Introduction
Lung cancer is one of the leading causes of cancer
mortality in numerous countries. In non-small cell lung cancer
(NSCLC), radical resection is generally recognized to be the most
effective treatment, provided that the tumor is resectable.
However, ~30–75% of patients with pathological stage IB to IIIA
disease who undergo complete resections suffer postoperative
recurrence (local recurrence or distant metastasis) (1,2). Several
of these patients may have micro-metastases that are not able to be
detected during pre- or intra-operative staging. Therefore, if such
micro-metastases could be controlled using adjuvant modalities
following surgery, it may be possible to improve the surgical
outcomes of patients with stage IB to IIIA NSCLC (1–4). In
fact, various large-scale phase III clinical trials have indicated
that adjuvant combined platinum-based chemotherapy improved the
overall survival (OS) and disease-free survival (DFS) rates of
patients with completely resected NSCLC (5–8).
Recently, individualized medication has served an
important role in improving chemotherapeutic outcomes, since the
effects of anti-cancer drugs are different among individuals. For
instance, individualized chemotherapy regimens for NSCLC are often
selected on the basis of a number of chemosensitivity-associated
biomarkers, including epidermal growth factor receptor (EGFR)
mutation status (9), anaplastic
lymphoma kinase (ALK) gene rearrangement status (10), excision repair cross-complementation
group 1 (ERCC1) status (11),
ribonucleotide reductase regulatory subunit M1 (RRM1) (12), class III β-tubulin (13,14), and
so on (15). Several recent clinical
studies have indicated that such biomarkers may help us to identify
subsets of patients that would benefit from adjuvant chemotherapy
(15).
In the past 20 years, an in vitro
chemosensitivity test, the collagen gel droplet embedded culture
drug sensitivity test (CD-DST), has been developed on the basis of
examinations of various types of malignant tumor at our (16,17) and
other (18–20) institutions. Our group has subjected
surgically resected samples to this test in order to aid the
selection of effective chemotherapy regimens for patients with
NSCLC. As a result, it was demonstrated that this test is useful
for selecting chemotherapy regimens in patients with NSCLC that
suffer postoperative recurrence (17,21). In
addition, Kawamura et al (22) used this test to select the most
appropriate chemotherapy regimens for patients with advanced NSCLC.
Of course, there have also been numerous studies in which this test
was used to aid the treatment of malignancies other than NSCLC,
indicating that the CD-DST may provide useful information that
would aid the development of individualized chemotherapy for
patients with various types of malignant disease (16–21); for
example, it could be used to provide information about
chemosensitivity-associated biomarkers such as those described
above (9–15).
Nevertheless, there have only been a few reports on
the clinical application of this test to aid regimen selection
during postoperative adjuvant chemotherapy (23,24). In
the present study, in order to determine the impact of in
vitro chemosensitivity test-guided adjuvant chemotherapy on
surgical outcomes, the association between CD-DST findings and the
effects of postoperative adjuvant chemotherapy in patients with
locally advanced p-stage IIIA NSCLC was retrospectively examined.
The results demonstrated that data derived from the CD-DST may aid
regimen selection during platinum-based adjuvant chemotherapy for
patients with p-stage IIIA NSCLC that undergo complete resection,
and that this may improve the surgical outcomes of such
patients.
Patients and methods
Patients
Between December 2007 and March 2012, 906 patients
underwent surgical resection for lung cancer at our institution
(the Osaka Medical Center for Cancer and Cardiovascular Diseases).
Of these patients, 107 were diagnosed with p-stage IIIA lung
cancer, and potentially curative surgery was performed in 93
patients in spite of the presence of locally advanced disease.
Patients that were treated with neo-adjuvant therapy prior to
surgery were excluded from the present study, and therefore a total
of 39 patients with NSCLC who received platinum-based adjuvant
chemotherapy after undergoing complete resection were enrolled.
Informed consent for the CD-DST was obtained preoperatively.
The clinicopathological characteristics of the
enrolled patients are summarized in Table I. Their mean age was 59 years old
(range, 39–76). Twenty-four patients were male and 15 were female.
All of the patients underwent a potentially curative lobectomy
combined with lymph node dissection. The histological diagnosis was
adenocarcinoma in 32 patients, squamous cell carcinoma in 4
patients, and other types in 3 patients (large cell carcinoma in 1
patient, and large cell neuroendocrine carcinoma in 2 patients). As
for the pathological (tumor-lymph node) TN stage, 9, 21, 1, 5, and
3 cases were classified as T1N2, T2N2, T3N1, T3N2, and T4N1
respectively, according to the 7th Edition of the TNM
classification. The EGFR mutation status of each primary tumor was
examined using transbronchial or surgical specimens in 35 cases. Of
these, 18 samples were positive for EGFR mutations (del746-750 in
11 samples, L858R in 6 samples, and L861Q in 1 sample). The 17
wild-type EGFR samples included three ALK-positive
adenocarcinomas.
 | Table I.Characteristics of NSCLC patients who
underwent adjuvant chemotherapy. |
Table I.
Characteristics of NSCLC patients who
underwent adjuvant chemotherapy.
| Characteristic | Total number of
tested patients (n=39) | Sensitive group
(n=25) | Non-sensitive group
(n=14) | Differences
(P-value) |
|---|
| Mean age, years
(range) | 59 (39–76) | 62 (46–76) | 55 (39–65) | 0.06a |
| Sex
(male/female) | 24/15 | 15/10 | 9/5 |
|
| p-stage |
|
|
| 0.08 (T1,T2 vs.
