Introduction
Pancreatic acinar cell carcinoma (PACC) is a rare
tumor of the pancreas, comprising only 1% of all pancreatic
malignancies (1). PACC usually
presents with non-specific symptoms, such as abdominal pain and
weight loss (2), but jaundice is
less frequent compared with ductal carcinoma, as the tumor rarely
arises in the head of the pancreas (3). The first report of PACC described by
Berner was characterized by fever, polyarthritis, subcutaneous fat
nodular necrosis and eosinophilia (4). These characteristics are collectively
referred to as lipase hypersecretion syndrome (LHS), occurring due
to lipase hypersecretion by PACC cells (5). Although PACC may be difficult to
diagnose, subcutaneous fat necrosis, referred to as pancreatic
panniculitis, may represent an initial symptom pointing to the
diagnosis.
Despite the aggressive clinical behavior of PACC,
its prognosis is more favorable compared with that of pancreatic
ductal carcinoma, and surgical intervention is recommended if
possible (6). However, LHS is more
frequently encountered in patients with advanced disease, and its
presence is considered to be a negative prognostic factor, along
with a diameter of the primary tumor of >10 cm and metastatic
disease (7). In cases where curative
resection is not applicable due to advanced disease, chemotherapy
may be considered. Although no standard therapy for PACC has been
established to date due to the rarity of this disease,
5-fluorouracil (5-FU) is considered to be more effective compared
with gemcitabine (8).
We herein present the case of a patient who was
diagnosed with PACC accompanied by LHS who responded well to
treatment with the FOLFIRINOX regimen.
Case report
In January 2016, a 50-year-old man consulted a
dermatologist after developing edema of the thumb joints
bilaterally, with subsequent appearance of masses in the bilateral
lower extremities in February 2016. The patient had no significant
past medical history, was a social drinker and had smoked until the
age of 35 years. There were no reported allergies and no family
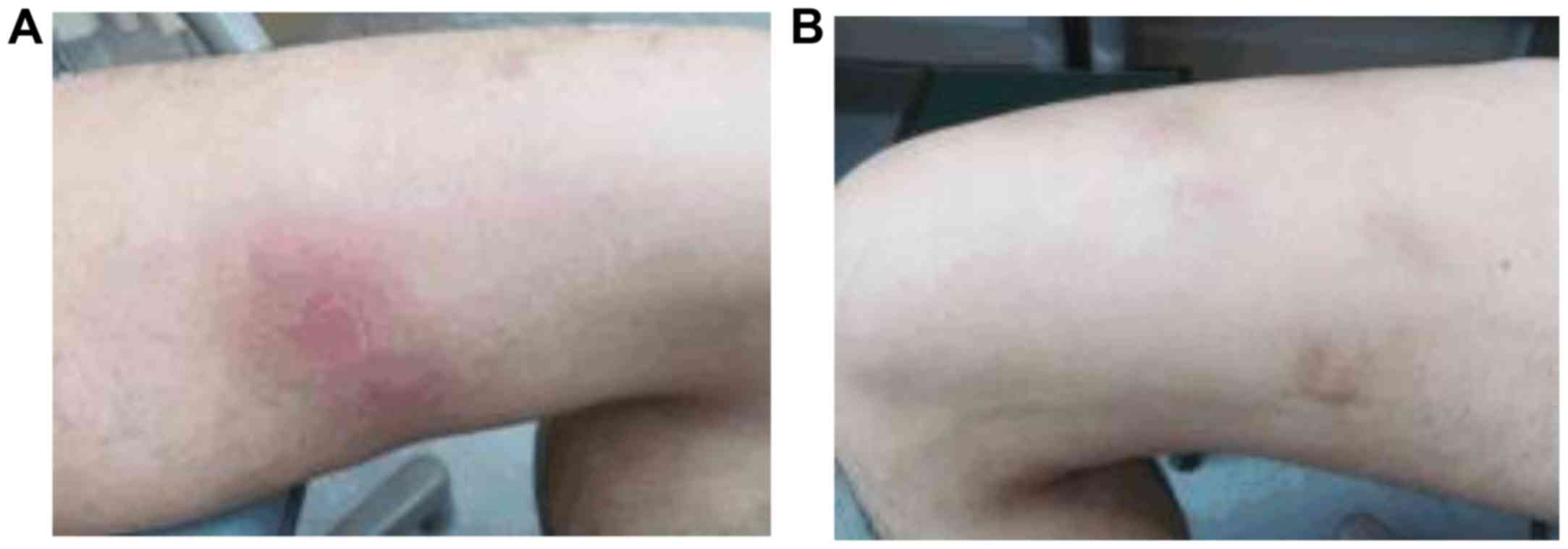
history of malignant tumors. The physical examination revealed
multiple subcutaneous masses in the lower extremities (Fig. 1), diagnosed as panniculitis by skin
biopsy. To further examine these masses, pelvic magnetic resonance
imaging (MRI) was performed and multiple intraperitoneal tumors
were incidentally detected. As the patient had been suffering from
a high fever since February, he was referred to the Kyushu
University Hospital (Fukuoka, Japan) for further investigation.
