Introduction
Breast cancer is the most common invasive cancer in
females and the data indicate that this cancer led to 458,503
deaths worldwide in 2008 (1). As a
number of studies are ongoing for identifying therapeutic
strategies to combat this form of cancer, the progress is slow
owing to its complexity. The resistance of breast cancer stem cells
to conventional therapies such as chemotherapy (2) increases this complexity, as these cells
are responsible for recurrence and disease progression. It has been
reported that, even at the early stages of the disease, a portion
of breast cancer cells may migrate to the bone marrow, and their
entry into the bone marrow is facilitated by mesenchymal stem cells
(3). These migrated cancer cells
remain dormant, becoming active later thereby causing recurrence or
advancement of the disease. CD3−CD56+ or
CD16+ natural killer (NK) cells that possess the ability
to identify and kill target cancer cells (4), particularly cancer stem cells (5,6) without
prior sensitization makes them an important player in
immunosurveillance and cancer defense in vivo. Therefore, NK
cell-based immunotherapy may be a promising therapeutic strategy
against cancer (4). There have been
several studies investigating the role of NK cells in anticancer
immunity and the outcome of cell-based immunotherapy in various
types of solid cancer (4,7–9). In
breast cancer, there have been reports on positive outcomes
following cell-based immunotherapy (10,11).
Chemotherapy has been reported to cause a decline in NK cell number
and function (12), which may
compromise their use in immunotherapy. In this context, the current
study reports on the use of NK cell-based autologous immune
enhancement therapy (AIET) in a patient with stage IIIA breast
cancer, in whom a positive outcome was observed after adding AIET
to conventional treatments. The observations concerning the
decrease in the NK cell in vitro growth expansion with
subsequent chemotherapy cycles in the patient are also
reported.
Case report
