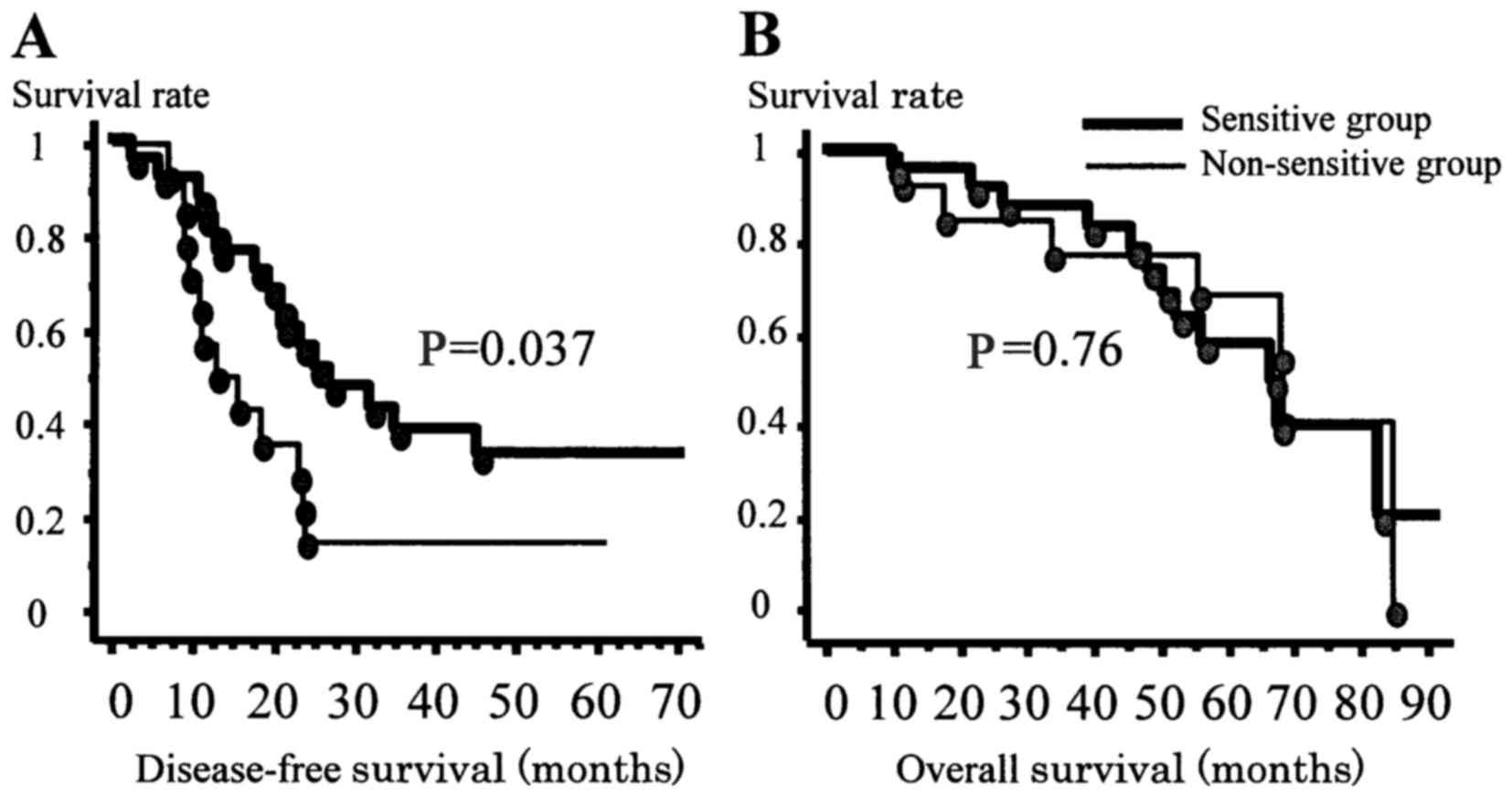
|
1
|
Le Chevalier T, Arriagada R, Pignon JP and
Scagliotti GV: Should adjuvant chemotherapy become standard
treatment in all patients with resected non-small-cell lung cancer?
Lancet Oncol. 6:182–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugimura H, Nichols FC, Yang P, Allen MS,
Cassivi SD, Deschamps C, Williams BA and Pairolero PC: Survival
after recurrent nonsmall-cell lung cancer after complete pulmonary
resection. Ann Thorac Surg. 83:409–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie Y and Minna JD: Non-small-cell lung
cancer mRNA expression signature predicting response to adjuvant
chemotherapy. J Clin Oncol. 28:4404–4407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leong D, Rai R, Nguyen B, Lee A and Yip D:
Advances in adjuvant systemic therapy for non-small-cell lung
cancer. World J Clin Oncol. 5:633–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J: International Adjuvant
Lung Cancer Trial Collaborative Group: Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winton T, Livingston R, Johnson D, Rigas
J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E,
et al: Vinorelbine plus cisplatin vs. observation in resected
non-small-cell lung cancer. N Engl J Med. 352:2589–2597. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douillard JY, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine
International Trialist Association [ANITA)]: A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE Collaborative Group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cameron L and Solomon B: New treatment
options for ALK-rearranged non-small cell lung cancer. Curr Treat
Options Oncol. 16:492015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
12
|
Gong W, Zhang X, Wu J, Chen L, Li L, Sun
J, Lv Y, Wei X, Du Y, Jin H and Dong J: RRM1 expression and
clinical outcome of gemcitabine-containing chemotherapy for
advanced non-small-cell lung cancer: A meta-analysis. Lung Cancer.