On admission, the Eastern Cooperative Oncology Group
(ECOG) performance status was 2 due to the persistent high fever.
Physical examination revealed subcutaneous masses in the right
chest wall and lower extremities. The blood test results were as
follows: White blood cell count, 8,960/µl (neutrophils, 91.4%); and
C-reactive protein, 9.43 mg/dl. The liver function was mildly
altered (aspartate aminotransferase, 80 U/l; alanine
aminotransferase, 229 U/l; alkaline phosphatase, 872 U/l; and
γ-glutamyltransferase, 118 U/l). Laboratory examination revealed
high serum concentrations of pancreatic exocrine enzymes, such as
lipase (327,500 U/l; normal range, 16–51 U/l), trypsin (68,400
ng/ml; normal range, 100–550 ng/ml), elastase 1 (3560 ng/dl; normal
range, <300 ng/dl) and pancreatic phospholipase A2 (23,500
ng/dl; normal range, 130–400 ng/dl), but the amylase levels
remained within the normal range (111 U/l). Pancreatic endocrine
enzymes, such as C-peptide (2.1 ng/ml) and gastrin (106 pg/ml) were
also within normal ranges. Tumor markers, such as α-fetoprotein
(30.3 ng/ml; normal range, <6.2 ng/ml), protein induced by
vitamin K absence or antagonist-II (87 mAU/ml; normal range, <40
mAU/ml), and neuron-specific enolase (17.9 ng/ml; normal range,
<10 ng/ml) were marginally elevated, but carcinoembryonic