Presenting complaints
In October 2012, a 29-year-old Indian female
presented to the Chennai Meenakshi Multispeciality Hospital Limited
(Chennai, India), reporting a history of pain and a lump in the
left breast since August 2012. The patient had given birth to a
child in July 2012.
Clinical findings
Following preliminary investigations, a Tru-Cut
biopsy in October 2012 revealed infiltrating lobular carcinoma of
the left breast. The cancer was aggressive and inflammatory in
nature, with rapidly progressing breast tenderness, pain and
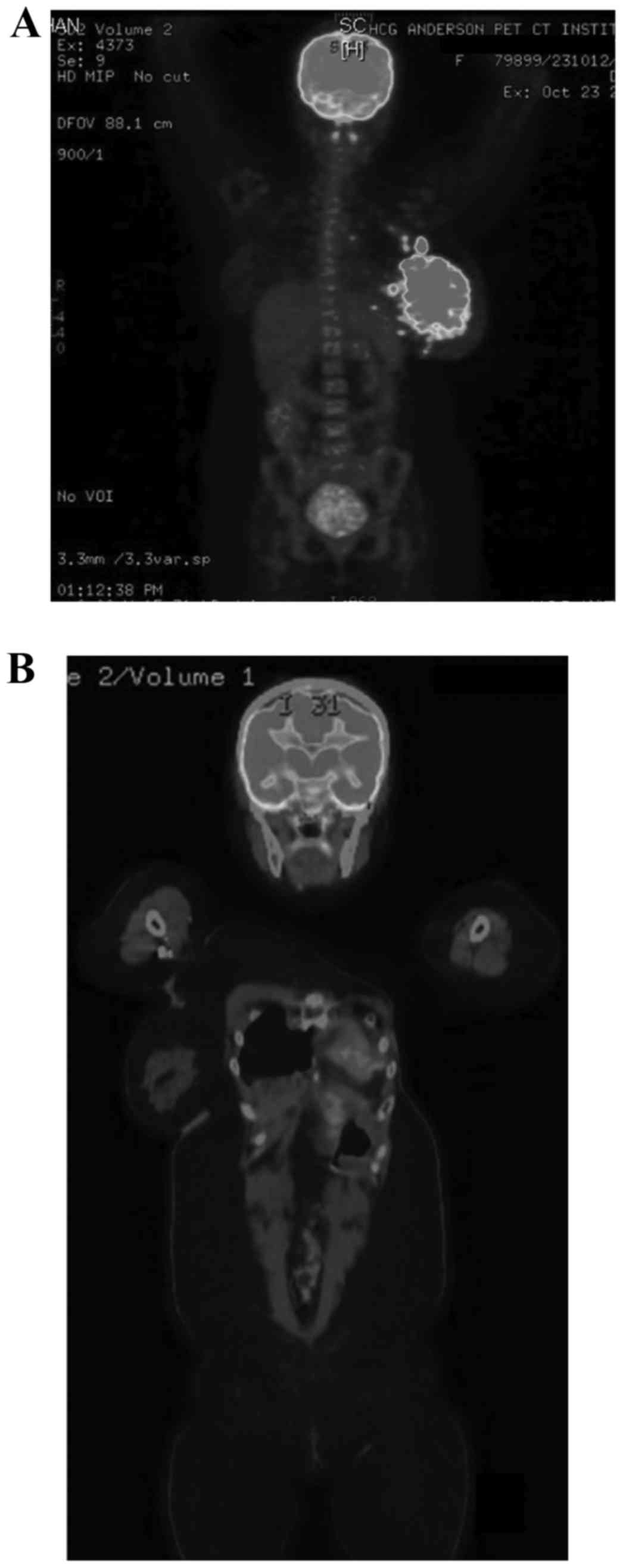
erythema. Whole-body positron emission tomography-computed
tomography (PET-CT) scanning revealed a metabolically active large
lobulated heterogeneously enhancing lesion (12.4×7.4 cm) (Fig. 1A), involving all quadrants of the
left breast with diffuse left breast skin thickening and multiple
discrete satellite nodules (<15 mm) surrounding the mass lesion
and metabolically active multiple left axillary, pectoral and left
upper internal mammary lymph nodes (Fig.
1A). Immunohistochemistry revealed histological characteristics
compatible with infiltrating ductal carcinoma of the breast (Bloom
& Richardson's provisional Grade 8) (13) ER-positive, PR-negative,
Her2/Neu-negative, Ki-67-positive (86%) and EGFR- and cytokeratin
5-negative. The patient underwent three cycles of preoperative
chemotherapy (between October 2012 and December 2012) with
doxorubicin, docetaxel and cyclophosphamide, followed by left
modified radical mastectomy (December 2012) followed by three
cycles of postoperative chemotherapy (between January and February
2013). The histopathological examination following the surgery and
chemotherapy established the tumor to be pT3 N2a Mx stage IIIA
(14).
Therapeutic focus and assessment
The patient simultaneously underwent 12 transfusions
of NK cell-based AIET (15–17) between November 2012 and February 2013
planned in accordance with the chemotherapy cycles. Approximately
200–210 ml of peripheral blood (PB) was withdrawn for the first 3
