Introduction
The number of individuals affected with breast
cancer has increased globally. In Japan, 89,400 females were newly
diagnosed with breast cancer and there were 13,800 mortalities
attributed to this disease in 2015 (1). Approximately 20% of breast cancer
patients have a family history and 5–10% (2,3) are
considered to be hereditary (hereditary breast cancer) HBC caused
by a germline mutation such as BRCA1, BRCA2,
TP53 or PTEN. Mutations in BRCA1/2 genes are
associated with hereditary breast and ovarian cancer syndrome
(HBOC), which is the most common form of HBC. It is also known that
germline mutations of DNA mismatch repair (MMR) genes, such as
MLH1, MSH2 and MSH6 are caused by Lynch
syndrome, which is a hereditary colorectal cancer syndrome, and
MLH1 particularly increases the risk of breast cancer
(4).
Women with HBOC have a 41–90% lifetime risk of
developing breast cancer (5) and
HBOC is characterized by the high risk of contralateral breast
cancer (6). In addition, the
cumulative risk for ovarian cancer is 8–59% and is higher in
BRCA1 mutation carriers compared with BRCA2 mutation
carriers (5–7). It was revealed that triple-negative
breast cancer, which is estrogen receptor/progesterone
receptor-negative and human epidermal growth factor receptor 2,
negative was associated with HBOC and that 36% of women with
early-onset triple-negative breast cancer (aged <40 years) had a
BRCA1 mutation and 27% of women with triple-negative breast
cancer diagnosed before the age of 50 years had a BRCA1
mutation (8). Male BRCA1/2
mutation carriers are also at increased risk of cancer. The
cumulative risk of breast cancer is 1.2% in male BRCA1
mutation carriers and 6.8% in male BRCA2 mutation carriers
(9), and the risk of prostate cancer
of BRCA2 mutation carriers is increased to ~3 times higher
than that of the general population (10,11).
Owing to the early onset and susceptibility of multiple cancer
associated with HBOC, the importance of surveillance from a younger
age has been emphasized (12). For
female carriers of a BRCA1/2 mutation, a monthly breast
self-examination from the age of 18, an annual breast MRI or
mammography between the ages of 25 and 29 and an annual breast MRI
and mammography over 30 years of age are recommended. However, the
usefulness of surveillance programs for cancer risks is not clear,
and an appropriate screening method has not been established.
Therefore, once a BRCA1/2 mutation is identified in cancer
patients and their families, the option of the risk reducing
mastectomy (RRM) and the risk reducing bilateral
salpingo-oophorectomy (RRSO) must be considered. In the
retrospective study, the 20-year survival rate for females with a
BRCA1/2 mutation who underwent contralateral prophylactic
mastectomy was 88% and for those who did not was 66% (13). Also, the results of meta-analysis
revealed that RRSO reduced ovarian cancer risk by 80% and breast
cancer risk by 50% in female BRCA1/2 mutation carriers
(14). Furthermore, in a short-term
follow-up study, RRSO reduced breast cancer-specific mortality by
90%, and gynecologic cancer-specific mortality by 95% and overall
mortality by 76% (15). In addition,
the use of the oral poly ADP-ribose polymerase inhibitor Lynparza™
(olaparib) for patients with advanced ovarian cancer with a
BRCA1/2 mutation was approved by the FDA in December 2014.
Although the clinical trial for this drug has not yet been
completed in Japan (16), it may
become a promising new drug for patients with
BRCA1/2-associated cancer once approved.
The frequency of variants of uncertain significance
(VUS) on the BRCA1/2 gene was 2–6 % in the Caucasian
population in the US (17,18) and this frequency is thought to be
greater in other countries with varying ethnic backgrounds due to
the small number of patients undergoing BRCA1/2 testing.
Therefore proper assessment of VUS has become a major issue in
clinical genetic testing. Furthermore, previous studies have
demonstrated that BRCA1/2 mutation-negative patients
harbored deleterious mutations in moderate-risk cancer genes such
as CHEK2, ATM, and PALB2 and MMR (19,20).
Despite an increasing number of HBC testing facilities, there is
little information available regarding attitudes towards precision
medicine for HBC in Japan.
