Introduction
Therapy with first-generation of epidermal growth
factor receptor (EGFR) tyrosine kinase inhibitors (TKI) shows
significant clinical response and provides progression-free
survival benefits vs. chemotherapy in patients with EGFR
mutant-positive non-small cell lung cancer (NSCLC) (1). However, most patients have progressive
disease after 9 to 14 months of treatment because of acquired
resistance to TKI (2). About 60% of
acquired resistance is attributable to EGFR T790M mutation
(2). Ascending doses of osimertinib
(AZD9291) have shown significant efficacy in patients with
T790M-positive advanced NSCLC with progressive disease (3,4).
However, the therapeutic efficacy and safety of osimertinib in
T790M-positive NSCLC patients with central nervous system (CNS)
metastasis and poor performance status is not completely clear.
In the present report we presented a case of
EGFR-T790M mutant positive NSCLC patient with CNS lesion and poor
performance status based on the criteria of the Eastern Cooperative
Oncology Group (ECOG-PS4) that effectively responded to osimertinib
therapy.
Case report
Seven years before admission, a 72-year old
non-smoker female, with no relevant family history, received the
diagnosis of lung adenocarcinoma, stage pT2N2M0 and underwent
partial resection of the upper and middle lobes of the right lung.
The post-surgical clinical stage was IIIA and genetic testing
disclosed EGFR exon 19 deletion. Thereafter, the patient was
treated with standard chemotherapy including cisplatin/carboplatin
in combination with gemcitabine but, during the follow-up
evaluation 3 years before admission, a metastatic tumor in the
right lung was detected by positron emission tomography (PET)
study. The patient was then treated with a combination of erlotinib
