Introduction
The clinical tumor size of middle-to-late-stage
primary liver cancer is estimated by its maximum diameter.
Hepatocellular carcinomas (HCCs) sized from >5 to <10 cm are
described as large, whereas those ≥10 cm as massive. Multimodal
comprehensive treatment has been used for unresectable large live
cancer, but without a distinct curative effect; the outcome of
massive HCC treated with non-surgical methods is not satisfactory.
Transcatheter arterial chemoembolization (TACE) is contraindicated
in patients with HCC invasion of the first branch or main trunk of
the portal vein, as it may cause extensive liver infarction,
resulting in poor prognosis (1).
Another limitation of TACE, as reported by Arata et al
(2), is that local control of HCCs
sized ≥5 cm is often difficult. These two limitations are
responsible for negative survival data in initial randomized
control trials of TACE (3). At
present, radiation therapy (RT) is applied as palliative treatment
for liver cancer, mainly as three-dimensional conformal RT
(3D-CRT). ‘Super gamma knife’ technology is one of the most
effective methods for solid tumors, has the advantages of high
precision and high single therapeutic dose, and it may improve the
effects of palliative care in terminal-stage liver cancer. In the
present study, 69 cases of primary unresectable liver cancer who
underwent gamma knife stereotactic body radiotherapy (SBRT) were
retrospectively analyzed to investigate the prognostic factors and
curative effect of this type of radiotherapy, providing the basis
for further clinical applications.
Patients and methods
Patient characteristics
This study included 69 patients with primary liver
cancer admitted to the 323 Hospital of People's Liberation Army
(Xi'an, China) between October, 2006 and October, 2010. A total of
22 patients received TACE prior to gamma knife therapy. All the
patients received RT doses of 50–60 Gy in daily fractions of 4–6
Gy. The eligibility criteria comprised the following: i)
Unresectable HCC with portal vein tumor thrombosis (PVTT) in the
first branch or the main trunk; ii) an HCC sized ≥10 cm; iii)
absence of extrahepatic metastasis (4) and no ascites or medical control of
ascites; and iv) an Eastern Cooperative Oncology Group performance
status of 0–2 (5). A total of 45 HCC
patients with portal vein tumor thrombosis (PVTT) were initially
diagnosed by liver biopsy histological examination and 24 patients
were initially diagnosed on dynamic helical computed tomography
(CT) using contrast medium or by magnetic resonance imaging (MRI)
and measurement of α-fetoprotein levels, then reconfirmed by CT
during hepatic arteriography and CT during arterial portography in
each case. Patients with diabetes were excluded. The patient
characteristics are summarized in Table
I. This study was approved by the Ethics Committee of the 323
Hospital of PLA and all the patients provided written informed
consent prior to enrolment.
 | Table I.Patient characteristics (n=69). |
Table I.
Patient characteristics (n=69).
| Characteristics | N (%) |
|---|
| Gender |
|
| Male | 53 (76.8) |
|
Female | 16 (23.2) |
| Age, years |
|
|
Median | 54 |
|
Range | 28–89 |
| T stage |
|
| T1 | 8 (11.6) |
| T2 | 12 (17.4) |
| T3 | 21 (30.4) |
| T4 | 28 (40.6) |
| PVTT | 45 (65.2) |
| Tumor size, cm |
|
| Mean | 11.0 (9.0–21.0) |
|
Median | 10.1 |
| Child- Pugh
class |
|
| A | 49 (71.0) |
| B | 15 (21.7) |
| C | 5 (7.3) |
| RT dose, Gy |
|
|
Median | 70.0 |
|
Range | 69.4–70.6 |
Treatment planning
RT was performed using the ‘super gamma-knife’ SGS-I
Stereotactic Gamma-Ray system (Huiheng Medical Inc., Shenzhen,
China), which focused on whole-body radiation, and the UNICORN 3D
Treatment Planning System (Huiheng Medical Inc.) for the design. RT
plans were reviewed using our department's treatment planning
software (Huiheng Medical Inc.). Individual gross tumor volumes
(GTVs) for each liver tumor and nodal lesion visualized on positron
emission tomography were generated on planning CTs. Both simulation
and treatment-planning CTs were performed during breath holding.
