49 | Branched | Conservative | 21 | 3 TACE | Progressive | Mortality |
| 19 | M | 51 | Cystic | Conservative | 9 | Radiofrequency
ablation and 1 TACE | Stable | Mortality |
| 20 | M | 43 | Branched | Conservative | 6 | Systemic
chemotherapy | Stable | Mortality |
Interventional treatment of the
biloma
Four patients with infection symptoms (jaundice,
upper abdominal pain and fever) were treated through percutaneous
transhepatic cholangial drainage (PTCD, n=1) and percutaneous
drainage (n=3) under ultrasonography or CT guidance.
Conservative medical treatment was performed in the
remaining 16 patients. During follow-up, the size of the biloma was
reduced, remained stable or slightly increased in 2, 10 and 2
patients, respectively. One patient underwent partial hepatectomy
for jaundice and the increase of biloma. The other patient
succumbed to progressive biloma and multiple organ failure.
Intrahepatic biloma formation made the repeat TACE
more challenging and riskier to perform in these patients. On
average, only 1 TACE procedure (range 0–3 procedures) was performed
in this group, and the sequential therapy included radioactive
125I seed implantation (n=2), microwave ablation (n=1),
radiofrequency ablation (n=2), percutaneous ethanol injection
(n=1), partial hepatectomy (n=1), systemic chemotherapy (n=1),
supportive therapy (n=2) and liver transplantation (n=1).
Survival analysis
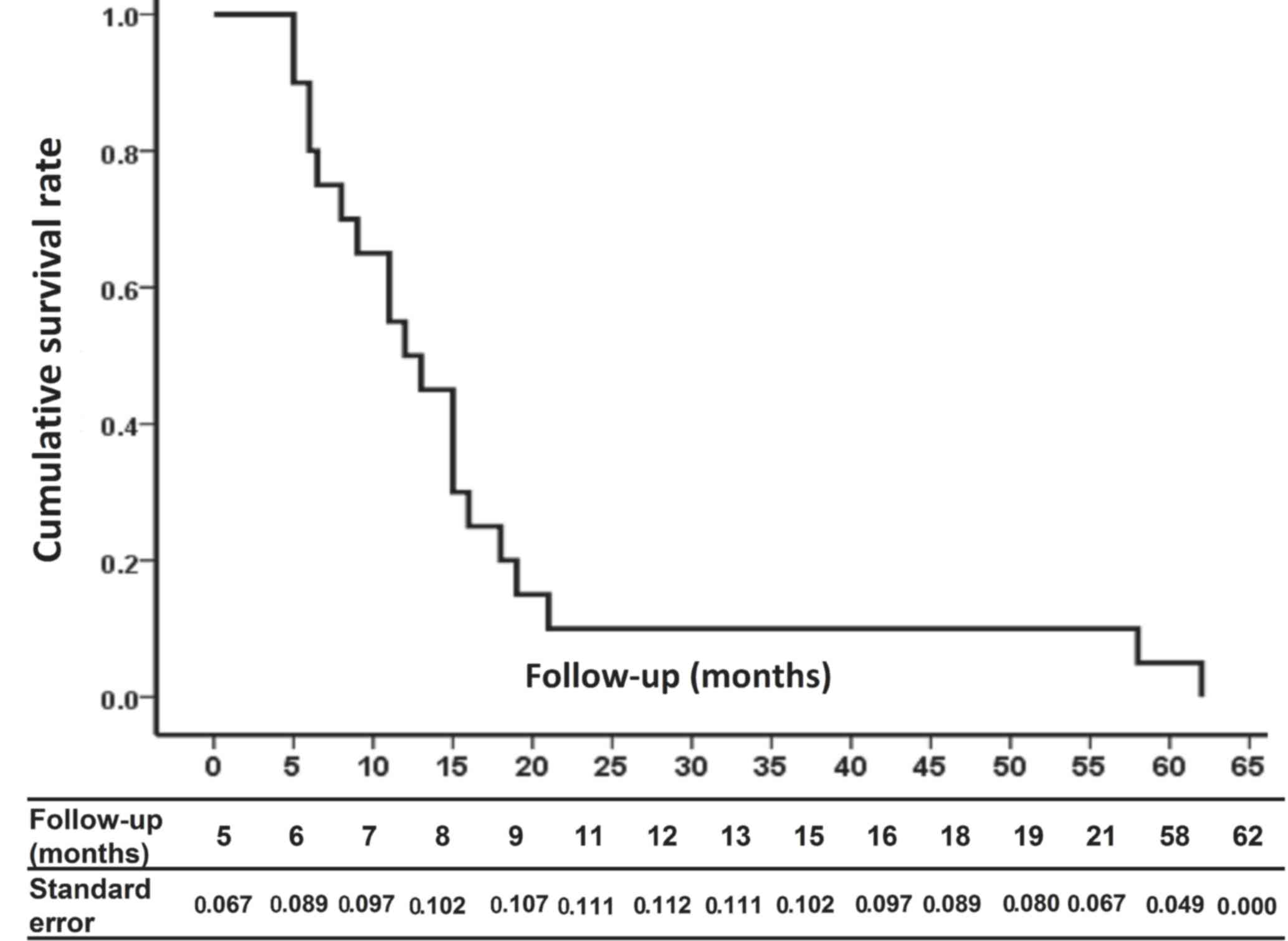
Kaplan-Meier curves revealed the cumulative survival
rate of intrahepatic biloma patients following TACE for HCC
(Fig. 3). The standard error of the
mean exceeded 10% from 8–15 months after formation of intrahepatic
biloma.
Discussion
Intrahepatic biloma may easily be confirmed using
enhanced CT or MRI. The incidence of intrahepatic biloma following
TACE varies greatly from 0.05% (12)
to 1.04% (in the present study) to 11.3% (13). The incidence discrepancy between the
present study and previous reports may be partially associated with