T3,T4) |
|
T1N2 | 9 | 6 | 3 |
|
|
T2N2 | 21 | 11 | 10 |
|
|
T3N1,T3N2,T4N1 | 9 | 8 | 1 |
|
| Histology |
|
|
| 0.63 (Sq vs.
non-sq) |
| Sq | 4 | 3 | 1 |
|
|
Adeno | 32 | 20 | 12 |
|
|
Others | 3 | 2 | 1 |
|
| EGFR status |
|
|
| 0.12 (wild-type vs.
mutant) |
|
Mutant | 18 | 14 | 4 |
|
|
Wild-type | 17 | 9 | 8 |
|
|
Unknown | 2 | 4 | 2 |
|
CD-DST data acquisition
CD-DST data for each patient's primary tumor were
obtained under preoperative informed consent. Note that the use of
CD-DST as a highly advanced medical technology was authorized by
the Japan Ministry of Health, Labour and Welfare in 2007. In
practice, consent was obtained prospectively where possible, which
also covered the postoperative treatment in cases in which locally
advanced disease was preoperatively predicted.
The CD-DST was performed as previously described by
Kobayashi (16) and Higashiyama
et al (17). In brief, after
the primary tumor had been resected, the fresh primary tumor
specimen was immediately minced using a scalpel and digested in a
cell dispersion enzyme solution (EZ; Kurabo Industries Ltd., Osaka,
Japan) for 2 h. The dispersed cancer cells were washed twice and
collected by centrifugation at room temperature, 250 × g for 3 min,
filtered through an 80-µm nylon mesh, and subsequently incubated in
a collagen gel-coated flask (CG-flask; Kurabo Industries Ltd.) in a
CO2 incubator at 37°C for 24 h. The viable cells that
adhered to the collagen gel were collected and suspended in
reconstructed type I collagen solution (Cellmatrix Type CD; Kurabo
Industries Ltd.) at a final density of 1×105 cells/ml.
Three drops of the collagen cell mixture (30 µl/drop) were placed
in each well of a 6-well multiplate, and allowed to gel at 37°C in
a CO2 incubator for 1 h. As a control, this process was
repeated using a 60-mm dish. The final cell concentration was
~3×103 cells/collagen gel droplet. Culture medium [DF
medium containing 10% fetal bovine serum (both from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA)] was overlaid on each
well, and the plate was incubated overnight in a CO2
incubator at 37°C.
Anti-cancer drugs (the drugs and their dosages are
described below) were added, and subsequently the plates were
incubated for 1 h (gemcitabine; GEM) or 24 h (other drugs). After
the removal of the medium containing the anti-cancer drugs, each
well was rinsed twice, overlaid with serum-free culture medium
(PCM-2; Kurabo Industries Ltd.), and incubated for 7 days. The
medium was changed on the fourth day of the incubation period. At
the end of the incubation period, neutral red was added to each
well at a final concentration of 50 µg/ml, and the colonies in the
collagen gel droplets were stained for 3 h. The collagen droplets
in the 60-mm dish were stained immediately prior to exposure (day
1). Thereafter, each collagen droplet was fixed with 10% neutral
formalin buffer, washed in water, air-dried, and subjected to image
analysis. When the optical density of the control group was >5,
the test was regarded a ‘success’. In vitro sensitivity was
expressed as the T/C ratio (%), where T and C are the total cell
numbers of the treated and the control group, respectively. For
each anti-cancer drug, a T/C ratio (%) of ≤50% was considered to
indicate sensitivity.
The anti-cancer drugs and dosages tested in the
CD-DST were as follows: 0.2 µg/ml cisplatin (CDDP), 2.0 µg/ml
carboplatin (CBDCA), 0.1 µg/ml docetaxel (DOC), 1.0 µg/ml
paclitaxel (PTX), 0.05 µg/ml vinorelbine (VNR), 8.0 µg/ml GEM, and
7.0 µg/ml pemetrexed disodium (PEM).
Examined adjuvant chemotherapy
regimens
At our institution, adjuvant chemotherapy for
locally advanced NSCLC, particularly p-stage IIIA NSCLC, is
generally performed using a platinum-based (usually a doublet)
regimen within the first 10 weeks following surgery. According to
the guidelines used in Japan, a platinum-based chemotherapeutic
method, such as CDDP plus VNR (CDDP+VNR) or CBDCA plus PTX
(CBDCA+PTX), is usually selected (5,25).
Therefore, CDDP+VNR or CBDCA+PTX was selected in cases in which the
surgical specimen was found to be sensitive to one or more of these
drugs during the CD-DST. When the surgical specimen was judged to
be more sensitive to other drugs, regimens containing these drugs
were selected, proving that the patient's physical condition
allowed it. These patients were defined as the sensitive group. In
contrast, when the CD-DST did not identify any appropriate
combinations, the CDDP+VNR or CBDCA+PTX regimen was selected. These
patients were defined as the non-sensitive group. In addition, one
patient in the latter group received the CDDP+DOC regimen (26) at their own request.
The regimens used were as follows: CDDP+VNR in 30
patients, CDDP plus DOC (CDDP+DOC) in 2 patients, CDDP plus GEM
(CDDP+GEM) in 1 patient (27,28),
CDDP plus PEM (CDDP+PEM) in 1 patient (28), CBDCA+PTX in 3 patients, CBDCA plus
GEM (CBDCA+GEM) in 1 patient (27),
and CBDCA plus DOC (CBDCA+DOC) in 1 patient (29) (Table
II). All regimens were started at the standard doses reported
in the guidelines (Table III).
Three chemotherapy cycles were generally planned, and, where
possible, a fourth was administered. During chemotherapy,
toxicities were evaluated using the National Cancer Institute
Common Terminology Criteria for Adverse Events (CTC-AE) Version
4.0.
 | Table II.Adjuvant chemotherapy regimens
performed, and the number of treatment cycles administered. |
Table II.
Adjuvant chemotherapy regimens
performed, and the number of treatment cycles administered.
| Treatment | Sensitive group
(n=25) | Non-sensitive group
(n=14) |
|---|
| Chemotherapy
regimen |
|
|
|
CDDP+VNR | 19 (76%) | 11 (78.6%) |
|
CDDP+DOC | 1 | 1 |
|
CDDP+GEM | 1 | 0 |
|
CDDP+PEM | 1 | 0 |
|
CBDCA+PTX | 1 | 2 |
|
CBDCA+GEM | 1 | 0 |
|
CBDCA+DOC | 1 | 0 |
| No. of completed
chemotherapy cycles |
|
|
|
One | 2 | 0 |
|
Two | 4 | 0 |
|
Three | 1 | 1 |
|
Four | 18 | 13 |
| Tolerability rate
(%)a | 76% | 100% |
 | Table III.Univariate analysis of DFS- and
OS-associated factors. |
Table III.