Patients and methods
Study subjects and questionnaire
A total of 120 medical facilities were selected for
this survey because those facilities may be confirmed on the
website to be providing the BRCA1/2 tests for patients with
cancer and their families. The written information on this survey
and self-administered questionnaire were sent to the facilities
between September and October 2015. The questionnaire contained the
following items: i) the outline of the respondents' facilities, ii)
the respondents' characteristics such as their affiliation and
specialty, iii) the number of clients who visited for genetic
counseling and/or received genetic testing for HBC in the most
recent one-year period, iv) the current status of implementation of
RRM and RRSO and v) the requirements for improving access to
genetic counseling. The subjects filled in the questionnaire if
they consented to participate in the study. The collected data were
calculated as totals, means, medians or percentages and the free
descriptive answers were categorized. The study protocol was
approved by the Medical Research Ethics Committee of Tokyo Medical
and Dental University (Tokyo, Japan) (no. 2231).
Results
Background information of the
facilities and the health care professionals
The questionnaire was sent to 120 medical
facilities, and a total of 83 health care professionals
participated (response rate, 69.2%). Approximately one half of the
respondents belonged to university hospitals (N=40, 48.2%) and to
the division of medical genetics (N=37, 44.6%), and the majority
were genetics professionals (N=55, 67.3%), such as clinical
geneticists and genetic counselors (Table I).
 | Table I.The outline of the facilities and the
respondents' characteristics. |
Table I.
The outline of the facilities and the
respondents' characteristics.
| A, Summary of
facility |
|---|
|
|---|
|
| N | % |
|---|
| Type |
|
|
|
University hospital | 40 | 48.2 |
| Cancer
center | 8 | 9.6 |
| Other
hospital | 26 | 31.3 |
|
Clinic | 8 | 9.6 |
|
Other | 1 | 1.2 |
| Number of beds |
|
|
|
<50 | 8 | 9.6 |
|
<100 | 6 | 7.2 |
|
<200 | 4 | 4.8 |
|
<500 | 19 | 22.9 |
|
<1,000 | 39 | 47.0 |
|
>1,000 | 7 | 8.4 |
|
| B, Number of breast
cancer surgeries/year |
|
|
| N | % |
|
|
non-performance | 8 | 9.6 |
|
<50 | 13 | 15.7 |
|
<200 | 36 | 43.4 |
|
<500 | 24 | 29.0 |
|
>500 | 2 | 2.4 |
|