the patient population, embolic material, anticancer drugs and the
TACE procedure.
The liver is dually supplied by hepatic arterial and
portal venous blood; however, HCC is primary supplied by the
former. Therefore, TACE preferentially interrupts the blood supply
of HCC cells and controls tumor growth. Unlike the normal liver
parenchyma, the intrahepatic bile ducts are supplied exclusively by
the hepatic arterial branches that form a vascular plexus
(peribiliary capillary plexus) around the bile ducts. During the
TACE procedure, the peribiliary capillary plexus is filled with
iodized oil or other embolic material. Therefore, ischemia of the
intrahepatic bile ducts may easily occur following TACE (13–15).
Liver cirrhosis has a notable role in the formation
of intrahepatic biloma following TACE. In the cirrhotic liver,
hypertrophy of the peribiliary capillary plexus may function as a
portoarterial shunt and compensate for the occluded arterial flow;
therefore, the hypertrophied peribiliary capillary plexus in the
cirrhotic liver is able to prevent the ischemic injury of bile
ducts during TACE (13,16,17). On
the other hand, the high incidence of bile duct injury in patients
with metastatic tumors and normal liver morphology also indicates
the protective role of the hypertrophied peribiliary plexus around
the bile ducts (9,18). The low incidence (1.04%) in this
study may be explained as the majority of patients had HCC
originating from the cirrhotic liver with hepatitis B virus
infection.
The other risk factors for intrahepatic biloma may
include portal vein thrombosis, biliary obstruction and
inflammation, the total number of TACE procedures undergone, and
the total volume of iodized oil, anticancer drugs, microspheres,
gelfoam and drug-eluting beads administered and undergoing
segmental or subsegmental TACE (13,18–21).
However, in the present study, with the exception of portal vein
invasion in half of the patients, there was no obvious trend of
repeat TACE procedures, embolic materials and anticancer drugs.