The patients were instructed to breathe quietly, as RT was
repeatedly delivered during breath holding at end-expiration for
10–15 sec at a time. Using these data, the whole liver, main tumor,
PVTT, and other hepatic tumors were contoured for each patient with
reference to the MRI or diagnostic enhanced CT images taken within
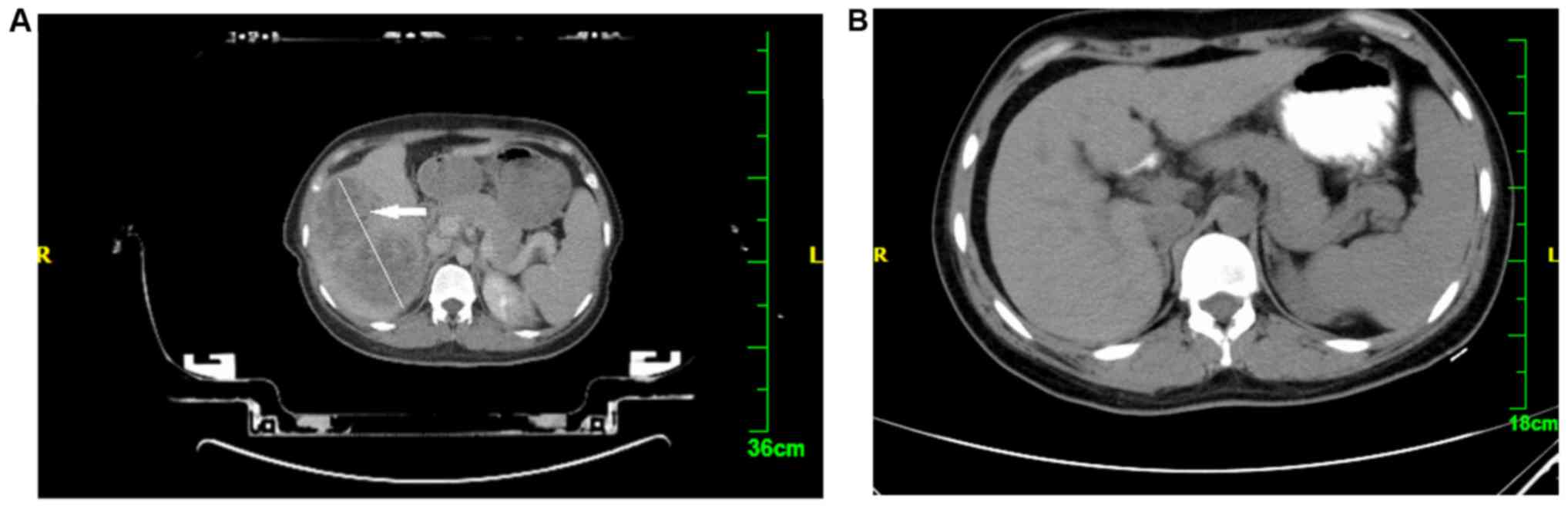
a week prior to treatment planning (Fig.