Univariate analysis of DFS- and
OS-associated factors.
|
|
|
| P-value (log-rank
test) |
|---|
|
|
|
|
|
|---|
| Variable |
|
| DFS | OS |
|---|
| Age (years) | ≤60 (n=15) | >60 (N=24) | 0.33 | 0.11 |
| Sex | Male (n=24) | Female (N=15) | 0.36 | 0.018 |
| T-stage | T3,T4 (n=9) | T1,T2 (N=30) | 0.64 | 0.69 |
| Histology | Sq (n=4) | Non-Sq (N=35) | 0.63 | 0.054 |
| EGFR status | Mutant (n=18) | Wild-type
(N=17) | 0.065 | 0.95 |
| Regimen
(CD-DST) | Non-sensitive
(n=14) | Sensitive
(n=25) | 0.037 | 0.76 |
| Regimen
(CDDP/CBDCA) | CDDP-based
(n=34) | CBDCA-based
(n=5) | 0.81 | 0.56 |
| Chemotherapy
completeness | No (n=6) | Yes (n=33) | 0.55 | 0.14 |
Follow-up and statistical
analyses
The follow-up examinations carried out after the
adjuvant chemotherapy were generally performed as follows: During
the first 36 months after the operation, systemic and local
screening examinations were performed using blood tests, chest
computed tomography (CT) scans were routinely obtained every 6
months, and fluoro-2-deoxyglucose positron emission tomography
(FDG-PET) scans were generally performed every year. Brain CT or
magnetic resonance imaging scans were performed as required. During
the first 24 months, such examinations were performed with
particular care. From the third postoperative year onwards, such
intensive examinations were performed once a year at least.
The time of the initial recurrence was determined
based on the onset of clinical symptoms, the detection of blood
test abnormalities (e.g., the patient's serum carcinoembryonic
antigen level), or the detection of recurrent lesions on imaging,
and the DFS period was defined as the period between the operation
and the time of the initial recurrence. When a tumor recurred, the
initial recurrence site was also evaluated. DFS and OS curves were
calculated using the Kaplan-Meier method, and differences were
determined using the log-rank test. The Cox proportional hazards
regression model was used to perform a multivariate analysis of
factors associated with favorable DFS. Statistical analyses were
performed using Fisher's exact probability test or the unpaired
t-test. P<0.5 was considered to indicate a statistically
significant difference.
Results
CD-DST data and the types of adjuvant
chemotherapy performed
In order to examine the associations between the
findings of the CD-DST and the adjuvant chemotherapy regimens
performed, the patients were classified into the following two
groups as described above: The sensitive group (25 patients), which
were treated with regimens including one or two anti-cancer drug(s)
that were indicated to be effective by the CD-DST (i.e.,
CD-DST-selected drugs), and the non-sensitive group (14 patients),
who were treated with chemotherapy regimens that did not include
any CD-DST-selected anti-cancer drugs.
Table I features the
characteristics of the patients in each group. The sensitive group
included slightly older patients than the non-sensitive group
(P=0.06). The sensitive group also exhibited more aggressive
T-factors than the non-sensitive group (P=0.08). There were no
significant differences in sex, histology, or EGFR mutation status
between the groups.
Table II shows a
summary of the adjuvant chemotherapy regimens performed, and the
numbers of cycles administered in each group. The CDDP+VNR regimen
was performed in 19 patients (76%) in the sensitive group, and 11
patients (78.6%) in the non-sensitive group, and the ratio of the
frequency of the CDDP+VNR regimen to the frequency of other
regimens did not differ between the groups. With regard to the
number of chemotherapy cycles administered, 19 patients in the
sensitive group (76%) and 14 in the non-sensitive group (100%)
received ≥3 cycles of platinum-based adjuvant chemotherapy, and
there was also a significant difference in the frequency of
adjuvant chemotherapy completion between the groups. In the
sensitive group, 6 patients received incomplete chemotherapy (only
1 cycle in 2 patients and 2 cycles in 4 patients) because of Grade
3 or 4 hematological toxicities in 2 patients, a severe pulmonary
infection in 1 patient, refusal in 2 patients, and cardiac failure
in 1 patient. These 6 patients received no additional adjuvant
chemotherapy until recurrent disease occurred. It was necessary
that appropriate dose reductions of each regimen were performed in
certain patients in each group.
DFS and OS
Follow-up examinations were conducted in August
2015. At this time, the patients' follow-up periods ranged from
10.3 to 91.0 months (median: 55.6 months), and recurrent disease
occurred in 28 patients. A total of 18 patients had succumbed to
cancer, and 1 had died of another disease without suffering
recurrence. Among the survivors, the follow-up period ranged from
28.7 to 91.0 months (median: 59.7 months).
The 2-year, 3-year, and 5-year DFS rates for all
patients were 40.5, 29.2, and 26.0%, respectively, and the median
DFS period was 21.9 months. The DFS curves of the two groups are
shown in Fig. 1A. The 2-year,
3-year, and 5-year DFS rates of the sensitive group were 55.7,
37.7, and 32.3%, respectively, and the median DFS period was 25.3
months. The 2-year, 3-year, and 5-year DFS rates of the
non-sensitive group were 14.3, 14.3, and 14.3%, respectively, and
the median DFS period was 15.4 months. The DFS of the two groups
differed significantly (P=0.037); i.e., the sensitive group
exhibited a significantly improved DFS (Fig. 1A).