Potentially due to the low incidence and lack of prospective
studies of intrahepatic biloma following TACE, these risk factors
are not widely recognized.
In the majority of cases, intrahepatic biloma may be
treated conservatively. However, for moderate to severe signs of
infection with or without increases in the size of the biloma,
promptly percutaneous drainage or partial hepatectomy even internal
drainage combined with antibiotics should be applied first
(9,22–24). If
it is not treated in a timely and appropriate manner, it may result
in severe systemic infection, and even a bronchobiliary fistula or
biliopleural fistula and multiple organ failure (23,25). In
the current series, one patient succumbed to progressive biloma and
septic shock.
Repeat TACE becomes more challenging and is
associated with increased risk in patients with intrahepatic
biloma. On average, only 1 TACE (range, 0–3) procedure per patient
was performed in this series after formation of intrahepatic
biloma, and the sequential therapy included local treatment
(radioactive 125I, microwave or radiofrequency ablation,
percutaneous ethanol injection, partial hepatectomy), systemic
chemotherapy (folinic acid, 5-Fluorouracil and oxaliplatin,
sorafenib), supportive therapy and liver transplantation.
In conclusion, whilst severe intrahepatic biloma
following TACE is rare, the procedure must be performed cautiously
with superselection of the hepatic artery. If intrahepatic biloma
occurs, careful observations must be made during follow-up. Timely
and appropriate management including percutaneous drainage, partial
hepatectomy and antibiotic administration must be performed in the
case of signs of infection.
Acknowledgments
This study was supported by a grant from National
Natural Science Foundation of China (grant no. 81301978), Ph.D.
Programs Foundation of Ministry of Education of China (grant no.
20130171120105), Young Teacher Cultivation Project of Sun Yat-sen
University (grant no. 13YKPY39) and Guangdong Province Medical
Science Foundation (grant no. B2013162).
References
|
1
|
Lencioni R and Crocetti L: Local-regional
treatment of hepatocellular carcinoma. Radiology. 262:43–58. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xue TC, Xie XY, Zhang L, Yin X, Zhang BH
and Ren ZG: Transarterial chemoembolization for hepatocellular
carcinoma with portal vein tumor thrombus: A meta-analysis. BMC
Gastroenterol. 13:602013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM,
Poon RT, Fan ST and Wong J: Randomized controlled trial of
transarterial lipiodol chemoembolization for unresectable
hepatocellular carcinoma. Hepatology. 35:1164–1171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lo GH: The use of transarterial
chemoembolization in hepatiocellular carcinoma 2 cm or smaller. Am
J Gastroenterol. 110:1962015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chern MC, Chuang VP, Liang CT, Lin ZH and
Kuo TM: Transcatheter arterial chemoembolization for advanced
hepatocellular carcinoma with portal vein invasion: Safety,
efficacy, and prognostic factors. J Vasc Interv Radiol. 25:32–40.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz
A, Ryu RK, Baker TB, Ibrahim SM, Abecassis MI, Miller FH, Sato KT,
et al: Chemoembolization for hepatocellular carcinoma:
Comprehensive imaging and survival analysis in a 172-patient
cohort. Radiology. 255:955–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakamoto I, Iwanaga S, Nagaoki K, Matsuoka
Y, Ashizawa K, Uetani M, Fukuda T, Okimoto T, Okudaira S, Omagari
K, et al: Intrahepatic biloma formation (bile duct necrosis) after
transcatheter arterial chemoembolization. AJR Am J Roentgenol.