| C, Respondent's
characteristics |
|
|
| N | % |
|
| Division |
|
|
| Medical
genetics | 37 | 44.6 |
| Breast
surgery | 28 | 33.7 |
|
Obstetrics &
gynecology | 8 | 9.6 |
|
Other | 10 | 12.0 |
| Specialty |
|
|
|
Clinical geneticist | 42 | 50.6 |
| Other
physician | 26 | 31.3 |
| Genetic
counselor | 13 | 15.7 |
|
Nurse | 2 | 2.4 |
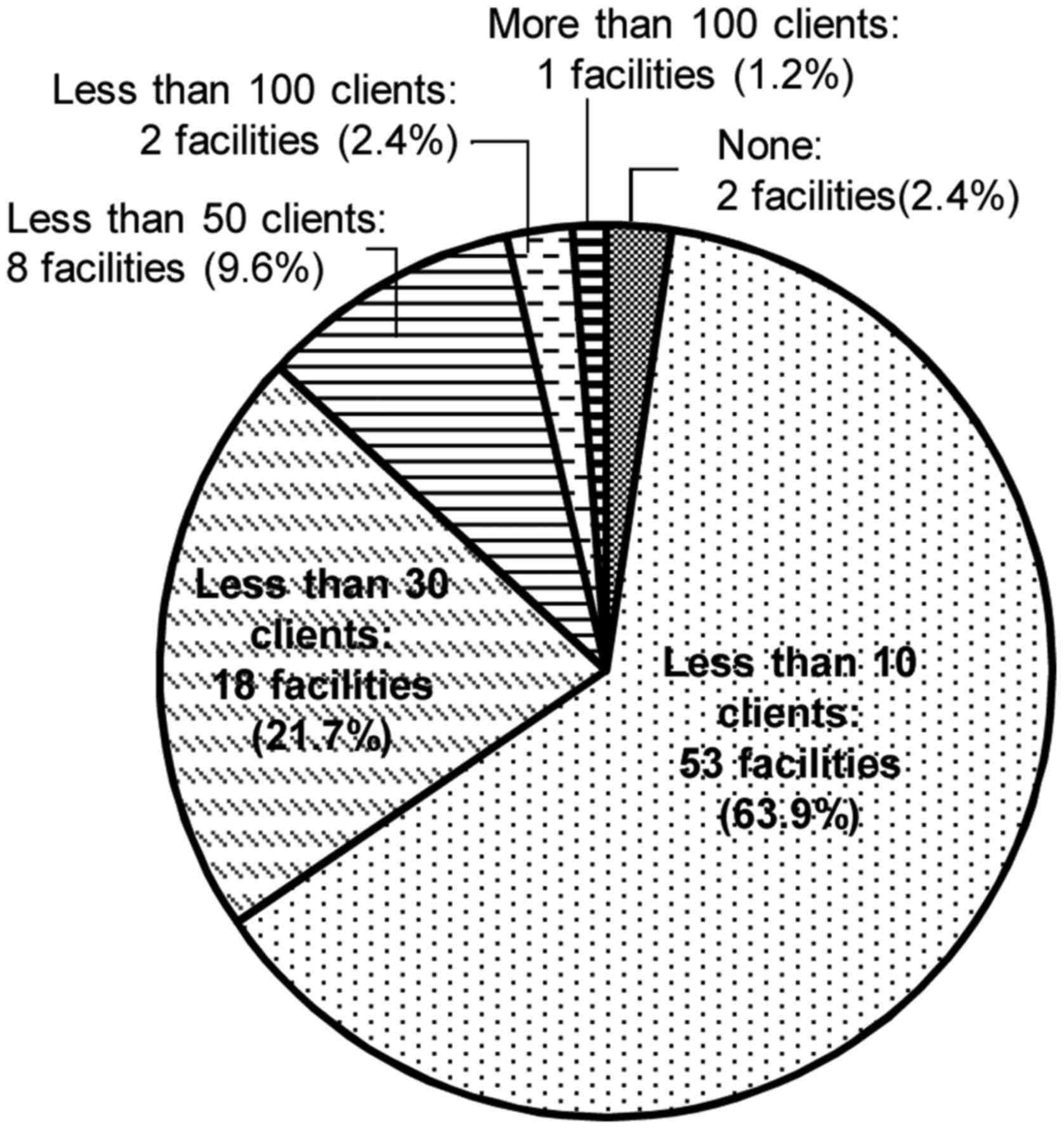
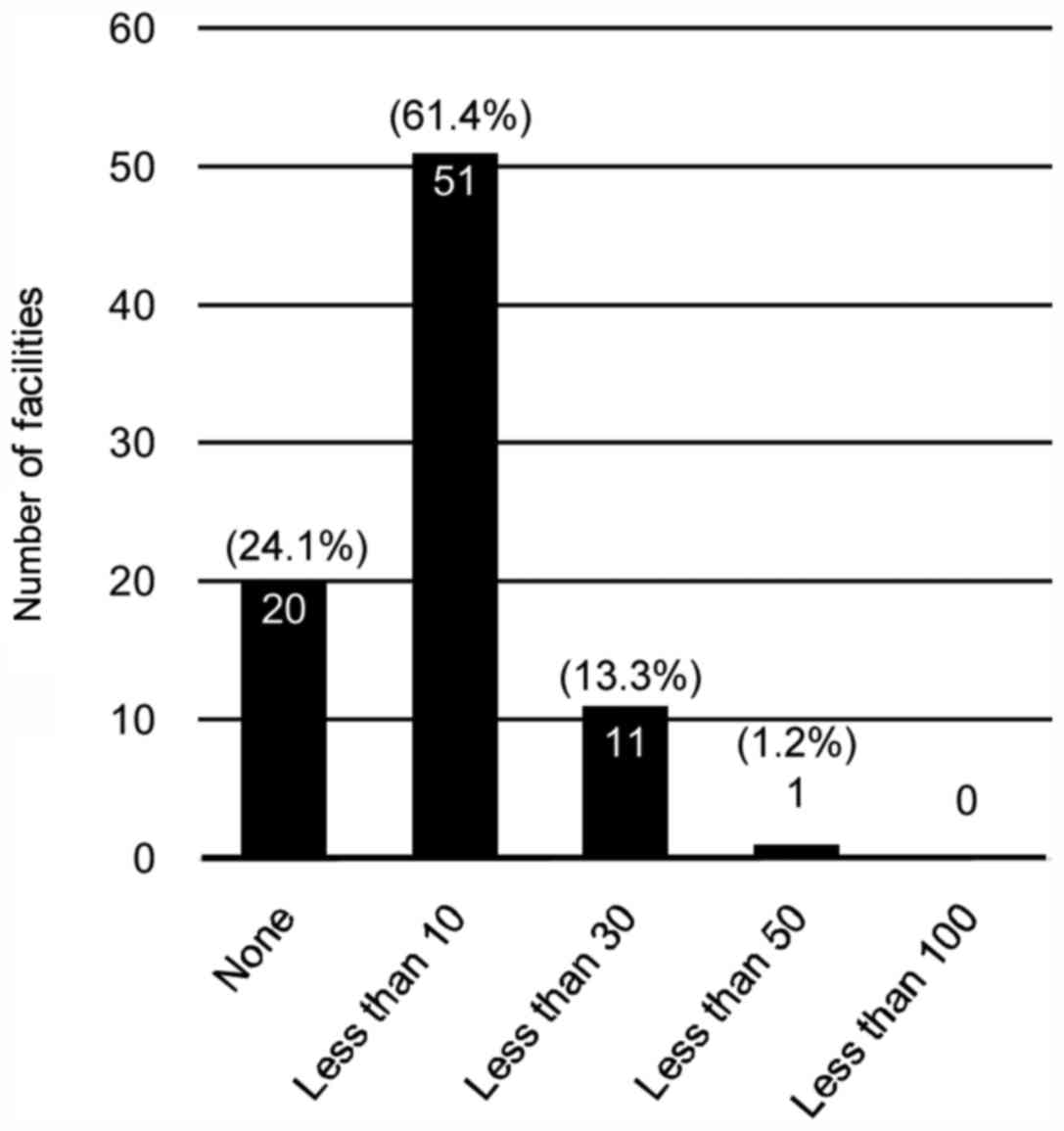
The number of clients who underwent
genetic testing
In 66.3% (N=55) of the respondent's facilities, the
number of patients who visited genetic counseling regarding HBC in
the most recent one-year period was <10 (Fig. 1). In total, 24.1% (N=20) of the
facilities did not perform any genetic testing (Fig. 2). Even in the facility where >100
clients visited genetic counseling, the number who received
implementation of genetic testing was <50.
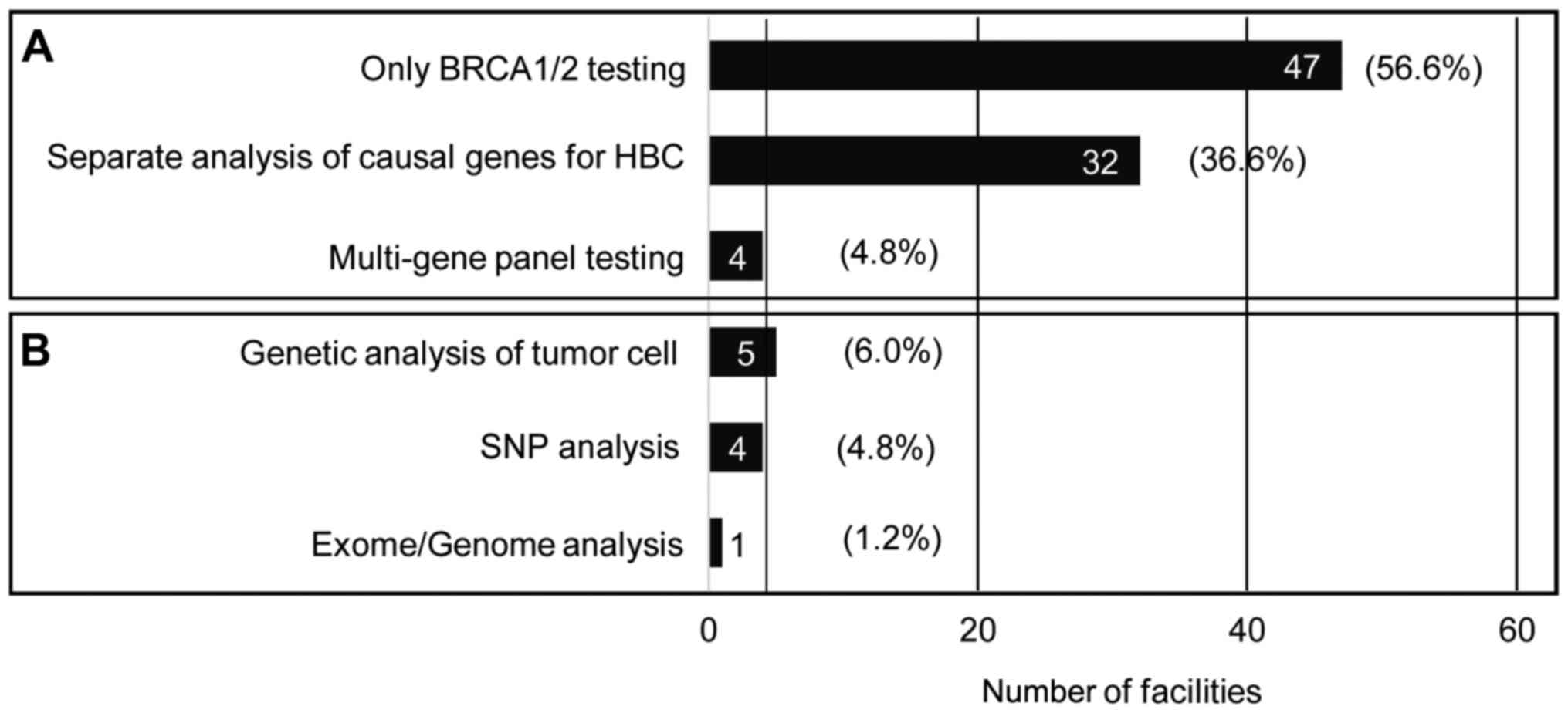
Of all the respondents' facilities, 60.2% dealt with
only BRCA1/2 testing and 38.6% with other targeted genetic
testing. Only four facilities (4.8%) provided multigene panel
testing for HBC.
Status of implementation of RRM and
RRSO
RRM was implemented in 19.3% (N=16) of the