75:374–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sève P and Dumontet C: Is class III
beta-tubulin a predictive factor in patients receiving
tubulin-binding agents? Lancet Oncol. 9:168–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirai Y, Yoshimasu T, Oura S, Ota F, Naito
K, Nishiguchi H, Hashimoto S and Okamura Y: Is class III
beta-tubulin a true predictive marker of sensitivity to vinorelbine
in non-small cell lung cancer? Chemosensitivity data evidence.
Anticancer Res. 31:999–1005. 2011.PubMed/NCBI
|
|
15
|
Wallerek S and Sørensen JB: Biomarkers for
efficacy of adjuvant chemotherapy following complete resection in
NSCLC stages I–IIIA. Eur Respir Rev. 24:340–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higashiyama M, Oda K, Okami J, Maeda J,
Kodama K, Imamura F, Minamikawa K, Takano T and Kobayashi H:
Prediction of chemotherapeutic effect on postoperative recurrence
by in vitro anticancer drug sensitivity testing in non-small cell
lung cancer patients. Lung Cancer. 68:472–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takebayashi K, Mekata E, Sonoda H, Shimizu
T, Endo Y and Tani T: Clinical potential of the anticancer drug
sensitivity test for patients with synchronous stage IV colorectal
cancer. Cancer Chemother Pharmacol. 72:217–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naitoh H, Yamamoto H, Murata S, Kobayashi
H, Inoue K and Tani T: Stratified phase II trial to establish the
usefulness of the collagen gel droplet embedded culture-drug
sensitivity test (CD-DST) for advanced gastric cancer. Gastric
Cancer. 17:630–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Nakatani A, Inou T, Washio M, Sakuyama N, Sato T,
Kishine K, et al: Individualized chemotherapy for colorectal cancer
based on the collagen gel droplet-embedded drug sensitivity test.
Oncol Lett. 4:621–624. 2012.PubMed/NCBI
|
|
21
|
Higashiyama M, Kodama K, Yokouchi H,
Takami K, Nakagawa H, Imamura F, Minamigawa K and Kobayashi H:
Cisplatin-based chemotherapy for postoperative recurrence in
non-small cell lung cancer patients: Relation of the in vitro
chemosensitive test to clinical response. Oncol Rep. 8:279–283.
2001.PubMed/NCBI
|
|
22
|
Kawamura M, Gika M, Abiko T, Inoue Y,
Oyama T, Izumi Y, Kobayashi H and Kobayashi K: Clinical evaluation
of chemosensitivity testing for patients with unresectable
non-small cell lung cancer (NSCLC) using collagen gel droplet
embedded culture drug sensitivity test (CD-DST). Cancer Chemother
Pharmacol. 59:507–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maejima K, Tokunaga A, Kiyama T, Kanno H,
Bou H, Watanabe M, Suzuki H and Uchida E: Chemosensitivity test for
5-fluorouracil and 5-chloro-2, 4-dihydroxypyridine predicts outcome
of gastric cancer patients receiving S-1 postoperatively. Gastric
Cancer. 13:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanahashi M, Niwa H, Yukiue H, Suzuki E,
Haneda H and Yoshii N: Adjuvant chemotherapy based on the in vitro
histoculture drug response assay for non-small cell lung cancer
improves survival. J Thorac Oncol. 5:1376–1381. 2010. View Article : Google Scholar
|
|
25
|
Chang WJ, Sun JM, Lee JY, Ahn JS, Ahn MJ
and Park K: A retrospective comparison of adjuvant chemotherapeutic
regimens for non-small cell lung cancer (NSCLC): Paclitaxel plus
carboplatin versus vinorelbine plus cisplatin. Lung Cancer.
84:51–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubota K, Watanabe K, Kunitoh H, Noda K,
Ichinose Y, Katakami N, Sugiura T, Kawahara M, Yokoyama A, Yokota
S, et al: Phase III randomized trial of docetaxel plus cisplatin
versus vindesine plus cisplatin in patients with stage IV
non-small-cell lung cancer: The Japanese Taxotere lung cancer study
group. J Clin Oncol. 22:254–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uramoto H, Nakanishi R, Nagashima A,
Uchiyama A, Inoue M, Osaki T, Yoshimatsu T, Sakata H, Nakanishi K
and Yasumoto K: A randomized phase II trial of adjuvant
chemotherapy with bi-weekly carboplatin plus paclitaxel versus
carboplatin plus gemcitabine in patients with completely resected
non-small cell lung cancer. Anticancer Res. 30:4695–4699.