1A). Outlining of the target area-GTV was performed by a
medical physicist and the planning target volume (PTV) was extended
0.5 cm outside the GTV. The organs at risk included the normal
liver, duodenal pancreas, kidney and spinal cord. It was ensured
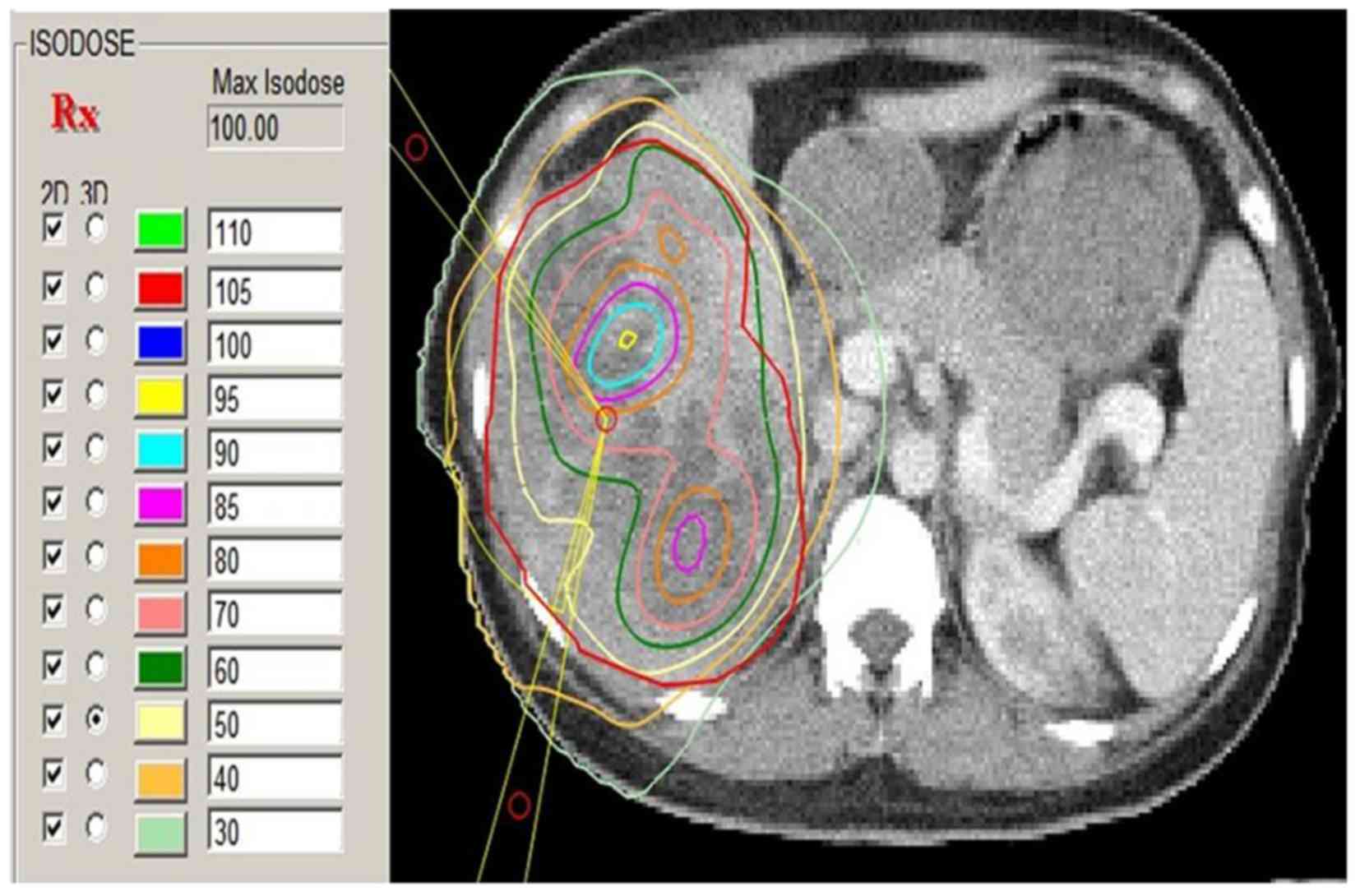
that the 50% isodose curve covered the PTV and that the radiation
delivered to normal tissue did not exceed the tolerance dose
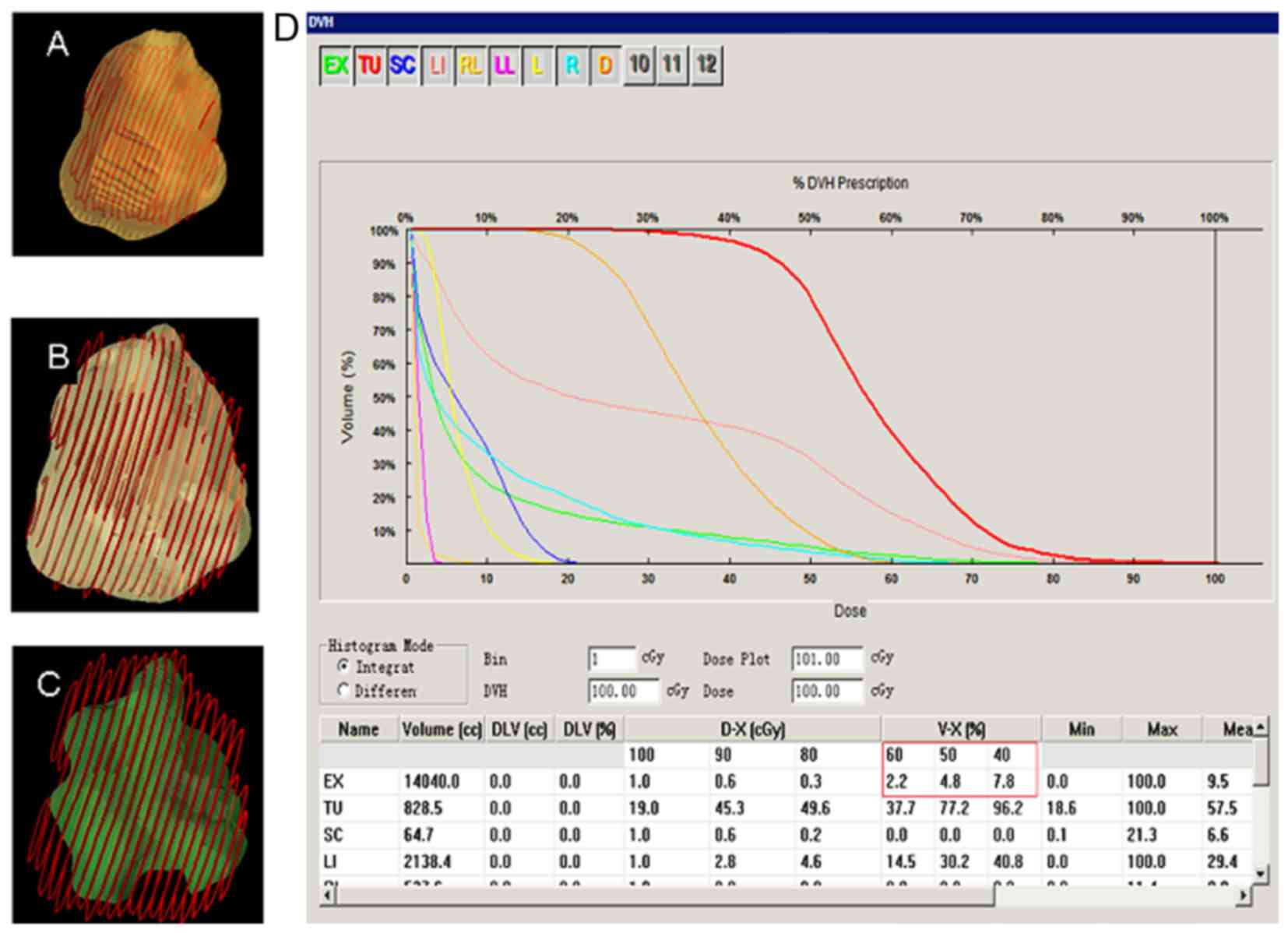
(Fig. 2). A dose-volume histogram
(DVH) was drawn to evaluate the square and optimize the radiation
scheme. The RT prescription dose was as follows: 4–6 Gy/fx, for a
total of 9–12 times, 2–5 times/week, up to a total dose of 50–60
Gy. Organs of interest were avoided and the treatment plan
optimization index was determined with DVH; 40 or 50% DVH
surrounded the PTV (Fig. 3). The
dose administered to adjacent target organs did not exceed 25 Gy
(tolerance dose). During treatment, the patients were administered
static drops of liquorice anhydride, while the use of chemicals was
avoided.