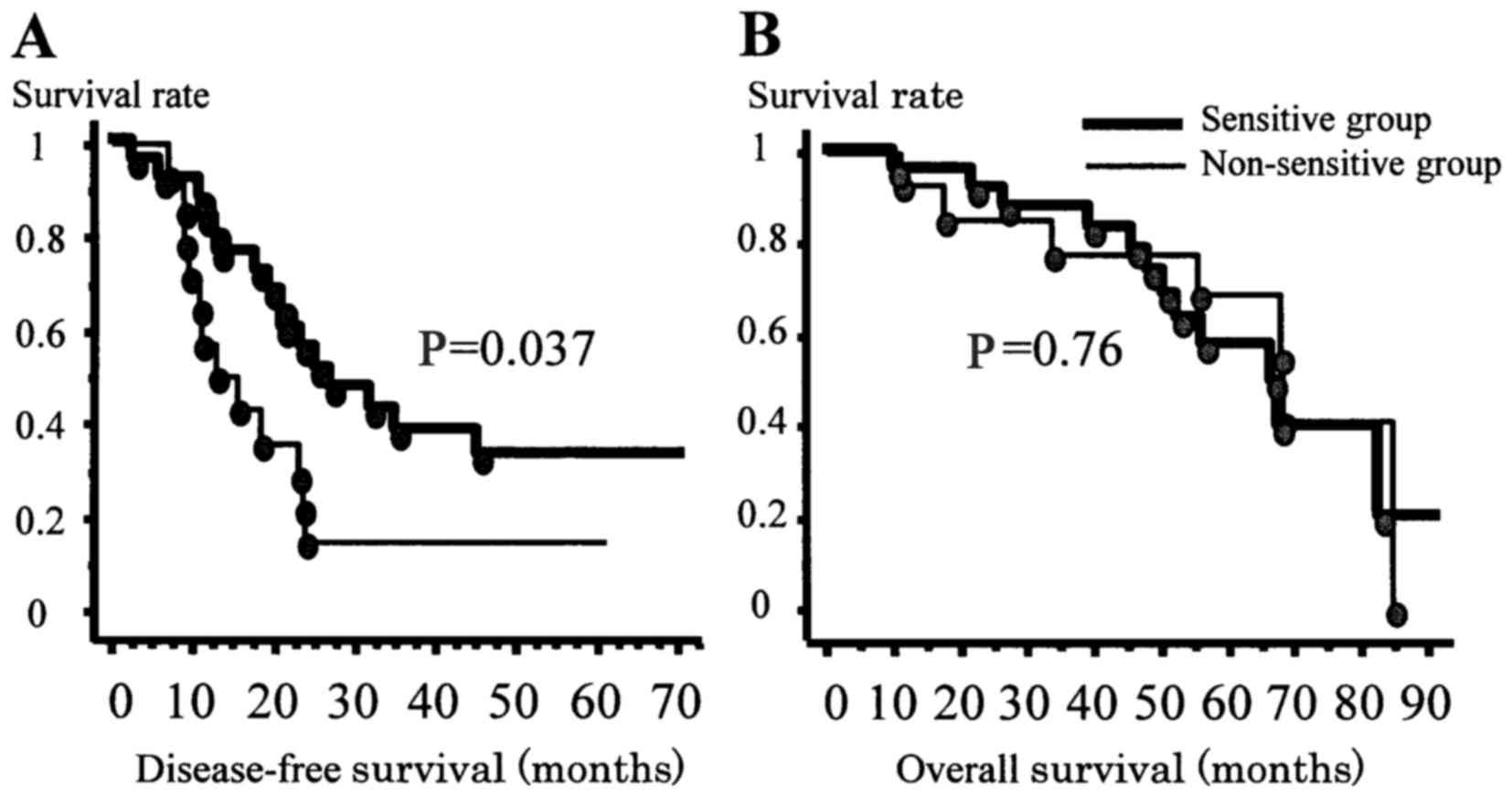 | Figure 1.DFS and OS curves of the two groups
of enrolled patients. (A) DFS curves of the two groups (i.e., the
sensitive and non-sensitive groups). The 2-year, 3-year, and 5-year
DFS rates of the sensitive group were 55.7, 37.7, and 32.3%,
respectively. The 2-year, 3-year, and 5-year DFS rates of the
non-sensitive group were 14.3, 14.3, and 14.3, respectively. The
sensitive group exhibited a significantly improved DFS (P=0.037).
(B) OS curves of the two groups. No significant difference in the
OS was identified between these groups (P=0.76). |
The 2-year, 3-year, and 5-year OS rates for all
patients were 87.2, 84.5, and 62.1%, respectively, and the median
OS period was 67.6 months. The OS curves of the two groups are
shown in Fig. 1B. No significant
difference in the OS was identified between these groups
(P=0.76).
Prognostic analysis
Representative candidates were selectively analyzed
to identify factors associated with favorable DFS or OS in this
series. The analysis included age, sex, T-factor, histology, EGFR
status, the administration of regimens involving CD-DST-selected
anti-cancer drugs, the administration of CDDP-based or CBDCA-based
regimens, and the number of chemotherapy cycles completed. A
summary of the results of the univariate analyses is shown in
Table III. Regarding DFS, only the
administration of regimens involving CD-DST-selected drugs was
found to be a prognostic factor (P=0.037), although EGFR status was
demonstrated to be a marginally significant factor (P=0.065). By
contrast, sex and histology were strongly associated with OS. As
described above, the administration of regimens involving
CD-DST-selected drugs did not have any effect on OS.
Table IV shows a
summary of the results of the multivariate analysis of
DFS-associated prognostic factors. According to this analysis,
among the 35 patients in the present study (excluding the four
patients for whom no information regarding EGFR status was
available), regimens involving CD-DST-selected drugs and EGFR
status were found to be independent predictors of DFS.
 | Table IV.Multivariate analysis of
DFS-associated prognostic factors among NSCLC patients (n=35) with
p-stage IIIA disease who underwent adjuvant chemotherapy. |
Table IV.