181:79–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iavarone M, Sangiovanni A, Forzenigo LV,
Massironi S, Fraquelli M, Aghemo A, Ronchi G, Biondetti P, Roncalli
M and Colombo M: Diagnosis of hepatocellular carcinoma in cirrhosis
by dynamic contrast imaging: The importance of tumor cell
differentiation. Hepatology. 52:1723–1730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guiu B, Deschamps F, Aho S, Munck F,
Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger
M, et al: Liver/biliary injuries following chemoembolisation of
endocrine tumours and hepatocellular carcinoma: Lipiodol vs.
drug-eluting beads. J Hepatol. 56:609–617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan
Y, Chen Y, Ge N, Ma Z, Wu Z, et al: Study of severe and rare
complications of transarterial chemoembolization (TACE) for liver
cancer. Eur J Radiol. 59:407–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu JS, Kim KW, Jeong MG, Lee DH, Park MS
and Yoon SW: Predisposing factors of bile duct injury after
transcatheter arterial chemoembolization (TACE) for hepatic
malignancy. Cardiovasc Intervent Radiol. 25:270–274. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung J, Yu JS, Chung JJ, Kim JH and Kim
KW: Haemodynamic events and localised parenchymal changes following
transcatheter arterial chemoembolisation for hepatic malignancy:
Interpretation of imaging findings. Br J Radiol. 83:71–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spina JC, Ulla M, Yeyati EL, Kucharczyk
MC, Irusta H, Savluk JL and García-Mónaco R: MDCT findings after
hepatic chemoembolization with DC-beads: What the radiologist needs
to know. Abdom Imaging. 38:778–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demachi H, Matsui O, Kawamori Y, Ueda K
and Takashima T: The protective effect of portoarterial shunts
after experimental hepatic artery embolization in rats with liver
cirrhosis. Cardiovasc Intervent Radiol. 18:97–101. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zipprich A, Loureiro-Silva MR, D'Silva I
and Groszmann RJ: The role of hepatic arterial flow on portal
venous and hepatic venous wedged pressure in the isolated perfused
CCl4-cirrhotic liver. Am J Physiol Gastrointest Liver Physiol.
295:G197–G202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhagat N, Reyes DK, Lin M, Kamel I, Pawlik
TM, Frangakis C and Geschwind JF: Phase II study of
chemoembolization with drug-eluting beads in patients with hepatic
neuroendocrine metastases: High incidence of biliary injury.
Cardiovasc Intervent Radiol. 36:449–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen MJ, Lin CC, Chang WH and Yang FS:
Biloma following repeated transcatheter arterial embolization and
complicated by intrahepatic duct stones: A case report. World J
Gastroenterol. 11:4764–4765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung JW, Park JH, Han JK, Choi BI, Han
MC, Lee HS and Kim CY: Hepatic tumors: Predisposing factors for
complications of transcatheter oily chemoembolization. Radiology.
198:33–40. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naumann M, Bonsall R and Gupta R:
Chemoembolization with drug-eluting beads complicated by
intrahepatic biloma. Semin Intervent Radiol. 28:212–217. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maruyama T, Mori A, Tatebe H, Sakai K,
Isono N, Ohashi N, Inoue H, Takegoshi S and Okuno M: A novel
technique for the internal drainage of extrahepatic biloma
complicating transarterial embolization of hepatocellular
carcinoma. J Gastroenterol. 42:783–786. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akazawa S, Omagari K, Amenomori M,
Nishiyama H, Mizuta Y and Kohno S: Bronchobiliary fistula
associated with intrahepatic biloma after transcatheter arterial
chemoembolization for hepatocellular carcinoma. J Hepatol.
40:1045–1046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Z, Li G, Ai X, Luo B, Wen Y, Zhao Z,
Dong S and Guan J: Hepatic and biliary damage after transarterial
chemoembolization for malignant hepatic tumors: Incidence,
diagnosis, treatment, outcome and mechanism. Crit Rev Oncol
Hematol. 79:164–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Butt AS, Mujtaba G, Anand S and Krishnaiah
M: Management of biliopleural fistula after transarterial
chemoembolization of a liver lesion. Can J Gastroenterol.
24:281–283. 2010. View Article : Google Scholar : PubMed/NCBI
|