Curative effect evaluation
Efficacy evaluation was performed according to the
World Health Organization curative effect evaluation standards of
solid tumors (4). Three months after
RT, the patients were evaluated by abdominal MRI. After treatment
completion, reexamination was performed every 3 months for 1 year
and every 6 months thereafter. Reexamination included blood tests,
liver and kidney function tests, serum electrolyte levels,
abdominal MRI and abdominal ultrasound.
Statistical analysis
SPSS software, version 19 (SPSS Inc., Chicago, IL,
USA) was used to perform the statistical analyses. The curative
effect observation index was overall survival estimated with
Kaplan-Meier curve analysis.
Results
Curative effect
A total of 8 patients succumbed to the disease
within 3 months after gamma knife treatment and were not included
in the evaluation of the curative effect. Among the remaining 61
patients, 8 had non-cancerous ascites, hepatomegaly, a 2-fold
increase in alkaline phosphatase levels and/or at least a 5-fold
increase in transaminase levels, which it was diagnosed as
radiation-induced liver disease (RILD), according to the diagnostic
standards reported in the literature (6,7). A total
of 7 patients achieved a complete response (CR), 29 achieved a
partial response (PR), 24 had stable disease (SD) and 9 developed
progressive disease (PD), including 5 cases of local progression
and 4 of intrahepatic metastasis. The total effectiveness rate (CR
+ PR) was 59.0% (36/61), the intrahepatic metastasis rate was 14.8
% (9/61) and the incidence of RILD was 13.1% (8/61).
Survival analysis
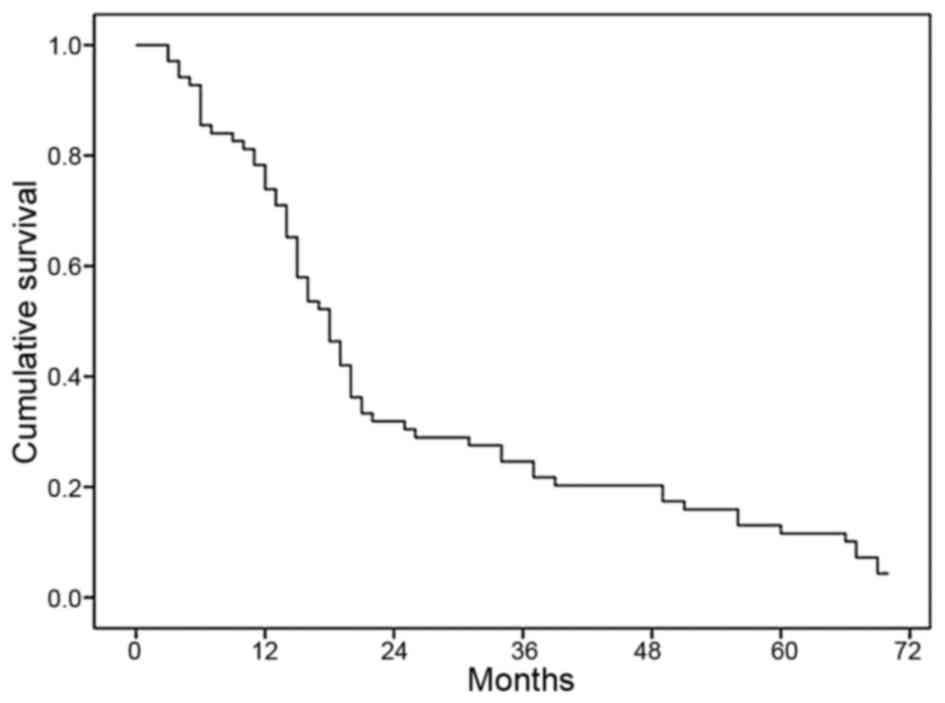
The overall survival of patients with massive
primary liver cancer following RT is shown in Fig. 4. The median survival was 17.4 months
for all patients included in the study and the 1-, 2- and 3-year
overall survival rates were 71, 30 and 22%, respectively.