and onartuzumab and then followed up on a maintenance therapy with
oral erlotinib (100 mg). Nine months before admission PET
examination disclosed the presence of bone metastasis and the
patient was then treated with four cycles of chemotherapy including
carboplatin and pemetrexed followed by a maintenance therapy with
pemetrexed. One month previous to admission, the patient complained
of dizziness and a serum test showed increased values of
carcinoembryonic antigen (CEA). A magnetic resonance image of the
brain revealed multiple brain metastasis with neurological
alterations. Combined therapy with docetaxel and ramucirumab was
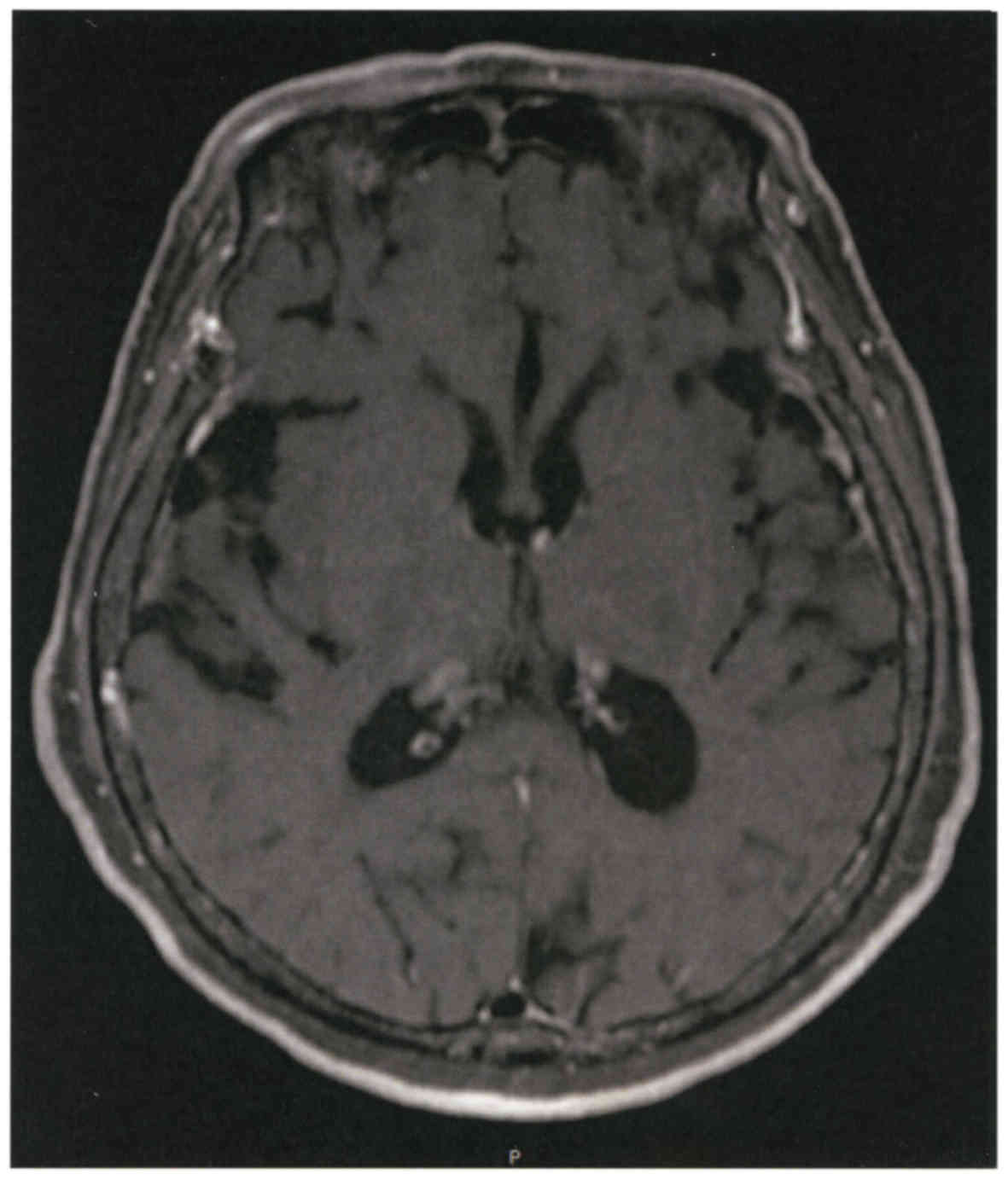
started but a subsequent magnetic resonance imaging showed
enlargement of ventricles (Fig. 1).
Based on these observations the malignant disease was judged as
being in progressive stage, and the performance status of the
patient was grade 4 based on the criteria of the Eastern
Cooperative Oncology Group (ECOG). At this clinical stage, the
patient was referred to our hospital.
On admission, the patient was lucid, heart rate
71/min, blood pressure 164/85 mmHg, oxygen saturation 97% at room
air, respiratory and heart sounds were normal, neck stiffness or
lymph node enlargement was absent. The chest X-ray and whole body
computed tomography (CT) revealed multiple bone metastasis in
vertebra and right iliac but no metastasis in the lungs, lymph
nodes or abdominal cavity. The blood values of serum albumin were
3.2 mg/dl, lactate dehydrogenase 430 IU/l, sodium 130 mEq/l and CEA
20.0 ng/ml.
Intravenous hyperalimentation was initiated because
the patient was unable to take meals due to poor performance status
and nausea. The diagnosis of NSCLC with EGFR exon 19 deletion,
resistance to erlotinib, the presence of CNS lesions and the poor