Multivariate analysis of
DFS-associated prognostic factors among NSCLC patients (n=35) with
p-stage IIIA disease who underwent adjuvant chemotherapy.
| Variable | Comparison | Odds ratio | 95% confidence
interval | P-value |
|---|
| EGFR status | Wild-type vs.
mutant | 0.337 | 0.139–0.818 | 0.016 |
| Regimen
(CD-DST) | Non-sensitive vs.
sensitive | 3.152 | 1.315–7.554 | 0.010 |
Recurrence pattern following adjuvant
chemotherapy
The differences in the initial recurrence patterns
of the sensitive and non-sensitive groups were analyzed. Table V shows a summary of the recurrence
site data for each group. The two groups exhibited similar
recurrence rates (68% vs. 79%). The rate of nodal recurrence was
slightly lower in the sensitive group (P=0.08) than in the
non-sensitive group, and a similar trend was observed for brain
recurrence (P=0.09).
 | Table V.Sites of recurrence in each
group. |
Table V.
Sites of recurrence in each
group.
|
| Sensitive group
(n=25, %) | Non-sensitive group
(n=14, %) |
|---|
| No. of cases of
recurrence | n=17 (68) | n=11 (79) |
| Initial recurrence
site |
|
|
| Intrathoracic |
|
|
|
Nodea | 3 (12) | 5 (36) |
|
Pleura | 2 (8) | 0 |
|
Lung | 6 (24) | 2 (14) |
|
Surgical margin | 1 (4) | 0 |
| Extrathoracic |
|
|
|
Brain | 1 (4) | 3 (21) |
|
Bone | 1 (4) | 0 |
|
Spleen | 1 (4) | 0 |
|
Systemic | 2 (8) | 1 (7) |
Discussion
Several recent large-scale clinical trials have
shown that postoperative platinum-based adjuvant chemotherapy
improves the DFS and OS of patients with p-stage IB to IIIA NSCLC
(5–8). For instance, the International Adjuvant
Lung Cancer Collaborative Trial Group (IALT) demonstrated that the
administration of three to four courses of CDDP-based adjuvant
chemotherapy after complete resection for p-stage I to IIIA NSCLC
improved the 5-year OS rate by 4.1%, and the 5-year DFS rate by
5.1% (5). The JBR.10 study also
revealed that CDDP+VNR adjuvant chemotherapy for completely
resected stage IB to II NSCLC improved OS by 15% (6). The adjuvant Navelbine International
Trialist Association (ANITA) trial reported that adjuvant
chemotherapy for stage IB to IIIA NSCLC resulted in an 8.6%
increase in the 5-year OS rate, and an 8.7% rise in the 5-year DFS
rate (7). In addition, it also
resulted in a significant difference in OS in patients with N1 or
N2 disease (7). Furthermore,
according to a study of the Lung Adjuvant Cisplatin Evaluation
(LACE) database, combined CDDP-based adjuvant chemotherapy
(CDDP+VNR) improved the OS and DFS rates of completely resected
patients, particularly those with stage II or III NSCLC (8). Therefore, adjuvant chemotherapy
involving a platinum-based regimen is now the standard treatment
for locally advanced NSCLC around the world (4). However, the optimal platinum-based
regimen has yet to be elucidated, in terms of the platinum agent
itself, as well as the best anti-cancer drug to pair it with. At
present, VNR is the most commonly available anti-cancer drug that
is suitable for pairing with platinum-based agents (6–8), but, if
possible, more effective individualized drugs should be selected.
In addition to these anti-cancer drugs, several studies of
molecular targeting medicines, such as EGFR inhibitors, have been
performed in the adjuvant setting (30–33). In
light of the fact that activating mutations in the EGFR gene are
strongly correlated with responsiveness to EGFR inhibitors
(30,31,33),
recent (ongoing) Japanese studies of EGFR inhibitors have only
involved patients with tumors expressing EGFR mutations.
In order to improve the efficacy of adjuvant
chemotherapy for patients who undergo surgical resection, it is
very important to select patients and regimens in an appropriate
manner. There are various characteristics that may be taken into
account during patient selection, including disease stage, age,
tumor histology, risk classification, biomarkers, genetics, and so
forth, and it would be useful to be able to accurately identify
subgroups of patients with NSCLC who would derive the greatest
benefit from individualized chemotherapy regimens (1–4,15). Several studies have reported that
individualized adjuvant chemotherapy is possible for patients with
NSCLC undergoing surgery. For example, ERCC1, a biomarker that is
useful for predicting sensitivity to CDDP, has been reported to aid
regimen selection (11,34). Low ERCC1 expression has been
suggested to predict increased sensitivity to CDDP-based
chemotherapy, as it is results in saturation of the enzyme complex
(11). Olaussen et al
(34) demonstrated that, among
ERCC1-negative patients, the chemotherapy group exhibited
significantly longer DFS rates compared with the observation group,
whereas no significant difference in survival was detected among
the ERCC1-positive patients, indicating that CDDP-based
chemotherapy should be administered to ERCC1-negative patients.
Thus, ERCC-1 could be a useful prognostic and predictive marker;
however, a recent report questioned its practical utility (35). Other biomarkers of tumor
chemosensitivity to cytotoxic anti-cancer drugs, for example, RRM1,
which is a marker of GEM sensitivity, have been identified by
experimental and clinical studies, but there are few clinical data
regarding the use of chemosensitivity markers to aid regimen
selection during adjuvant chemotherapy for NSCLC (12).
On the other hand, in vitro chemosensitivity
tests for cytotoxic anti-cancer drugs, such as the CD-DS, the
histoculture drug response assay (HDRA), the MTT assay, and the
adenosine triphosphate-based tumor chemosensitivity assay
(ATP-TCA), are promising regimen selection techniques (23,24,36–39). In
Japan, the three former tests were used as highly advanced medical
technologies between September 2007 and March 2012 (since April
2012, they have been classified as medical services under the
Japanese health insurance system). As described in the
Introduction, these tests are now widely used in clinical practice
during the treatment of lung cancer and other malignancies
(17–24). In fact, there have been many reports
about the utility of these tests during chemotherapy for various
types of advanced and recurrent malignancies (17–24,37–40).