respondents' facilities and RRSO in 24.1% (N=20) but ~30% of the
facilities that have not implemented RRM and RRSO are currently
considering introducing these surgeries (26.9 and 28.6%,
respectively) (Table II). In total,
6.0% (N=5) and 18.1% (N=15) of the facilities, respectively, had
experience in performing these surgeries and it was therefore
identified that RRSO may be more frequently performed than RRM in
Japan.
 | Table II.The availability and use of RRM and
RRSO. |
Table II.
The availability and use of RRM and
RRSO.
|
|
|
|
| Number of uses
(%) |
|---|
|
|
|
|
|
|
|---|
|
| Unavailable, N
(%) | Under
consideration, N (%) | Available, N
(%) | 0 | <5 | <10 |
|---|
| RRM | 67 | 18 | 16 | 11 | 5 | 0 |
|
| (80.7) | (26.9) | (19.3) | (68.8) | (31.3%) | (−) |
| RRSO | 63 | 18 | 20 | 5 | 13 | 2 |
|
| (75.9) | (28.6) | (24.1) | (25.0) | (65.0) | (10.0) |
Access to genetic medicine
The respondents were asked about the barriers that
impeded visiting genetic counseling, excluding the issues of the
high-costs of genetic testing. The majority of the genetic
counselors (N=7, 58.3%) recognized the need for education of
medical staff in order to improve the access to genetic counseling.