antigen (0.9 ng/ml), carbohydrate antigen (CA) 19–9 (34.7 U/ml),
DUPAN-II (66 U/ml) and pro-gastrin-releasing peptide (37.1 pg/ml)
were within normal ranges. Fluorodeoxyglucose-positron emission
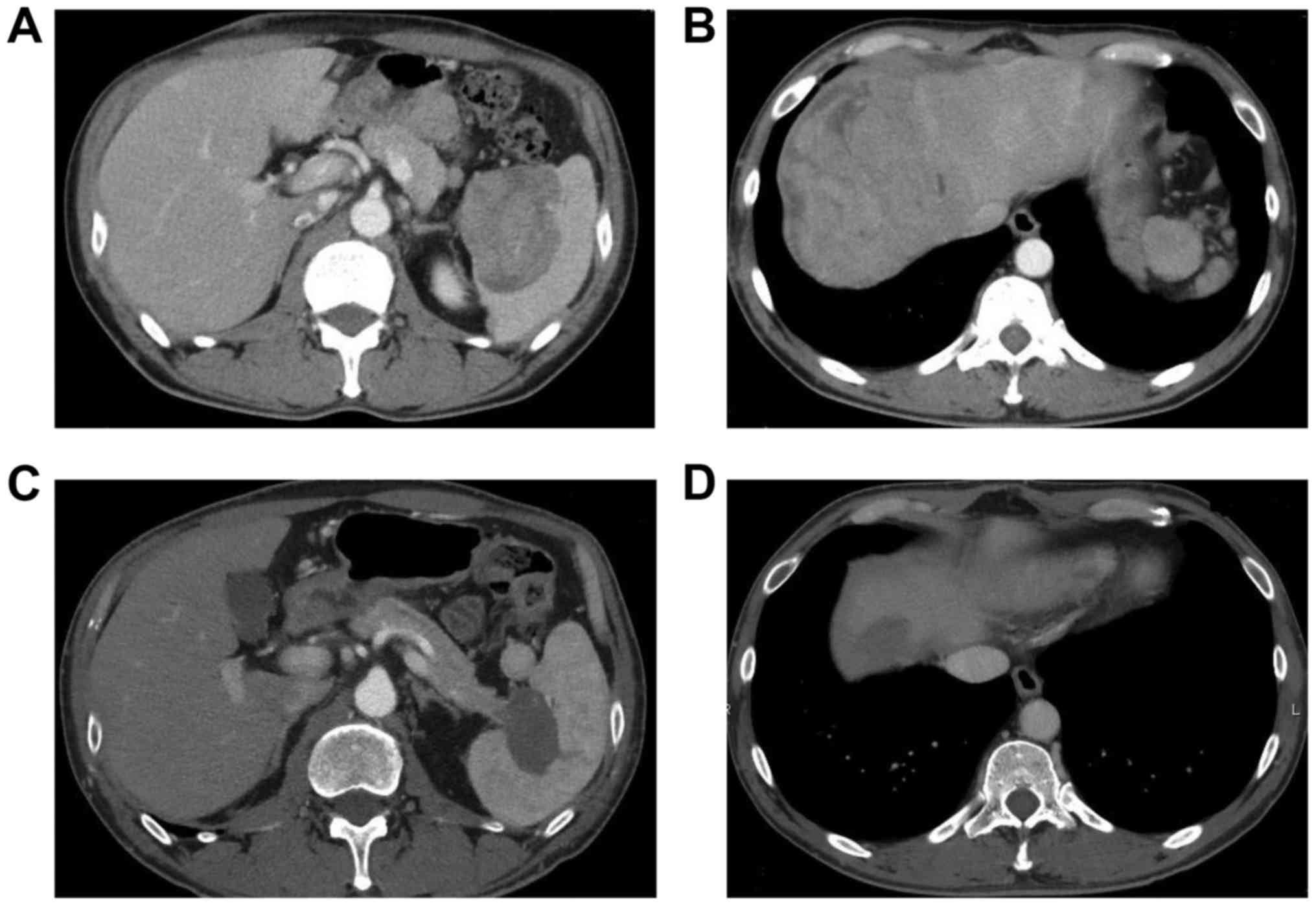
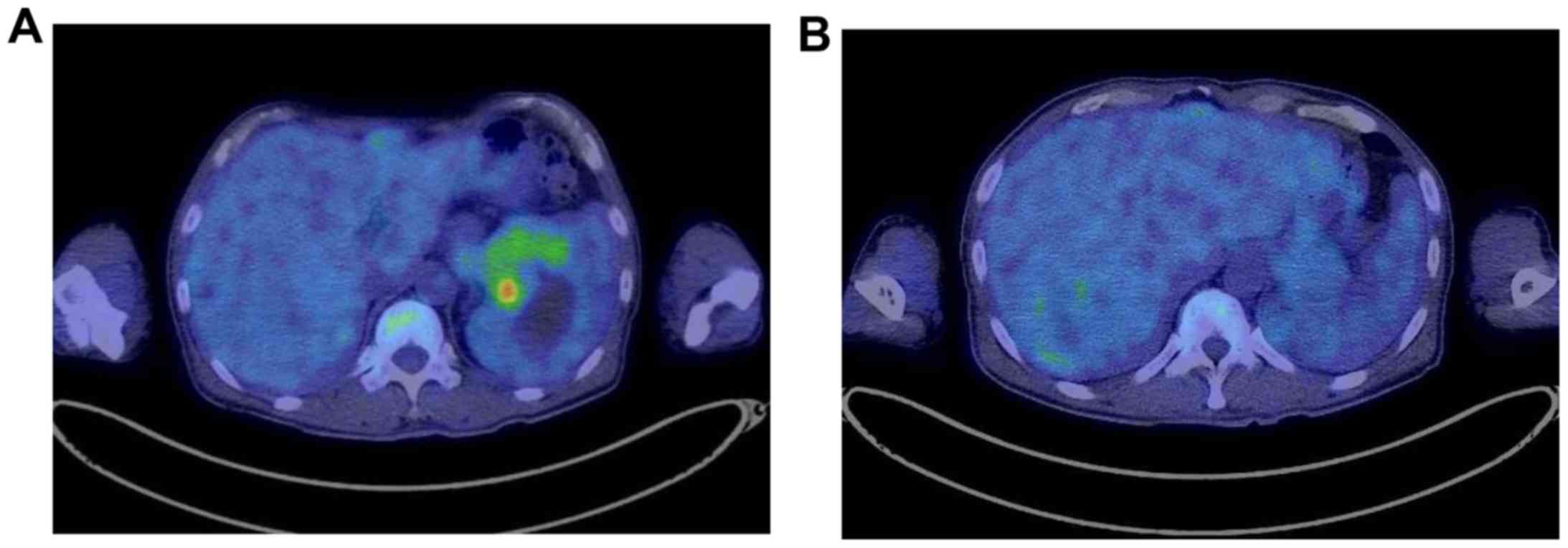
tomography/computed tomography (CT) revealed high-uptake mass
lesions in the pancreatic tail, in the periphery of the liver and
in the pelvis (Figs. 2 and 3). A transcutaneous biopsy of a liver
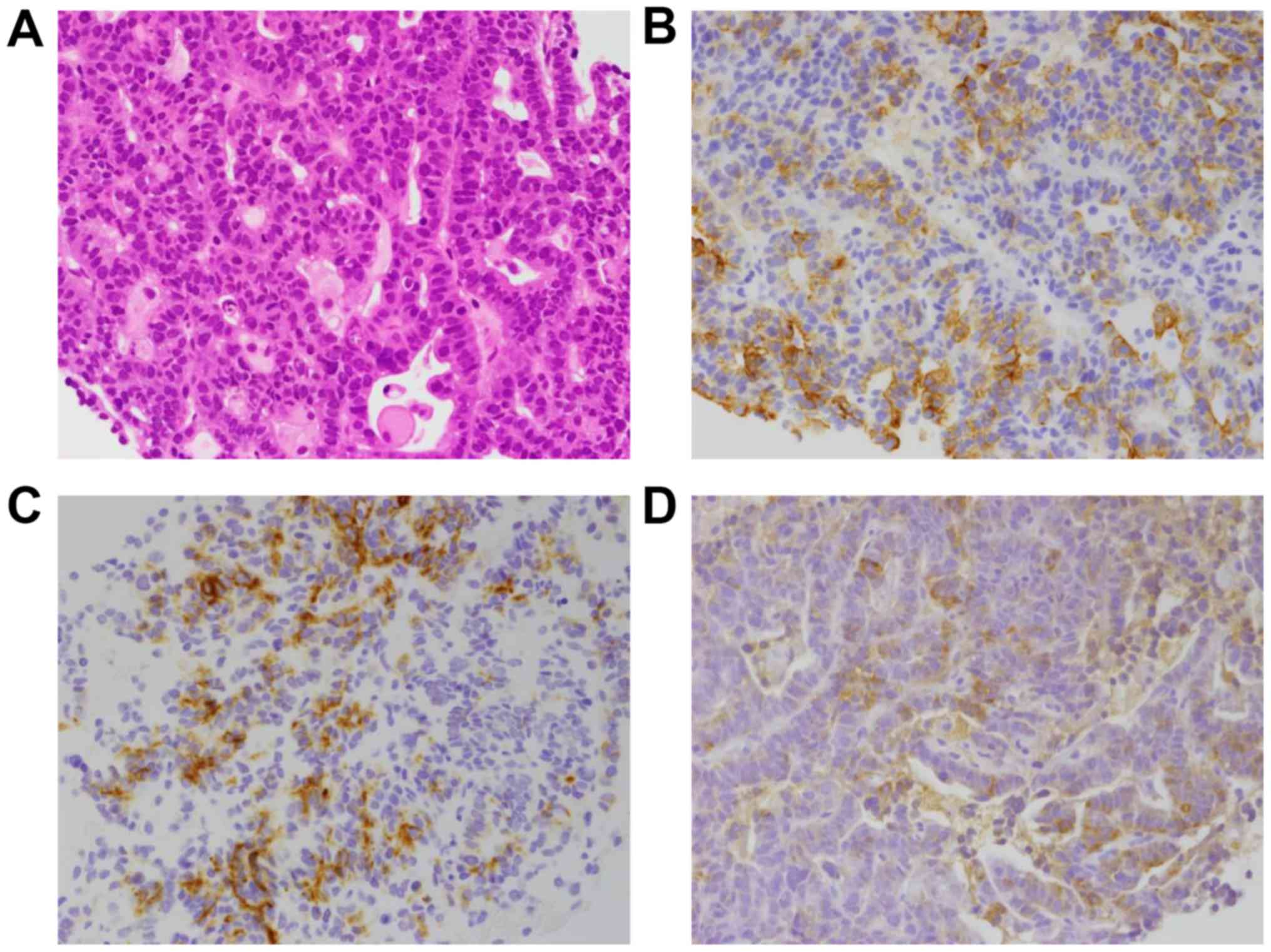
lesion was conducted, and histological examination revealed
proliferation of atypical cells arranged in a tubular or glandular
pattern (Fig. 4A).