cycles (3 transfusions in 1 cycle, 9 transfusions in total) and
then for the 10th transfusion, only 40 ml of PB was withdrawn as
the patient's general health condition was low. A quantity of 185
ml of PB was withdrawn for the 11 and 12th transfusions. For each
AIET transfusion, the NK cells isolated from peripheral blood
mononuclear cells were culture-expanded in vitro, employing
autologous plasma without using feeder layers or animal or
allogeneic serum, based on previously described protocols (11,15–17) for
10–12 days before being infused into the patient. The patient also
underwent 5,400 cGy in 27 fractions of radiotherapy between March
2013 and April 2013.
Follow-up and outcomes
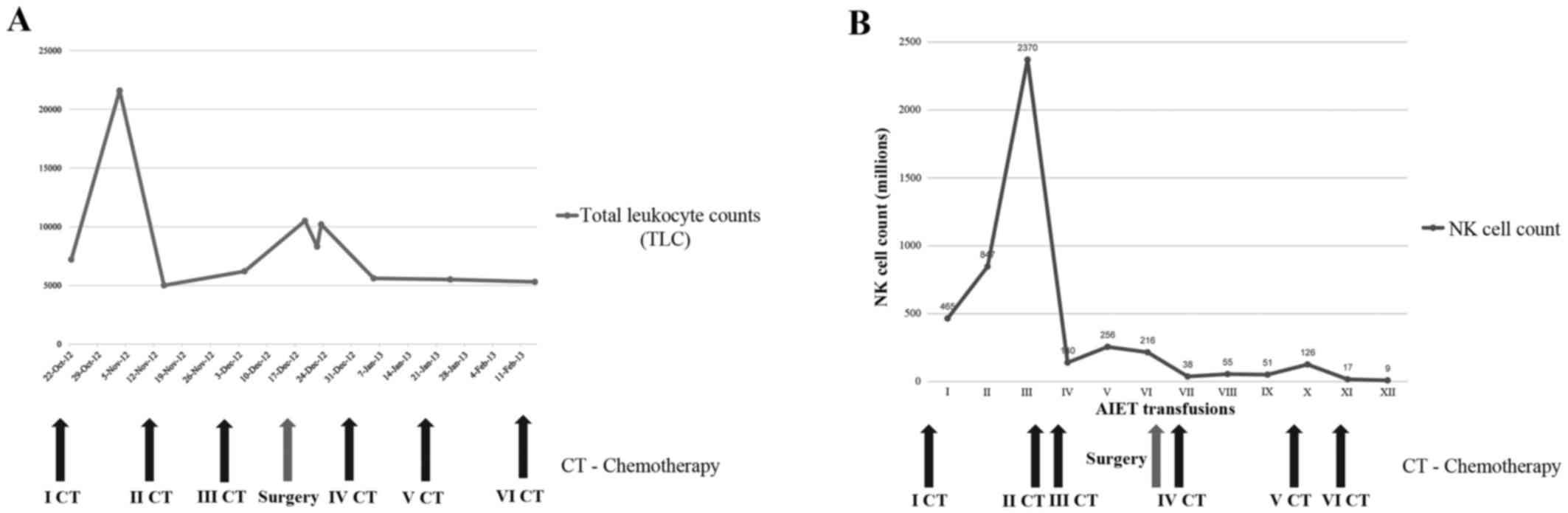
Fig. 2A presents the
total number of leukocytes, which remained close to normal
throughout the therapy. Fig. 2B
shows the declining NK cell counts following their in vitro
expansion, parallel to subsequent chemotherapy cycles, and the
decrease became more profound after the surgery. There were no
adverse reactions following the AIET. Despite the decreased NK
cells counts after in vitro expansion, there was a
subjective improvement in the quality of life after AIET and the
patient reported improved tolerance to the side effects of the
chemotherapy, possibly as AIET was administered concurrently. The
patient has been disease-free for >28 months and a PET-CT scan
in February 2014 identified no evidence of recurrence. Follow-up
scans in June 2014 and March 2015 (Fig.
1B) also reported that there was no evidence of any mass lesion
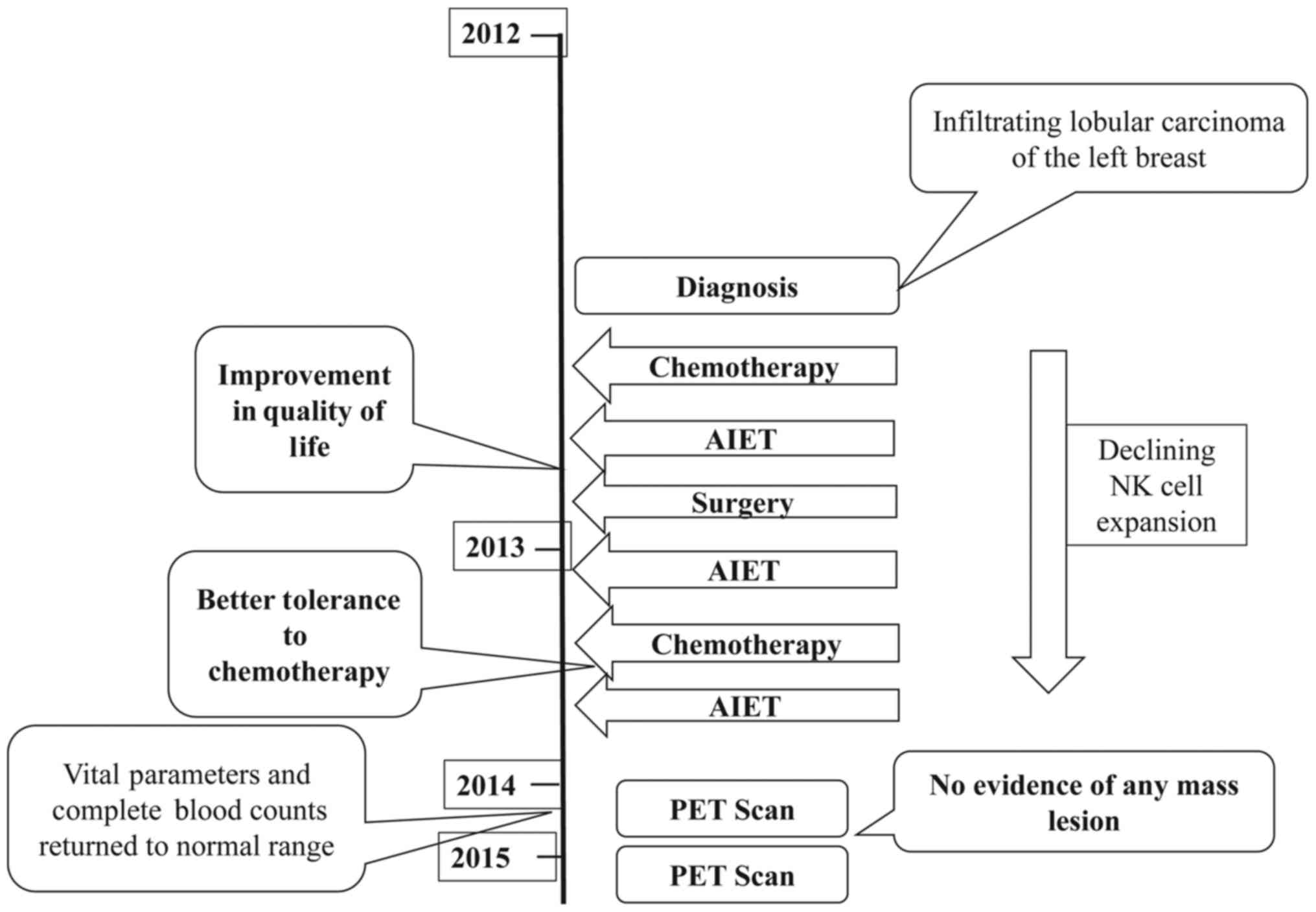
or large lymph nodes in the abdomen or the pelvis. Fig. 3 depicts the timeline of the
interventions and outcomes.
Informed consent and ethics
policy
Verbal and written informed consent was obtained
from the patient for the inclusion of her medical and treatment
history within this case report. The study was performed in
accordance with the ethical standards laid down in the 1964