2010.PubMed/NCBI
|
|
28
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aoki T, Ebihara A, Yogo Y, Suemasu K and
Sakamaki F: Analysis of continuous first-line treatment with
docetaxel and carboplatin for advanced non-small cell lung cancer.
Oncol Lett. 7:1771–1777. 2014.PubMed/NCBI
|
|
30
|
Janjigian YY, Park BJ, Zakowski MF,
Ladanyi M, Pao W, D'Angelo SP, Kris MG, Shen R, Zheng J and Azzoli
CG: Impact on disease-free survival of adjuvant erlotinib or
gefitinib in patients with resected lung adenocarcinomas that
harbor EGFR mutations. J Thorac Oncol. 6:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D'Angelo SP, Janjigian YY, Ahye N, Riely
GJ, Chaft JE, Sima CS, Shen R, Zheng J, Dycoco J, Kris MG, et al:
Distinct clinical course of EGFR-mutant resected lung cancers:
Results of testing of 1118 surgical specimens and effects of
adjuvant gefitinib and erlotinib. J Thorac Oncol. 7:1815–1822.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goss GD, O'Callaghan C, Lorimer I, Tsao
MS, Masters GA, Jett J, Edelman MJ, Lilenbaum R, Choy H, Khuri F,
et al: Gefitinib versus placebo in completely resected
non-small-cell lung cancer: Results of the NCIC CTG BR19 study. J
Clin Oncol. 31:3320–3326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv C, An C, Feng Q, Ma Y, Li S, Wang J,
Zhang J, Wang X, Yan S, Fang J, et al: A retrospective study of
stage I to IIIa lung adenocarcinoma after resection: What is the
optimal adjuvant modality for patients with an EGFR mutation? Clin
Lung Cancer. 16:e173–e181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Friboulet L, Olaussen KA, Pignon JP,
Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour
L, Pirker R, et al: ERCC1 isoform expression and DNA repair in
non-small-cell lung cancer. N Engl J Med. 368:1101–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurbacher CM and Cree IA: Chemosensitivity
testing using microplate adenosine triphosphate-based luminescence
measurements. Methods Mol Med. 110:101–120. 2005.PubMed/NCBI
|
|
37
|
Moon YW, Choi SH, Kim YT, Sohn JH, Chang
J, Kim SK, Park MS, Chung KY, Lee HJ and Kim JH: Adenosine
triphosphate-based chemotherapy response assay (ATP-CRA)-guided
platinum-based 2-drug chemotherapy for unresectable nonsmall-cell
lung cancer. Cancer. 109:1829–1835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshimasu T, Oura S, Hirai I, Tamaki T,
Kokawa Y, Hata K, Ohta F, Nakamura R, Kawago M, Tanino H, et al:
Data acquisition for the histoculture drug response assay in lung
cancer. J Thorac Cardiovasc Surg. 133:303–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanigawa N, Yamaue H, Ohyama S, Sakuramoto
S, Inada T, Kodera Y, Kitagawa Y, Omura K, Terashima M, Sakata Y,
et al: Exploratory phase II trial in a multicenter setting to
evaluate the clinical value of a chemosensitivity test in patients
with gastric cancer (JACCRO-GC 04, Kubota memorial trial). Gastric
Cancer. 19:350–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakamura R, Saikawa Y, Kubota T, Kumagai
A, Kiyota T, Ohashi M, Yoshida M, Otani Y, Kumai K and Kitajima M:
Role of the MTT chemosensitivity test in the prognosis of gastric
cancer patients after postoperative adjuvant chemotherapy.
Anticancer Res. 26:1433–1437. 2006.PubMed/NCBI
|
|
41
|
Fujita Y, Hiramatsu M, Kawai M, Nishimura
H, Miyamoto A and Tanigawa N: Histoculture drug response assay
predicts the postoperative prognosis of patients with esophageal
cancer. Oncol Rep. 21:499–505. 2009.PubMed/NCBI
|
|
42
|
Higashiyama M, Okami J, Maeda J, Tokunaga
T, Fujiwara A, Kodama K, Imamura F and Kobayashi H: Differences in
chemosensitivity between primary and paired metastatic lung cancer
tissues: In vitro analysis based on the collagen gel droplet
embedded culture drug test (CD-DST). J Thorac Dis. 4:40–47.
2012.PubMed/NCBI
|
|
43
|
Maniwa Y, Yoshimura M, Hashimoto S, Takata
M and Nishio W: Chemosensitivity of lung cancer: Differences
between the primary lesion and lymph node metastasis. Oncol Lett.
1:345–349. 2010. View Article : Google Scholar : PubMed/NCBI
|