Such tests were used to examine surgical specimens, and this
revealed that they were clinically useful for regimen selection
during chemotherapy for patients with advanced and recurrent NSCLC
(17,21), but scant information is available
regarding the clinical application of these tests in the adjuvant
chemotherapy setting (23,24). Recently, Tanahashi et al
(24) reported an interesting
observation regarding the use of in vitro chemosensitivity
tests during adjuvant chemotherapy for patients undergoing surgery
for lung cancer: The OS of the patients treated with two
HDRA-positive drugs was significantly better (P=0.03) compared with
that of patients treated with one HDRA-positive drug or
HDRA-negative drugs, indicating that adjuvant chemotherapy based on
in vitro chemosensitivity test data may have a strong
positive influence on the surgical outcomes of patients with NSCLC.
However, no significant differences in DFS were detected among the
patients in the latter series. By contrast, the present study
revealed that adjuvant chemotherapy regimens that included at least
one CD-DST-selected drug resulted in significantly more favorable
DFS rates in patients with NSCLC with stage IIIA disease (P=0.036)
than did regimens that did not involve any CD-DST-selected drugs.
However, the use of such regimens did not have any impact on OS. In
the present study, multivariate analysis revealed that CD-DST data
may be used to improve the DFS rate in patients treated with
adjuvant chemotherapy. The present study had a similar design to
that performed by Tanahashi et al (24), although the in vitro
chemosensitivity test method differed, as did the patients' disease
stages, i.e., the patients enrolled by Tanahashi et al
(24) had stage II or worse disease,
whereas all of the patients in the present study had stage IIIA
disease. In addition, the patients were tested at different points
in their clinical courses: Since our study included more recent
cases, recurrent lesions could have been treated differently (e.g.,
with molecular targeting agents) compared with the cases observed
in the study of Tanahashi et al (24). Such factors may have been responsible
for the differences in patient outcomes observed between our study
and those of Tanahashi's group (24). Although there were differences
between the findings of these studies, it was clearly demonstrated
that the data obtained with in vitro chemosensitivity tests
is correlated with surgical outcomes following complete resection
in patients with NSCLC.
Maejima et al (23) reported that a good correlation exists
between in vitro sensitivity to S-1 and the outcomes of
gastric cancer patients who undergo complete resection followed by
adjuvant chemotherapy with S-1. In that study, the CD-DST was used
as an in vitro sensitivity test to examine the sensitivity
of surgical samples to 5-fluorouracil and
5-chloro-2,4-dihydroexpyridine. In their prospective study, the
high-sensitivity group exhibited higher 3-year OS and DFS rates
compared with the low-sensitivity group. Thus, the CD-DST data
exhibited a stronger correlation with DFS. It is noteworthy that,
according to most recent report of the Japan multicenter
exploratory phase II trial (JACCRO-GC 04) (39), similar results were also observed,
indicating that chemosensitivity testing of surgical specimens
appears to be a promising approach to selecting adjuvant
chemotherapy regimens in patients with gastric cancer. Another
similar study of gastric cancer demonstrated that the MTT assay is
useful for regimen selection (40).
Similarly, Fujita et al (41)
identified that sensitivity testing is useful for selecting
regimens for adjuvant chemotherapy for patients with locally
advanced esophageal cancer.
In the present study, the patterns of recurrence
that arose in each group after adjuvant chemotherapy for locally
advanced NSCLC were also analyzed. An interesting difference was
detected between the sensitive and non-sensitive groups: The
sensitive group tended to develop fewer recurrent nodal or brain
lesions than the non-sensitive group. Our group has previously
emphasized the usefulness of CD-DST data for selecting chemotherapy
regimens, especially for patients that suffer postoperative nodal
recurrence (17). In addition, our
group demonstrated how, in the case of certain anti-cancer drugs,
few differences were observed between the chemosensitivity of the
primary NSCLC tissue and the associated lymph node metastases
(42). Several experimental studies
have detected unexpected differences in chemosensitivity between
primary and metastatic tumors (42,43).
These results, and our previous data (17,42),
appear to agree with the findings of the present study regarding
nodal recurrence. By contrast, it was not possible explain our
findings regarding brain recurrence. Regardless, further analyses
of this topic are required, since the inter-group differences in
recurrence patterns detected in the present study were not
statistically significant.
The DFS results obtained in the present study, i.e.,
that the sensitive group displayed a more favorable prognosis,
could be explained by slower tumor growth or a lower grade of tumor
malignancy. According to several reports (11,12,24,34),
in vitro chemosensitivity to certain anti-cancer drugs is
strongly associated with the grade of tumor malignancy. As
described above, ERCC-1 was found to be a prognostic marker, as
well as a predictor, of chemotherapeutic efficacy (12,34).
However, our firm opinion is that the differences in DFS between
the two groups were due to variations in the efficacy of adjuvant
chemotherapy, as there were no apparent differences in the
background characteristics of the two groups, and OS was not
influenced by the CD-DST status. Therefore, taking into
consideration both our previous and present findings (17,21), the
CD-DST data are more important as a predictor of the efficacy of
anti-cancer drugs, which impacts on DFS, rather than as a
prognostic indicator of OS.
The present study had a retrospective design, but
was conducted in the clinical setting. It had several limitations.
First, it involved a small number of patients, and secondly, all of
the patients had stage IIIA disease. The current treatment strategy
for patients with locally advanced NSCLC may be about to change.
For example, adjuvant therapy using molecular targeting agents
could become the standard regimen for patients with locally
advanced NSCLC whose tumors are positive for EGFR mutations
(30,31,33). In
addition, in vitro chemosensitivity test-guided
platinum-based regimen selection could be used in the clinical
setting during the treatment of a limited population of patients
with NSCLC, i.e., those who express the wild-type EGFR, in the
future. Despite these limitations, at the very least it could be
said that CD-DST data provide important information for improving
the efficacy of adjuvant chemotherapy in patients with stage IIIA
NSCLC.