Declaration of Helsinki and all subsequent revisions.
Discussion
In a study that examined the prognosis of
inflammatory breast cancer (IBC) by screening nearly 3,650 patients
with breast cancer, the median progression-free survival period was
17 months. This study was from the All India Institute of Medical
Sciences (AIIMS), a large tertiary care center in India (18) and this may be considered as
representative of Indian data on IBC, which is relevant to the
present case, as the patient described is of Indian ethnicity. The
patients in the AIIMS study underwent conventional therapies such
as chemotherapy, modified radical mastectomy, radiotherapy and
hormonal therapy as indicated. The patient described in the present
case is having a disease-free survival period of >28 months
following a combination therapy of chemotherapy, radiotherapy and
surgery along with AIET, and is continuously under follow-up. The
longer disease-free survival period may be attributable to the
combination of AIET with the conventional therapies, which requires
further validation in larger number of patients.
The complex nature of breast cancer biology, due to
its genetic and hormonal influence, is further complicated by the
migration of its cells to bone marrow even at an early stage
(3), which leads to later recurrence
of the disease. More complex is IBC, which has a very aggressive
presentation, with the majority of patients ultimately succumbing
to the disease. The present study examined in vitro NK cell
expansion during administration of cell-based AIET concurrently
with chemotherapy and surgery in a patient with stage IIIA IBC. The
observation from the data is that, in this patient, the in
vitro expansion of NK cells from PB withdrawn prior to the
start of the chemotherapy cycles is higher (I–III transfusions),
whereas with progressive chemotherapy, the quantity of NK cells
following in vitro expansion decreased. In particular, after
the surgery and the fourth cycle of chemotherapy, the expansion of
NK cells decreased markedly (Fig.