On the other hand, the clinical geneticists answered that the
education of patients with cancer and their families (N=14, 33.3%),
as well as the education of medical staff (N=14), was necessary. In
addition, respondents thought of the following factors as other
barriers to participating in genetic counseling: Negative feelings
toward hereditary diseases (N=8), the lack of human resources such
as physician's consultation time regarding HBC (N=6), and the
number of genetic counselors (N=5) and the clinical and genetic
data among Japanese populations (N=5).
Discussion
In the past few years, the awareness of HBC in Japan
has rapidly grown, partly in reaction to the announcement of a US
actress that she underwent a double mastectomy to avoid the risk of
breast cancer. Nonetheless, as presented in the current study,
66.3% of facilities who responded to this survey in Japan answered
that the number of clients receiving HBC-associated genetic
counseling was <10 in the last year, and 24.1% answered no
genetic testing was performed in the past year. As genetic
screening for HBC is not covered by public health insurance in
Japan, clients taking the test must pay the cost in full (~200,000
JPY). These financial burdens on patients may be one of the
negative factors.
Though the pattern of occurrence of breast and
ovarian cancer in a pedigree is characteristic of HBOC, a mutation
in the BRCA1/2 gene may be identified in more than ninety
percent of these pedigrees (21);
patients with Li-Fraumeni syndrome, Cowden syndrome and
Peutz-Jeghers syndrome have a higher risk of breast cancer
(22–24), and MMR, CHECK2,
PALB2 and ATM gene mutations also increase breast
cancer risk (25). Therefore, during
the genetic counseling for HBC, a risk assessment to identify the
genes including BRCA1/2 that must be examined from the
patterns of cancer development in probands and within a family is
essential. The current survey revealed that over sixty percent of
the facilities provided the BRCA1/2 test alone. As presented
in Fig. 3, approximately one half
(N=23, 47.9%) of the university hospitals and cancer hospitals
offered only BRCA1/2 tests for HBC. The services of genetic
counseling and genetic testing based on a comprehensive risk
assessment of HBC may not be available in these facilities.
Although the multi-gene cancer panel test is very useful to analyze
the causal genes for HBC, the test has not been widely used in the
Japanese clinical setting.
Another issue identified by this survey is a lack of
education for health care providers, patients with cancer and their
families. In particular, ~60% of the genetic counselors recognized
the needs for education of medical staff. Previous studies have
revealed that the physicians' recommendation and referral to
genetic counseling significantly affected the patients' motivation
for receiving genetic counseling (26–28).
Particularly in Asia, the physician's referral was a strong
motivator (26–28). Accordingly, there is an urgent
requirement for the additional education of physicians regarding
appropriate referrals for cancer genetics.
Furthermore, certain respondents were concerned
about the lack of clinical and genetic data on HBC in Japan. Only
260 individuals with a strong family history of breast cancer at 8
institutions in Japan were recorded and analyzed regarding
BRCA1/2 genes by the end of March 2012 (29). The small sample size of this study
limits its clinical significance and the results of on-going study
are required. Recently, the rate of VUS results among those
undergoing the BRCA1/2 genetic testing in the US has
decreased 2.1% from 13%, which was accomplished by numerous efforts
directed to the determining the pathogenicity of variants in over
one million samples tested over 20 years (18). Therefore, accumulation of data from
the Japanese population is essential for effective use of genetic
testing.
The overall interpretation of VUS is currently
reported in 2.1% of patients undergoing genetic analysis for HBOC
at Myriad Genetic Laboratories (9,10). This
represents a decline from around 13% over the past decade. The
dramatic decline in the percentage of patients receiving a VUS
result reflects the impact of targeted efforts directed at
determining the pathogenicity of variants, as well as the
availability of data from an increased number of individuals
undergoing testing for HBOC (10).
When a mutation is identified in patients and their
unaffected family members, they are provided the option of RRM and
RRSO in order to reduce future risk of cancer. However, as
presented in Table II, RRM and RRSO
are not available in the majority of the facilities in japan. The
current study indicates that a comprehensive medical care system is
necessary to offer a wider range of options for patients.
In conclusion, the status of genetic medicine for
HBC in Japan has yet to be established. To the best of our
knowledge, the present study is the first to examine the frequency
of use of genetic testing, RRM and RRSO in Japan. Furthermore, the
type of genetic testing was mostly limited to BRCA1/2
testing. Clinical practitioners may offer the appropriate testing
corresponding to the medical history of patients and their family.