In conclusion, the present study suggested that
CD-DST data obtained from surgical specimens may provide important
information for regimen selection during platinum-based adjuvant
chemotherapy for patients who undergo complete resection for
locally advanced stage IIIA NSCLC. The CD-DST-guided selection of
platinum-based regimens could have a favorable impact on the DFS of
such patients. In order to estimate the clinical usefulness of
in vitro chemosensitivity tests, such as the CD-DST, HDRA,
and ATP-TCA, further comparisons of the effects of in vitro
chemosensitivity test-guided regimens with those of conventional
regimens in patients with NSCLC should be performed in a randomized
control study.
Acknowledgments
We would like to thank Mrs. E. Yoneima for her
technical assistance and advice.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
CD-DST
|
collagen gel droplet embedded culture
drug sensitivity test
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
EGFR
|
epidermal growth factor receptor
|
|
ERCC1
|
excision repair cross-complementation
group 1
|
|
RRM1
|
ribonucleotide reductase regulatory
subunit M1
|
|
CDDP
|
cisplatin
|
|
CBDCA
|
carboplatin
|
|
DOC
|
docetaxel
|
|
PTX
|
paclitaxel
|
|
VNR
|
vinorelbine
|
|
GEM
|
gemcitabine
|
|
PEM
|
pemetrexed disodium
|
|
HDRA
|
histoculture drug response assay
|
|
ATP-TCA
|
adenosine triphosphate-based tumor
chemosensitivity assay
|
|
CT
|
computed tomography
|
References
|
1
|
Le Chevalier T, Arriagada R, Pignon JP and
Scagliotti GV: Should adjuvant chemotherapy become standard
treatment in all patients with resected non-small-cell lung cancer?
Lancet Oncol. 6:182–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugimura H, Nichols FC, Yang P, Allen MS,
Cassivi SD, Deschamps C, Williams BA and Pairolero PC: Survival
after recurrent nonsmall-cell lung cancer after complete pulmonary
resection. Ann Thorac Surg. 83:409–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie Y and Minna JD: Non-small-cell lung
cancer mRNA expression signature predicting response to adjuvant
chemotherapy. J Clin Oncol. 28:4404–4407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leong D, Rai R, Nguyen B, Lee A and Yip D:
Advances in adjuvant systemic therapy for non-small-cell lung
cancer. World J Clin Oncol. 5:633–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J: International Adjuvant
Lung Cancer Trial Collaborative Group: Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winton T, Livingston R, Johnson D, Rigas
J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E,
et al: Vinorelbine plus cisplatin vs. observation in resected
non-small-cell lung cancer. N Engl J Med. 352:2589–2597. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douillard JY, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine
International Trialist Association [ANITA)]: A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE Collaborative Group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cameron L and Solomon B: New treatment
options for ALK-rearranged non-small cell lung cancer. Curr Treat
Options Oncol. 16:492015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
12
|
Gong W, Zhang X, Wu J, Chen L, Li L, Sun
J, Lv Y, Wei X, Du Y, Jin H and Dong J: RRM1 expression and
clinical outcome of gemcitabine-containing chemotherapy for
advanced non-small-cell lung cancer: A meta-analysis. Lung Cancer.
75:374–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sève P and Dumontet C: Is class III
beta-tubulin a predictive factor in patients receiving
tubulin-binding agents? Lancet Oncol. 9:168–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirai Y, Yoshimasu T, Oura S, Ota F, Naito
K, Nishiguchi H, Hashimoto S and Okamura Y: Is class III
beta-tubulin a true predictive marker of sensitivity to vinorelbine
in non-small cell lung cancer? Chemosensitivity data evidence.
Anticancer Res. 31:999–1005. 2011.PubMed/NCBI
|
|
15
|
Wallerek S and Sørensen JB: Biomarkers for
efficacy of adjuvant chemotherapy following complete resection in
NSCLC stages I–IIIA. Eur Respir Rev. 24:340–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higashiyama M, Oda K, Okami J, Maeda J,
Kodama K, Imamura F, Minamikawa K, Takano T and Kobayashi H:
Prediction of chemotherapeutic effect on postoperative recurrence
by in vitro anticancer drug sensitivity testing in non-small cell
lung cancer patients. Lung Cancer. 68:472–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takebayashi K, Mekata E, Sonoda H, Shimizu
T, Endo Y and Tani T: Clinical potential of the anticancer drug
sensitivity test for patients with synchronous stage IV colorectal
cancer. Cancer Chemother Pharmacol. 72:217–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naitoh H, Yamamoto H, Murata S, Kobayashi
H, Inoue K and Tani T: Stratified phase II trial to establish the
usefulness of the collagen gel droplet embedded culture-drug
sensitivity test (CD-DST) for advanced gastric cancer. Gastric
Cancer. 17:630–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Nakatani A, Inou T, Washio M, Sakuyama N, Sato T,
Kishine K, et al: Individualized chemotherapy for colorectal cancer
based on the collagen gel droplet-embedded drug sensitivity test.
Oncol Lett. 4:621–624. 2012.PubMed/NCBI
|
|
21
|
Higashiyama M, Kodama K, Yokouchi H,
Takami K, Nakagawa H, Imamura F, Minamigawa K and Kobayashi H:
Cisplatin-based chemotherapy for postoperative recurrence in
non-small cell lung cancer patients: Relation of the in vitro
chemosensitive test to clinical response. Oncol Rep. 8:279–283.
2001.PubMed/NCBI
|
|
22
|
Kawamura M, Gika M, Abiko T, Inoue Y,
Oyama T, Izumi Y, Kobayashi H and Kobayashi K: Clinical evaluation
of chemosensitivity testing for patients with unresectable
non-small cell lung cancer (NSCLC) using collagen gel droplet
embedded culture drug sensitivity test (CD-DST). Cancer Chemother
Pharmacol. 59:507–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maejima K, Tokunaga A, Kiyama T, Kanno H,
Bou H, Watanabe M, Suzuki H and Uchida E: Chemosensitivity test for
5-fluorouracil and 5-chloro-2, 4-dihydroxypyridine predicts outcome
of gastric cancer patients receiving S-1 postoperatively. Gastric
Cancer. 13:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanahashi M, Niwa H, Yukiue H, Suzuki E,
Haneda H and Yoshii N: Adjuvant chemotherapy based on the in vitro
histoculture drug response assay for non-small cell lung cancer
improves survival. J Thorac Oncol. 5:1376–1381. 2010. View Article : Google Scholar
|
|
25
|
Chang WJ, Sun JM, Lee JY, Ahn JS, Ahn MJ
and Park K: A retrospective comparison of adjuvant chemotherapeutic
regimens for non-small cell lung cancer (NSCLC): Paclitaxel plus
carboplatin versus vinorelbine plus cisplatin. Lung Cancer.