2B). There are mixed reports in the literature on the effects
of chemotherapy on NK cells. Although certain reports suggest that
chemotherapy reduces the count and cytotoxicity of NK cells
(12,19), another suggested an increase in NK
cell cytotoxicity following chemotherapy (20). The present case report suggests that
there is decrease in the in vitro expansion of NK cells with
subsequent chemotherapy cycles. Although earlier studies have
discussed the effects of chemotherapy on either the count or the
in vitro cytotoxicity of the NK cells (12,19,20), to
the best of our knowledge the current report is the first to
provide details on the in vitro expansion potential of the
NK cells, which has clinical significance as these expanded cells
were used for immunotherapy in the patient described herein. Tai
et al (21) reported that
surgical stress induces dysfunction of NK cells, impairing their
cytotoxic ability, thereby promoting tumor metastasis. The present
report is in line with the Tai et al study, in which,
following surgery, there was a significant decrease in the quantity
of NK cells after in vitro expansion. Their cytotoxicity may
also have been affected, which requires further study. The present
report is of interest not only for the development of more focused
therapies, but also for developing targeted therapies that will not
compromise the cells of the immune system, as high densities of
cytotoxic immune cells have been correlated with good prognosis in
cancer (22–24), and proper functioning of immune cells
such as NK cells is necessary to prevent metastasis (21). However, despite reduced NK cell
quantities after in vitro expansion, the patient in the
present case has remained disease-free for >28 months, which
suggests that AIET, even with compromised NK cell function, may be
able to contribute to a favorable prognosis. This may be due to the
effects of the infused NK cells on encountered cancer cell targets,
including circulating tumor cells, as NK cells have been observed
to lyse circulating tumour emboli efficiently (25). Starting NK cell-based AIET
immediately after diagnosis, as in the present case, may be
advantageous, as these NK cells may also act on breast cancer cells
that migrate to the bone marrow during the early stages of the
disease, as well as targeting treatment-resistant breast cancer
stem cells (3,5,6).
Therefore, an assessment of NK cell expansion potential and
cytotoxicity following conventional therapies in several patients
may elucidate the potential loopholes of the immune system through
which cancer is able to evade these immune cells, leading to
disease recurrence and resistance to treatment. Further studies
focusing on improving or modifying current therapeutic strategies
for cancer so that they do not compromise the application of
autologous immune cells must be performed, after which the approach
of combining AIET with conventional therapies may be suggested for
similar cases with proper validation.
In conclusion, combining NK cell-based AIET with
surgery and chemotherapy was associated with >28 months of
disease-free survival in a patient with stage IIIA IBC. The in
vitro expansion potential of NK cells gradually declined with
subsequent dosages of chemotherapy and markedly after surgery in
this patient, which warrants an assessment of the immune system
during therapies that compromise immunity to allow spread of
cancer, and also to properly validate the timings of starting such
immune-enhancing therapies. In addition, targeted cancer therapies
that do not compromise the immune system are urgently required.
Acknowledgements
The authors would like to thank the M/S Chennai Cell
Cluster (CCC) for technical advice, the Loyola ICAM College of
Engineering Technology (LICET) and the Loyola Institute of Frontier
Energy (LIFE) for their support.
Glossary
Abbreviations
Abbreviations:
|
AIET
|
autologous immune enhancement
therapy
|
|
NK cells
|
natural killer cells
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
World Cancer Report. International Agency
for Research on Cancer. 2008, Available at. http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/index.phpApril
29–2016
|
|
2
|
Dittmer J and Rody A: Cancer stem cells in
breast cancer. Histol Histopathol. 28:827–838. 2013.PubMed/NCBI
|
|
3
|
Corcoran KE, Trzaska KA, Fernandes H,
Bryan M, Taborga M, Srinivas V, Packman K, Patel PS and Rameshwar
P: Mesenchymal stem cells in early entry of breast cancer into bone
marrow. PLoS One. 3:e25632008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terunuma H, Deng X, Dewan Z, Fujimoto S
and Yamamoto N: Potential role of NK cells in the induction of
immune responses: Implications for NK cell-based immunotherapy for
cancers and viral infections. Int Rev Immunol. 27:93–110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tseng HC, Arasteh A, Paranjpe A, Teruel A,
Yang W, Behel A, Alva JA, Walter G, Head C, Ishikawa TO, et al:
Increased lysis of stem cells but not their differentiated cells by
natural killer cells; de-differentiation or reprogramming activates
NK cells. PLoS One. 5:e115902010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin T, Wang G, He S, Liu Q, Sun J and Wang
Y: Human cancer cells with stem cell-like phenotype exhibit
enhanced sensitivity to the cytotoxicity of IL-2 and IL-15
activated natural killer cells. Cell Immunol. 300:41–45. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y and Kakizoe T: Adoptive immunotherapy to lower postsurgical
recurrence rates of hepatocellular carcinoma: A randomised trial.