Adverse reactions
The severity of the adverse reactions was scored for
each patient using the National Cancer Institute (NCI) Common
Toxicity Criteria (CTC), version 2.0 (6), the grading system of the Radiation
Therapy Oncology Group (RTOG) (7),
and the decrease in the white blood cell (WBC) and platelet (PLT)
counts. Other widely used scoring systems include those of the RTOG
and European Organization for Research and Treatment of Cancer, and
the Late Effects Normal Tissue Task Force (8). These scales include four separate
elements, representing subjective, objective, management and
analytical evaluation of injury (Table
II).
 | Table II.Criteria for grading by the toxicity
scoring system. |
Table II.
Criteria for grading by the toxicity
scoring system.
| Scoring system | 0 | 1 | 2 | 3 |
|---|
| NCI-CTC 2.0 | – | 1.26–2.5 times the
upper limit of normal ALT value | 2.6–5 times the upper
limit of normal ALT value | 5–10 times the upper
limit of normal ALT value |
| RTOG | No nausea and
vomiting | Nausea | Vomiting can be
controlled | – |
| LENT/SOMA | – | – | – | – |
| WBC decline in
grading (x109) | – | WBC at
(3–3.9)x109 | WBC at
(2–2.9)x109 | – |
| Platelet decline in
grading (x109) | – | 75–99 | 50–74 | 28–49 |
As shown in Table
III, 22 patients had acute liver adverse reactions classified
according to NCI-CTC 2.0 as grade 1 (n=5), grade 2 (n=13) and grade
3 (n=4). Treatment with compound glycyrrhizin injection was
administered to these patients and the liver function returned to
normal; however, in 8 cases the liver function did not recover and
the patients succumbed to the disease 3 months after RT. A total of
57 patients exhibited digestive tract reactions classified by RTOG
as grade 0 (n=15), 1 (n=23) and 2 (n=19). A decrease in the WBC
count was observed in 52 cases (grade 1 in 29 and grade 2 in 23
cases) and a decrease in the PLT count was observed in 35 cases
(grade 1 in 24, grade 2 in 8 and grade 3 in 3 cases).
 | Table III.Demographic and clinical
characteristics of the patients included in data analysis. |
Table III.
Demographic and clinical
characteristics of the patients included in data analysis.
|
| Scoring rate (n) |
|---|
|
|
|
|---|
| Variables | 0 | 1 | 2 | 3 |
|---|
| NCI-CTC 2.0 (n) | – | 5 | 13 | 4 |
| RTOG | 15 | 23 | 19 | – |
| WBC decline in
grading (x109) | – | 29 | 23 | – |
| Platelet decline in
grading (x109) | – | 24 | 8 | 3 |
Discussion
Historically, the liver has been considered to be a
relatively radiosensitive organ and it may be difficult to achieve
the radiation doses required to eradicate gross tumors without
causing RILD, which generally develops ~4–8 weeks following RT
(9). The higher incidence of RILD in
this study may be associated with the sizeable tumor and the single
integral high dose and high total dose of RT. The curative effect
of massive liver cancer was significant. As seen in Fig. 1, showing a pre- and post-treatment
CT, the liver displayed a sizeable shadow prior to treatment,
whereas the shadow completely disappeared following RT. The reason
for this significant shrinking or disappearance of the tumor may be
associated with high division of a single dose and the total
dose.
Child-Pugh classification, which was first
introduced by the Child in 1964 (10), is commonly used to quantitatively
assess liver reserve function in patients with cirrhosis. This
classification divides patients in different groups based on five
indicators (general status, ascites, serum bilirubin, serum albumin
and prothrombin time) scored with 1, 2 and 3 points. The score of
the five indicators is added (the lowest score is 5 points and the
highest score is 15 points) and liver reserve function is classed
as A, B or C, indicating three different levels of severity of
liver damage (the higher the score, the worse the liver reserve
function). Patient prognosis is considered to be associated with
liver function according to the Child-Pugh classification, with
patients classed as Child-Pugh A having a good prognosis.