84:51–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubota K, Watanabe K, Kunitoh H, Noda K,
Ichinose Y, Katakami N, Sugiura T, Kawahara M, Yokoyama A, Yokota
S, et al: Phase III randomized trial of docetaxel plus cisplatin
versus vindesine plus cisplatin in patients with stage IV
non-small-cell lung cancer: The Japanese Taxotere lung cancer study
group. J Clin Oncol. 22:254–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uramoto H, Nakanishi R, Nagashima A,
Uchiyama A, Inoue M, Osaki T, Yoshimatsu T, Sakata H, Nakanishi K
and Yasumoto K: A randomized phase II trial of adjuvant
chemotherapy with bi-weekly carboplatin plus paclitaxel versus
carboplatin plus gemcitabine in patients with completely resected
non-small cell lung cancer. Anticancer Res. 30:4695–4699.
2010.PubMed/NCBI
|
|
28
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aoki T, Ebihara A, Yogo Y, Suemasu K and
Sakamaki F: Analysis of continuous first-line treatment with
docetaxel and carboplatin for advanced non-small cell lung cancer.
Oncol Lett. 7:1771–1777. 2014.PubMed/NCBI
|
|
30
|
Janjigian YY, Park BJ, Zakowski MF,
Ladanyi M, Pao W, D'Angelo SP, Kris MG, Shen R, Zheng J and Azzoli
CG: Impact on disease-free survival of adjuvant erlotinib or
gefitinib in patients with resected lung adenocarcinomas that
harbor EGFR mutations. J Thorac Oncol. 6:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D'Angelo SP, Janjigian YY, Ahye N, Riely
GJ, Chaft JE, Sima CS, Shen R, Zheng J, Dycoco J, Kris MG, et al:
Distinct clinical course of EGFR-mutant resected lung cancers:
Results of testing of 1118 surgical specimens and effects of
adjuvant gefitinib and erlotinib. J Thorac Oncol. 7:1815–1822.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goss GD, O'Callaghan C, Lorimer I, Tsao
MS, Masters GA, Jett J, Edelman MJ, Lilenbaum R, Choy H, Khuri F,
et al: Gefitinib versus placebo in completely resected
non-small-cell lung cancer: Results of the NCIC CTG BR19 study. J
Clin Oncol. 31:3320–3326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv C, An C, Feng Q, Ma Y, Li S, Wang J,
Zhang J, Wang X, Yan S, Fang J, et al: A retrospective study of
stage I to IIIa lung adenocarcinoma after resection: What is the
optimal adjuvant modality for patients with an EGFR mutation? Clin
Lung Cancer. 16:e173–e181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Friboulet L, Olaussen KA, Pignon JP,
Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour
L, Pirker R, et al: ERCC1 isoform expression and DNA repair in
non-small-cell lung cancer. N Engl J Med. 368:1101–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurbacher CM and Cree IA: Chemosensitivity
testing using microplate adenosine triphosphate-based luminescence
measurements. Methods Mol Med. 110:101–120. 2005.PubMed/NCBI
|
|
37
|
Moon YW, Choi SH, Kim YT, Sohn JH, Chang
J, Kim SK, Park MS, Chung KY, Lee HJ and Kim JH: Adenosine
triphosphate-based chemotherapy response assay (ATP-CRA)-guided
platinum-based 2-drug chemotherapy for unresectable nonsmall-cell
lung cancer. Cancer. 109:1829–1835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshimasu T, Oura S, Hirai I, Tamaki T,
Kokawa Y, Hata K, Ohta F, Nakamura R, Kawago M, Tanino H, et al:
Data acquisition for the histoculture drug response assay in lung
cancer. J Thorac Cardiovasc Surg. 133:303–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanigawa N, Yamaue H, Ohyama S, Sakuramoto
S, Inada T, Kodera Y, Kitagawa Y, Omura K, Terashima M, Sakata Y,
et al: Exploratory phase II trial in a multicenter setting to
evaluate the clinical value of a chemosensitivity test in patients
with gastric cancer (JACCRO-GC 04, Kubota memorial trial). Gastric
Cancer. 19:350–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakamura R, Saikawa Y, Kubota T, Kumagai
A, Kiyota T, Ohashi M, Yoshida M, Otani Y, Kumai K and Kitajima M:
Role of the MTT chemosensitivity test in the prognosis of gastric
cancer patients after postoperative adjuvant chemotherapy.
Anticancer Res. 26:1433–1437. 2006.PubMed/NCBI
|
|
41
|
Fujita Y, Hiramatsu M, Kawai M, Nishimura
H, Miyamoto A and Tanigawa N: Histoculture drug response assay
predicts the postoperative prognosis of patients with esophageal
cancer. Oncol Rep. 21:499–505. 2009.PubMed/NCBI
|
|
42
|
Higashiyama M, Okami J, Maeda J, Tokunaga
T, Fujiwara A, Kodama K, Imamura F and Kobayashi H: Differences in
chemosensitivity between primary and paired metastatic lung cancer
tissues: In vitro analysis based on the collagen gel droplet
embedded culture drug test (CD-DST). J Thorac Dis. 4:40–47.
2012.PubMed/NCBI
|
|
43
|
Maniwa Y, Yoshimura M, Hashimoto S, Takata
M and Nishio W: Chemosensitivity of lung cancer: Differences
between the primary lesion and lymph node metastasis. Oncol Lett.
1:345–349. 2010. View Article : Google Scholar : PubMed/NCBI
|