Immunohistochemical staining showed that the atypical cells were
positive for cytokeratin (CK)7, CK19 and lipase, but negative for
CK20 and thyroid transcription factor-1 (Fig. 4B), which led to the final diagnosis
of acinar cell carcinoma of the pancreatic tail, T4bN0M1, stage IV
according to the 7th edition of the TNM Classification of Malignant
Tumors (http://www.uicc.org/sites/main/files/private/TNM_Classification_of_Malignant_Tumours_Website_15%20MAy2011.pdf).
While a fever of 38°C persisted, the results of the
blood cultures were negative. It was concluded that the fever was
caused by the tumor, and administered acetaminophen or
non-steroidal anti-inflammatory agents only achieved temporary
declines in fever. Chemotherapy with the FOLFIRINOX regimen
(oxaliplatin at 85 mg/m2 on day 1, irinotecan at 180
mg/m2 on day 1, l-leucovorin at 200 mg/m2 on
day 1, 5-FU at 400 mg/m2 as a bolus infusion on day 1,
and 5-FU at 2400 mg/m2 as a 46-h continuous infusion,
every 2 weeks) was initiated at the end of March, 2016. Alleviation
of fever was soon achieved, the blood lipase levels also declined
and panniculitis completely resolved over 7 seeks. The patient
experienced no severe adverse events other than grade 1 malaise
according to the Common Terminology Criteria for Adverse Events,
version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf)
and the ECOG performance status became 0. At the start of the 8th
course of chemotherapy, the concentrations of pancreatic exocrine
enzymes were within normal ranges and CT revealed partial response
according to the Response Evaluation Criteria in Solid Tumors,
version 1.1 (https://ctep.cancer.gov/protocoldevelopment/docs/recist_guideline.pdf)
(Fig. 4). The patient has been
receiving FOLFIRINOX regimen for 12 months and maintained partial
response at the last follow-up visit (March 30, 2017). The patient
provided a signed informed consent regarding the publication of the
case details and associated images.
Discussion
PACC originates from the acinar cells of the
pancreas, the exocrine tissue in this organ. Although pancreatic
tissue consists predominantly of acinar cells (82% by volume)
(9), PACC represents only 1% of all
pancreatic malignancies (1). PACC is
morphologically and genetically distinct from common pancreatic
ductal adenocarcinoma, and genetic alterations in the adenoma
polyposis coli (APC)/β-catenin pathway of PACC cells are often
detected (10). Although the reason
remains unclear, ductal adenocarcinoma has been hypothesized to
arise from acinar cells via metaplasia to ductal cells, in a
process known as acinar-ductal metaplasia (ADM) based on genetic
instability (11). In fact, in
transforming growth factor-α transgenic mice, an animal model for
pancreatic cancer, acinar cells exhibited loss of their
characteristic zymogen granules and became transitional cells,
subsequently acquiring the characteristics of duct cells (12). In KrasG12D transgenic
mice, tumors predominantly originated not from ductal cells, but
from acinar cells through ADM (13).
These findings indicate that acinar cells represent the primary
origin of pancreatic neoplasms.
Subcutaneous fat necrosis is most characteristic in
PACC, but its pathogenesis has not been fully elucidated. One
possible mechanism is that trypsin overproduced together with
lipase alters the permeability of blood vessels, allowing lipase to
hydrolyze lipids in the cell membrane and cytoplasm of adipocytes
(14). LHS occurs in only 10–15% of
patients with PACC and serum amylase activity is increased in only
30% of LHS cases (7). In the present
case, amylase was the only pancreatic exocrine enzyme within the
normal range.