Lancet. 356:802–807. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kono K, Takahashi A, Ichihara F, Amemiya
H, Iizuka H, Fujii H, Sekikawa T and Matsumoto Y: Prognostic
significance of adoptive immunotherapy with tumor-associated
lymphocytes in patients with advanced gastric cancer: A randomized
trial. Clin Cancer Res. 8:1767–1771. 2002.PubMed/NCBI
|
|
9
|
Egawa K: Immuno-cell therapy of cancer in
Japan. Anticancer Res. 24:3321–3326. 2004.PubMed/NCBI
|
|
10
|
Takada M, Terunuma H, Deng X, Dewan MZ,
Saji S, Kuroi K, Yamamoto N and Toi M: Refractory lung metastasis
from breast cancer treated with multidisciplinary therapy including
an immunological approach. Breast Cancer. 18:64–67. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Domschke C, Ge Y, Bernhardt I, Schott S,
Keim S, Juenger S, Bucur M, Mayer L, Blumenstein M, Rom J, et al:
Long-term survival after adoptive bone marrow T cell therapy of
advanced metastasized breast cancer: Follow-up analysis of a
clinical pilot trial. Cancer Immunol Immunother. 62:1053–1060.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotsakis A, Sarra E, Peraki M, Koukourakis
M, Apostolaki S, Souglakos J, Mavromanomakis E, Vlachonikolis J and
Georgoulias V: Docetaxel-induced lymphopenia in patients with solid
tumors: A prospective phenotypic analysis. Cancer. 89:1380–1386.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bloom HJ and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumors. 7th edition.
Wiley-Blackwell; Oxford: 2009
|
|
15
|
Premkumar S, Dedeepiya VD, Terunuma H,
Senthilkumar R, Srinivasan T, Reena HC, Preethy S and Abraham SJ:
Cell based autologous immune enhancement therapy (AIET) after
radiotherapy in a locally advanced carcinoma of the cervix. Case
Rep Oncol Med. 2013:9030942013.PubMed/NCBI
|
|
16
|
Manjunath SR, Ramanan G, Dedeepiya VD,
Terunuma H, Deng X, Baskar S, Senthilkumar R, Thamaraikannan P,
Srinivasan T, Preethy S and Abraham SJ: Autologous immune
enhancement therapy in recurrent ovarian cancer with metastases: A
case report. Case Rep Oncol. 5:114–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dedeepiya V, Terunuma H, Deng X, Baskar S,
Manjunath S, Senthilkumar R, Murugan P, Thamaraikannan P,
Srinivasan T, Preethy S and Abraham SJ: A comparative analysis of
in vitro expansion of natural killer cells of a patient with
autoimmune haemolytic anaemia and ovarian cancer with patients with
other solid tumours. Oncol Lett. 3:435–440. 2012.PubMed/NCBI
|
|
18
|
Gogia A, Raina V, Deo SV, Shukla NK,
Mohanti BK and Sharma DN: Inflammatory breast cancer: A single
centre analysis. Asian Pac J Cancer Prev. 15:3207–3210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sevko A, Sade-Feldman M, Kanterman J,
Michels T, Falk CS, Umansky L, Ramacher M, Kato M, Schadendorf D,
Baniyash M and Umansky V: Cyclophosphamide promotes chronic
inflammation-dependent immunosuppression and prevents antitumor
response in melanoma. J Invest Dermatol. 133:1610–1619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsavaris N, Kosmas C, Vadiaka M,
Kanelopoulos P and Boulamatsis D: Immune changes in patients with
advanced breast cancer undergoing chemotherapy with taxanes. Br J
Cancer. 87:21–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tai LH, de Souza CT, Bélanger S, Ly L,
Alkayyal AA, Zhang J, Rintoul JL, Ananth AA, Lam T, Breitbach CJ,
et al: Preventing postoperative metastatic disease by inhibiting
surgery-induced dysfunction in natural killer cells. Cancer Res.
73:97–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fridman WH, Remark R, Goc J, Giraldo NA,
Becht E, Hammond SA, Damotte D, Dieu-Nosjean MC and Sautès-Fridman
C: The immune microenvironment: A major player I human cancers. Int
Arch Allergy Immunol. 164:13–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sznurkowski JJ, Zawrocki A and Biernat W:
Subtypes of cytotoxic lymphocytes and natural killer cells
infiltrating cancer nests correlate with prognosis in patients with
vulvar squamous cell carcinoma. Cancer Immunol Immunother.
63:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shafer D, Smith MR, Borghaei H, Millenson
MM, Li T, Litwin S, Anad R and Al-Saleem T: Low NK cell counts in
peripheral blood are associated with inferior overall survival in
patients with follicular lymphoma. Leuk Res. 37:1213–1235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanna N and Fidler IJ: Role of natural
killer cells in the destruction of circulating tumor emboli. J Natl
Cancer Inst. 65:801–809. 1980. View Article : Google Scholar : PubMed/NCBI
|