The use of the multigene panel testing for HBC may be useful for
patients who do not exhibit family history of various cancers other
than HBOC. Therefore, the current status of genetic services and
the issues regarding HBC were investigated and it was identified
that clinical cancer genetics requires further development in
Japan.
Acknowledgements
The present study was supported by a grant from the
Health Care Science Institute. The authors are grateful to all of
the clinical health care providers who participated in the
study.
Glossary
Abbreviations
Abbreviations:
|
BRCA1 or BRCA2 gene
|
BRCA1/2
|
|
HBC
|
hereditary breast cancer
|
|
HBOC
|
hereditary breast and ovarian cancer
syndrome
|
References
|
1
|
Katanoda K, Kamo K, Saika K, Matsuda T,
Shibata A, Matsuda A, Nishino Y, Hattori M, Soda M, Ioka A, et al:
Short-Term projection of cancer incidence in Japan using an
age-period interaction model with spline smoothing. Jpn J Clin
Oncol. 44:36–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foulkes WD: Inherited Susceptibility to
Common Cancers. N Engl J Med. 359:2143–2153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pharoah PD, Antoniou A, Bobrow M, Zimmern
RL, Easton DF and Ponder BA: Polygenic susceptibility to breast
cancer and implications for prevention. Nat Genet. 31:33–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harkness EF, Barrow E, Newton K, Green K,
Clancy T, Lalloo F, Hill J and Evans DG: Lynch syndrome caused by
MLH1 mutations is associated with an increased risk of breast
cancer: A cohort study. J Med Genet. 52:553–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Risch HA, McLaughlin JR, Cole DE, Rosen B,
Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA and Narod SA:
Population BRCA1 and BRCA2 mutation frequencies and cancer
penetrances: A kin-cohort study in Ontario, Canada. J Natl Cancer
Inst. 98:1694–1706. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mavaddat N, Peock S, Frost D, Ellis S,
Platte R, Fineberg E, Evans DG, Izatt L, Eeles RA, Adlard J, et al:
Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from
prospective analysis of EMBRACE. J Natl Cancer Inst. 105:812–822.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S and Parmigiani G: Meta-analysis of
BRCA1 and BRCA2 penetrance. J Clin Oncol. 25:1329–1333. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fostira F, Tsitlaidou M, Papadimitriou C,
Pertesi M, Timotheadou E, Stavropoulou AV, Glentis S, Bournakis E,
Bobos M, Pectasides D, et al: Prevalence of BRCA1 mutations among
403 women with triple-negative breast cancer: Implications for
genetic screening selection criteria: A Hellenic Cooperative
Oncology Group Study. Breast Cancer Res Treat. 134:353–362. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tai YC, Domchek S, Parmigiani G and Chen
S: Breast cancer risk among male BRCA1 and BRCA2 mutation carriers.
J Natl Cancer Inst. 99:1811–1814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallagher DJ, Gaudet MM, Pal P, Kirchhoff
T, Balistreri L, Vora K, Bhatia J, Stadler Z, Fine SW, Reuter V, et
al: Germline BRCA mutations denote a clinicopathologic subset of
prostate cancer. Clin Cancer Res. 16:2115–2121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirchhoff T, Kauff ND, Mitra N, Nafa K,
Huang H, Palmer C, Gulati T, Wadsworth E, Donat S, Robson ME, et
al: BRCA Mutations and Risk of Prostate Cancer in Ashkenazi Jews.
Clin Cancer Res. 10:2918–2921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®) ‘Genetic/Familial High-Risk Assessment:
Breast and Ovarian’. https://www.nccn.org/professionals/physician_gls/f_guidelines.aspNovember
7–2016
|
|
13
|
Metcalfe K, Gershman S, Ghadirian P, Lynch
HT, Snyder C, Tung N, Kim-Sing C, Eisen A, Foulkes WD, Rosen B, et
al: Contralateral mastectomy and survival after breast cancer in
carriers of BRCA1 and BRCA2 mutations: Retrospective analysis. BMJ.