Of the symptoms of LHS, subcutaneous fat necrosis
referred to as pancreatic panniculitis is observed in 0.3–1% of all
patients with pancreatic disease, such as acute and chronic
pancreatitis and pancreatic neoplasms (15), and it is considered to be a
dermadrome.
Patients with pancreatitis and those with neoplastic
conditions tend to differ markedly, with tumor patients exhibiting
significantly higher serum lipase concentrations. Retrospective
analysis identified a serum lipase concentration of 4,414 U/l as
the optimal cut-off value, offering 73% sensitivity and 82.1%
specificity for the diagnosis of a neoplastic cause (16). These sensitivity and specificity
values are comparable with CA19-9 in ductal adenocarcinoma compared
with benign pancreatic diseases (17). These findings suggest that patients
with pancreatic panniculitis and high serum lipase concentrations
should be carefully examined for neoplastic disease. Of the
symptoms of LHS, severe fever and polyarthritis may worsen the
general condition of the patient. Octreotide has been reported to
alleviate the symptoms (16), but
treatment directed at the underlying pancreatic disease is
crucial.
Although no standard chemotherapy for advanced,
unrespectable PACC has been established to date, some studies
suggested a more favorable efficacy for 5-FU compare with
gemcitabine (8). Considering the
promising data for FOLFIRINOX against advanced pancreatic cancer
(18), the present patient was
treated with this regimen. Combination chemotherapy employing
oxaliplatin or irinotecan for the treatment of colorectal cancer
may be effective in PACC, as these tumors are associated with
abnormalities in the APC/β-catenin pathway, similar to colorectal
carcinomas, and FOLFOX therapy has also been administered to PACC
patients (19). In addition,
sustained antitumor activity from oxaliplatin monotherapy was shown
in basic research using a patient-derived tumor xenograft mouse
model (20). In that model,
irinotecan, gemcitabine and 5-FU also exhibited antitumor activity,
but the tumor rapidly grew once those drugs were withdrawn. These
findings suggested that oxaliplatin-based therapy may be one of the
most effective therapies for the treatment of PACC.
In the PRODIGE 4/ACCORD 11 trial, FOLFIRINOX
achieved significantly longer overall survival (OS) and
progression-free survival (PFS) and a higher response rate compared
with gemcitabine monotherapy (21).
As the eligibility criteria for that study included a performance
status of 0–1, FOLFIRINOX is recommended for patients with a good
general condition. In this case, the patient had a performance
status of 2 due to a persistent fever of 38°C and polyarthritis.
FOLFIRINOX was still administered, as the patient's condition was
predicted to recover if symptom relief was achieved. Soon after
treatment initiation, the symptoms induced by severe LHS were
relieved and the performance status improved to 0. This case
suggests that administering chemotherapy to patients with poor
performance status due to LHS should be considered, as symptom
relief by chemotherapy is expected to lead to improvement of the
performance status.
The patient presented herein was diagnosed with PACC
overproducing exocrine enzymes, such as lipase. Severe LHS was
well-controlled by FOLFIRINOX and shrinkage of the mass was also
observed. To the best of our knowledge, this represents the first
case in which FOLFIRINOX therapy achieved favorable control of
advanced PACC with LHS, and chemotherapy should be considered as a
viable option for those patients.
Acknowledgements
The authors would like to thank Dr Kazuko Imamura,
Department of Dermatology, Fukuoka Saiseikai Futsukaichi Hospital,
for providing pictures of the subcutaneous masses, as well as the
medical staff of each institution who contributed to the treatment
of the patient.
References
|
1
|
Holen KD, Klimstra DS, Hummer A, Gonen M,
Conlon K, Brennan M and Saltz LB: Clinical characteristics and
outcomes from an institutional series of acinar cell carcinoma of
the pancreas and related tumors. J Clin Oncol. 20:4673–4678. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butturini G, Pisano M, Scarpa A, D'Onofrio
M, Auriemma A and Bassi C: Aggressive approach to acinar cell
carcinoma of the pancreas: A single-institution experience and a
literature review. Langenbecks Arch Surg. 396:363–369. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klimstra DS, Heffess CS, Oertel JE and
Rosai J: Acinar cell carcinoma of the pancreas. A clinicopathologic
study of 28 cases. Am J Surg Pathol. 16:815–837. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berner O: Subkutane fettgewebsnekrose.