Intermediate- or advanced-staged liver cancer
patients may have cirrhosis and poor liver function, and the
majority of patients who are not considered to be candidates for
surgical treatment may receive comprehensive treatment. TACE has
been considered as the standard optimal treatment for unresectable
primary liver cancer (11), but its
curative effect is affected by the patient's general condition,
tumor progression and blood supply of tumor tissue. As residual
tumor cells remain in the majority of the lesions, the long-term
outcome is not satisfactory (12).
In recent years, TACE combined with conventional RT have achieved a
good curative effect. Seong et al (13) reported 30 cases treated with TACE
followed by conventional RT (normalized total dose, 44 Gy), and the
reported results were a median survival of 17 months and a 3-year
survival rate of 22.2%. In the present study, 69 patients with
massive liver cancer (≥10 cm), advanced TNM stage, poor liver
function and PVTT (45/61), were unable to receive conventional TACE
treatment. In such cases, RT is considered the best palliative
treatment option, but the patients' prognosis is poorer. Xiong
et al (14) reported on 69
cases of massive HCC treated with 3D-CRT (fraction dose 4–8 Gy, for
7–15 times, to a total dose of 53.6±6.6 Gy). The 1-, 2- and 3-year
survival rates were 41, 20 and 17%, respectively, but there was no
analysis of the curative effect of RT combined with TACE.
Therefore, the curative effect of TACE for massive liver cancer is
not satisfactory, and data on the curative effect of TACE combined
with RT have not yet been reported.
Super gamma knife SBRT is stereotactic radiosurgery
combined with 3D-CRT technology. Compared with traditional body
gamma knife accurate positioning, SBRT uses concentric dose control
rings at different distances from the PTV, with the dose increasing
from the outer to the inner ring. 3D-CRT may be used for larger
tumors, which is advantageous in terms of increasing the target
dose and protecting normal tissues. Due to the characteristics of
the focus and the high dose, SBRT significantly improves the
curative effect and reduces the incidence of the radiation
reactions and the extent of normal tissue damage. Dang et al
(15) reported the curative effect
of SBRT on hepatic hilar carcinoma and confirmed that the
technology of gamma knife treatment of primary liver cancer is a
safe and reliable treatment, effective in improving patient
survival. Normalized total dose (NTD) was defined as the
biologically equivalent total dose, normalized to 2 Gy per
fraction. The association of biologically effective dose (BED) with
NTD is illustrated as NTD=BED/[1+d/(α/β)] (16). According to Fletcher (17), if α/β=10 Gy, conventional single dose
(d)=2 Gy, the GTV dose should be as follows: When the NTD for small
lesions was 60 Gy, the BED was 72 Gy, and when the NTD for tumors
sized ≥10 cm was 50–60 Gy, the BED was 60–72 Gy, as the dose
gradually increased from the periphery to the center (Fig. 2). Although the NTD for tumors sized
≥10 cm is lower compared with the NTD for small lesions, the
opposite stands for the dose to the central area of the tumor, as
the single irradiation dose for tumors sized ≥10 cm is higher
compared with that for small lesions.
In this study, the dose to the periphery of the
tumor was 50–70 Gy, which was equivalent to an NTD of 65 Gy, but
the dose to the central area of the tumor may reach >90 Gy. The
therapeutic dose was lower than the conventional radiation dose.
The total effectiveness rate (CR+PR) was 51% and the 1-, 2- and
3-year overall survival rates were 45, 24 and 19%, respectively.
The median survival was 13 months, which was superior to that with
3D-CRT, due to the high central dose. However, there was an
increased incidence of adverse reactions, such as acute liver
toxicity and gastrointestinal reactions (27 and 73%, respectively),
a 63% decrease in the WBC count and a 41% decrease in the PLT
count.
Although gamma knife treatment for massive HCC
appears to be effective, its curative effect is not satisfactory.