348:g2262014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rebbeck TR, Kauff ND and Domchek SM:
Meta-analysis of risk reduction estimates associated with
risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation
carriers. J Natl Cancer Inst. 101:80–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Domchek SM, Friebel TM, Neuhausen SL,
Wagner T, Evans G, Isaacs C, Garber JE, Daly MB, Eeles R, Matloff
E, et al: Mortality after bilateral salpingo-oophorectomy in BRCA1
and BRCA2 mutation carriers: A prospective cohort study. Lancet
Oncol. 7:223–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto N, Nokihara H, Yamada Y, Goto Y,
Tanioka M, Shibata T, Yamada K, Asahina H, Kawata T, Shi X and
Tamura T: A phase I dose-finding and pharmacokinetic study of
olaparib (AZD2281) in Japanese patients with advanced solid tumors.
Cancer Sci. 103:504–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindor NM, Goldgar DE, Tavtigian SV, Plon
SE and Couch FJ: BRCA1/2 sequence variants of uncertain
significance: A primer for providers to assist in discussions and
in medical management. Oncologist. 18:518–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eggington JM, Bowles KR, Moyes K, Manley
S, Esterling L, Sizemore S, Rosenthal E, Theisen A, Saam J, Arnell
C, et al: A comprehensive laboratory-based program for
classification of variants of uncertain significance in hereditary
cancer genes. Clin Genet. 86:229–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Desmond A, Kurian AW, Gabree M, Mills MA,
Anderson MJ, Kobayashi Y, Horick N, Yang S, Shannon KM, Tung N, et
al: Clinical actionability of multigene panel testing for
hereditary breast and ovarian cancer risk assessment. JAMA Oncol.
1:943–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kapoor NS, Curcio LD, Blakemore CA,
Bremner AK, McFarland RE, West JG and Banks KC: Multigene panel
testing detects equal rates of pathogenic BRCA1/2 mutations and has
a higher diagnostic yield compared to limited BRCA1/2 analysis
alone in patients at risk for hereditary breast cancer. Ann Surg
Oncol. 22:3282–3288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ford D, Easton DF, Stratton M, Narod S,
Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J,
et al: Genetic heterogeneity and penetrance analysis of the BRCA1
and BRCA2 genes in breast cancer families. The Breast Cancer
Linkage Consortium. Am J Hum Genet. 62:676–89. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Birch JM, Hartley AL, Tricker KJ, Prosser
J, Condie A, Kelsey AM, Harris M, Jones PH, Binchy A, Crowther D,
et al: Prevalence and diversity of constitutional mutations in the
p53 gene among 21 Li-Fraumeni families. Cancer Res. 54:1298–1304.
1994.PubMed/NCBI
|
|
23
|
Pilarski R: Cowden syndrome: A critical
review of the clinical literature. J Genet Couns. 18:13–27. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hearle N, Schumacher V, Menko FH,
Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott
RJ, Lim W, et al: Frequency and spectrum of cancers in the
Peutz-Jeghers syndrome. Clin Cancer Res. 12:3209–3215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Apostolou P and Fostira F: Hereditary
breast cancer: The era of new susceptibility genes. Biomed Res Int.
2013.747–318. 2013.
|
|
26
|
Sussner KM, Jandorf L, Thompson HS and
Valdimarsdottir HB: Barriers and facilitators to BRCA genetic
counseling among at-risk Latinas in New York City. Psychooncology.
22:1594–1604. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anderson B, McLosky J, Wasilevich E,
Lyon-Callo S, Duquette D and Copeland G: Barriers and facilitators
for utilization of genetic counseling and risk assessment services
in young female breast cancer survivors. J Cancer Epidemiol.
2012:2987452012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chin TM, Tan SH, Lim SE, Iau P, Yong WP,
Wong SW and Lee SC: Acceptance, motivators and barriers in
attending breast cancer genetic counseling in Asians. Cancer Detect
Prev. 29:412–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakamura S, Takahashi M, Tozaki M,
Nakayama T, Nomizu T, Miki Y, Murakami Y, Aoki D, Iwase T,
Nishimura S, et al: Prevalence and differentiation of hereditary
breast and ovarian cancers in Japan. Breast Cancer. 22:462–468.
2015. View Article : Google Scholar : PubMed/NCBI
|