Virchow Arch Path Anat. 193:510–518. 1908. View Article : Google Scholar
|
|
5
|
Wang Y, Wang S, Zhou X, Zhou H, Cui Y, Li
Q and Zhang L: Acinar cell carcinoma: A report of 19 cases with a
brief review of the literature. World J Surg Oncol. 14:1722016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidt CM, Matos JM, Bentrem DJ,
Talamonti MS, Lillemoe KD and Bilimoria KY: Acinar cell carcinoma
of the pancreas in the United States: Prognostic factors and
comparison to ductal adenocarcinoma. J Gastrointest Surg.
12:2078–2086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riediger C, Mayr M, Berger H, Becker K,
Dobritz M, Kleeff J and Friess H: Transarterial chemoembolization
of liver metastases as symptomatic therapy of lipase hypersecretion
syndrome. J Clin Oncol. 30:e209–e212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simon M, Bioulac-Sage P, Trillaud H and
Blanc JF: FOLFOX regimen in pancreatic acinar cell carcinoma: Case
report and review of the literature. Acta Oncol. 51:403–405. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams JA: Regulation of acinar cell
function in the pancreas. Curr Opin Gastroenterol. 26:478–483.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo
CJ, Conlon K, Brennan M, Cameron JL and Klimstra DS: Genetic and
immunohistochemical analysis of pancreatic acinar cell carcinoma:
Frequent allelic loss on chromosome 11p and alterations in the
APC/beta-catenin pathway. Am J Pathol. 160:953–962. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmid RM: Acinar-to-ductal metaplasia in
pancreatic cancer development. J Clin Invest. 109:1403–1404. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagner M, Greten FR, Weber CK, Koschnick
S, Mattfeldt T, Deppert W, Kern H, Adler G and Schmid RM: A murine
tumor progression model for pancreatic cancer recapitulating the
genetic alterations of the human disease. Genes Dev. 15:286–293.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kopp JL, von Figura G, Mayes E, Liu FF,
Dubois CL, JP IV Morris, Pan FC, Akiyama H, Wright CV, Jensen K, et
al: Identification of Sox9-dependent acinar-to-ductal reprogramming
as the principal mechanism for initiation of pancreatic ductal
adenocarcinoma. Cancer Cell. 22:737–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vakiani E, Young RH, Carcangiu ML and
Klimstra DS: Acinar cell carcinoma of the pancreas metastatic to
the ovary: A report of 4 cases. Am J Surg Pathol. 32:1540–1545.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bogart MM, Milliken MC, Patterson JW and
Padgett JK: Pancreatic panniculitis associated with acinic cell
adenocarcinoma: A case report and review of the literature. Cutis.
80:289–294. 2007.PubMed/NCBI
|
|
16
|
Zundler S, Erber R, Agaimy A, Hartmann A,
Kiesewetter F, Strobel D, Neurath MF and Wildner D: Pancreatic
panniculitis in a patient with pancreatic-type acinar cell
carcinoma of the liver-case report and review of literature. BMC
Cancer. 16:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poruk KE, Gay DZ, Brown K, Mulvihill JD,
Boucher KM, Scaife CL, Firpo MA and Mulvihill SJ: The clinical
utility of CA 19–9 in pancreatic adenocarcinoma: Diagnostic and
prognostic updates. Curr Mol Med. 13:340–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Callata-Carhuapoma HR, Cour E Pato,
Garcia-Paredes B, Fernandez RM, Fernandez ML Mendoza, Fernandez AM,
De La Rosa CA, Lezama MJ Sotelo, Cabezas-Camarero S and Varela J
Sastre: Pancreatic acinar cell carcinoma with bilateral ovarian
metastases, panniculitis and polyarthritis treated with FOLFIRINOX
chemotherapy regimen. A case report and review of the literature.
Pancreatology. 15:440–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaudhary P, Ranjan G, Chaudhary A, Tiwari
AK and Arora MP: Acinar cell carcinoma: A rare pancreatic
malignancy. Clin Pract. 3:e182013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hall JC, Marlow LA, Mathias AC, Dawson LK,
Durham WF, Meshaw KA, Mullin RJ, Synnott AJ, Small DL, Krishna M,
et al: Novel patient-derived xenograft mouse model for pancreatic
acinar cell carcinoma demonstrates single agent activity of
oxaliplatin. J Transl Med. 14:1292016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|