As the majority of the patients are at an advanced stage, with
compromised liver function and poor general status, only few
patients may be suitable candidates for gamma knife treatment.
Further research for methods to improve the curative effect of
treatment is required.
References
|
1
|
Yamada R, Sato M, Kawabata M, Nakatsuka H,
Nakamura K and Takashima S: Hepatic artery embolization in 120
patients with unresectable hepatoma. Radiology. 148:397–401. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arata S, Tanaka K, Okazaki H, Kondo M,
Morimoto M, Saito S, Numata K, Nakamura S and Sekihara H: Risk
factors for recurrence of large HCC in patients treated by combined
TAE and PEI. Hepatogastroenterology. 48:480–485. 2001.PubMed/NCBI
|
|
3
|
A comparison of lipiodol chemoembolization
and conservative treatment for unresectable hepatocellular
carcinoma. Groupe d'Etude et de Traitement du Carcinome
Hépatocellulaire. N Engl J Med. 332:1256–1261. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
WHO, . Handbook for reporting the results
of cancer treatment. Offset Publication No. 48. World Health
Organization; Geneva: pp. 34–35. 1979
|
|
5
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin Onco.
12:649–655. 198:
|
|
6
|
Cancer Therapy Evaluation Program.
http://ctep.cancer.gov/reporting/ctc.htmlFebruary
25–2010
|
|
7
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavy JJ, Denekamp J, Letschert J,
Littbrand B, Mornex F, Bernier J, Gonzales-Gonzales D, Horiot JC,
Bolla M and Bartelink H: EORTC Late Effects Working Group. Late
effects toxicity scoring: The SOMA scale. Int J Radiat Oncol Biol
Phys. 31:1043–1047. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lawrence TS, Robertson JM, Anscher MS,
Jirtle RL, Ensminger WD and Fajardo LF: Hepatic toxicity resulting
from cancer treatment. Int J Radiat Oncol Biol Phys. 31:1237–1248.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shetty K, Rybicki L and Carey WD: The
Child-Pugh classification as a prognostic indicator for survival in
primary sclerosing cholangitis. Hepatology. 5:1049–1053. 1997.
View Article : Google Scholar
|
|
11
|
Glouse ME, Stokes KR, Kruskal JB, Perry
LJ, Stuart KE and Nasser IA: Chemoembolization for hepatocellular
carcinoma: Epinephrine followed by a doxorubicin-ethiodized oil
emulsion and gelatin sponge powder. J Vasc Interv Radiol.
4:717–725. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakai M, Sato M, Kawai N, Minamiguchi H,
Masuda M, Tanihata H, Takeuchi T, Terada M and Kishi K:
Hepatocellular carcinoma: Involvement of the internal mammary
artery. Radiology. 219:147–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seong J, Park HC, Han KH, Lee DY, Lee JT,
Chon CY, Moon YM and Suh CO: Local radiotherapy for unresectable
hepatocellular carcinom a patients who failed with transcatheter
arterial chemoembolization. Int J Radia Oncol Biol Phys.
47:1331–1335. 2000. View Article : Google Scholar
|
|
14
|
Xiong LS, Dong ZX, Liang JG, Long C and Li
YY: Three-dimensional conformal radiotherapy(3-DCRT) for 69 cases
of massive primary liver cancer. Chin Oncol. 16:478–479. 2006.
|
|
15
|
Dang YZ, Huang SG, Lu WL, Wu FW and Wang
QY: Curative effect of stereotactic body radiotherapy on hepatic
hilar carcinoma. Mol Clin Oncol. 2:1135–1138. 2014.PubMed/NCBI
|
|
16
|
Park C, Papiez L, Zhang S, Story M and
Timmerman RD: Universal survival curve and single fraction
equivalent dose: Useful tool in understanding potency of ablative
radiotherapy. Int J Radiat Oncol Biol Phys. 70:847–852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fletcher GH: Clinical dose-response curve
of subclinical aggregates of epithelial cells. J Radiol Electrol
Med Nucl. 53:201–206. 1972.PubMed/NCBI
|