performance status of the patient predicted a poor response to
therapy with first-generation EGFR-TKIs. The patient was then
treated only with best supportive care. In addition, positron
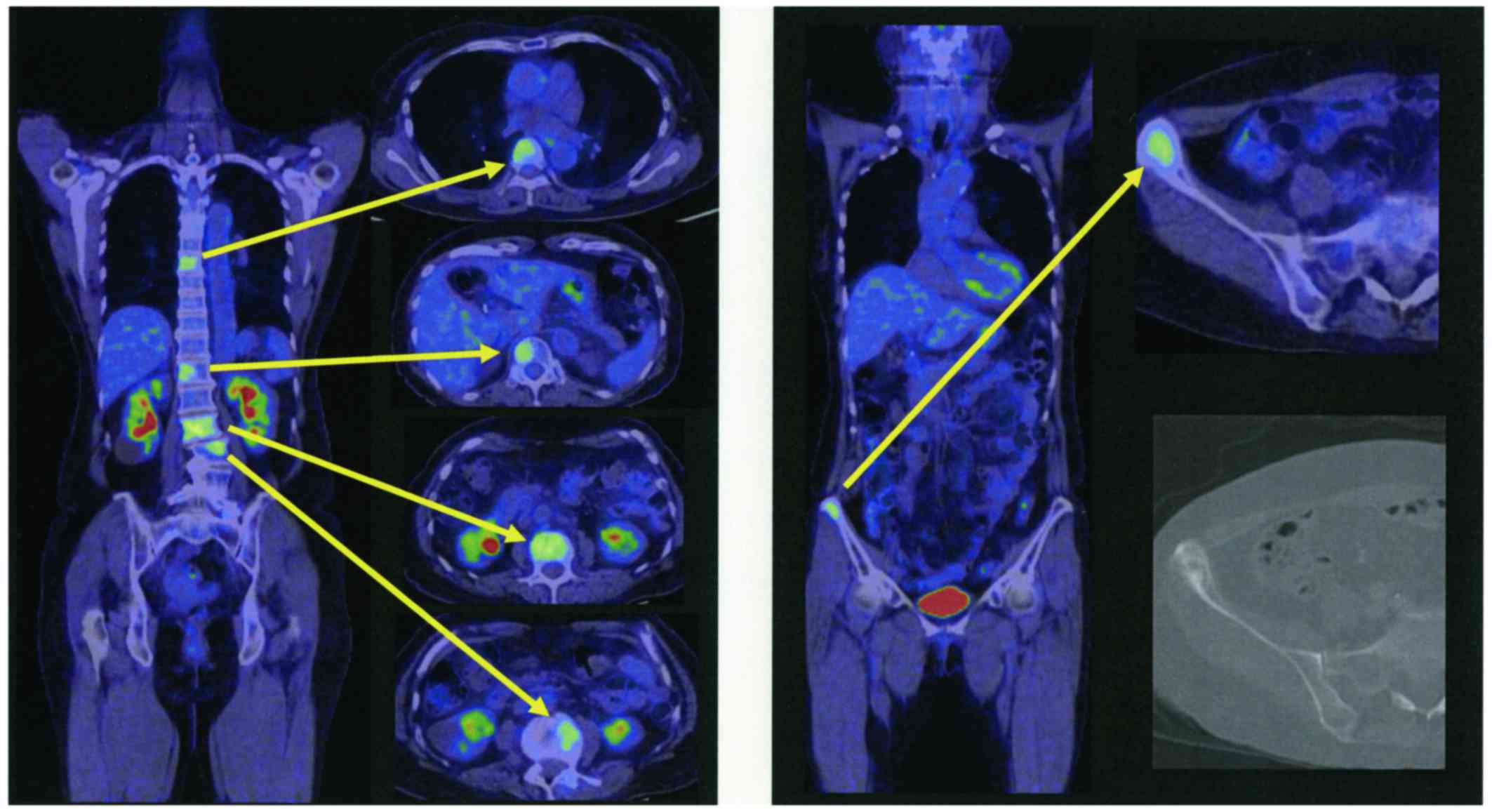
emission tomography CT (PET/CT) images from a previous hospital
(Fig. 2) revealed several metastatic
hot spots in vertebra (Th7, 12, L2, 3, 5) and right ilium. Because
the patient and her relatives preferred tumor-reductive therapy
rather than palliative treatment, we performed liquid biopsy using
plasma and cerebrospinal fluid (CSF) and tissue biopsy from the
right ilium on day 6 after admission to rule out EGFR-T790M-related
TKI resistance. The biopsy specimen analysis for EGFR mutation
performed by peptide nucleic acid-locked nucleic acid polymerase
chain reaction clamp was negative for all mutations in plasma
samples, positive for exon 19 deletion and for exon 21 mutation but
negative for T790M mutation in CSF samples. Cobas test (Cobas EGFR
Mutation Test, version 2) performed using CSF samples was positive
for exon 19 deletion, but negative for exon 21 and T790M mutations.
However, Cobas test performed using tissue from the right ilium was
positive for exon 19 deletion, negative for exon 21 mutation but
positive for T790M mutation.
Based on these findings, therapy with osimertinib
was initiated on day 9 after admission. Adverse effects including
diarrhea, rash, dry skin or tiredness were absent and appetite was
significantly increased. There was no hematological toxicity and a
magnetic resonance image examination revealed no progression of
central nervous system lesions, chest CT showed no drug-induced
pneumonia and her performance status gradually improved to grade 2.
She was discharged from the hospital. On day 20 of osimertinib
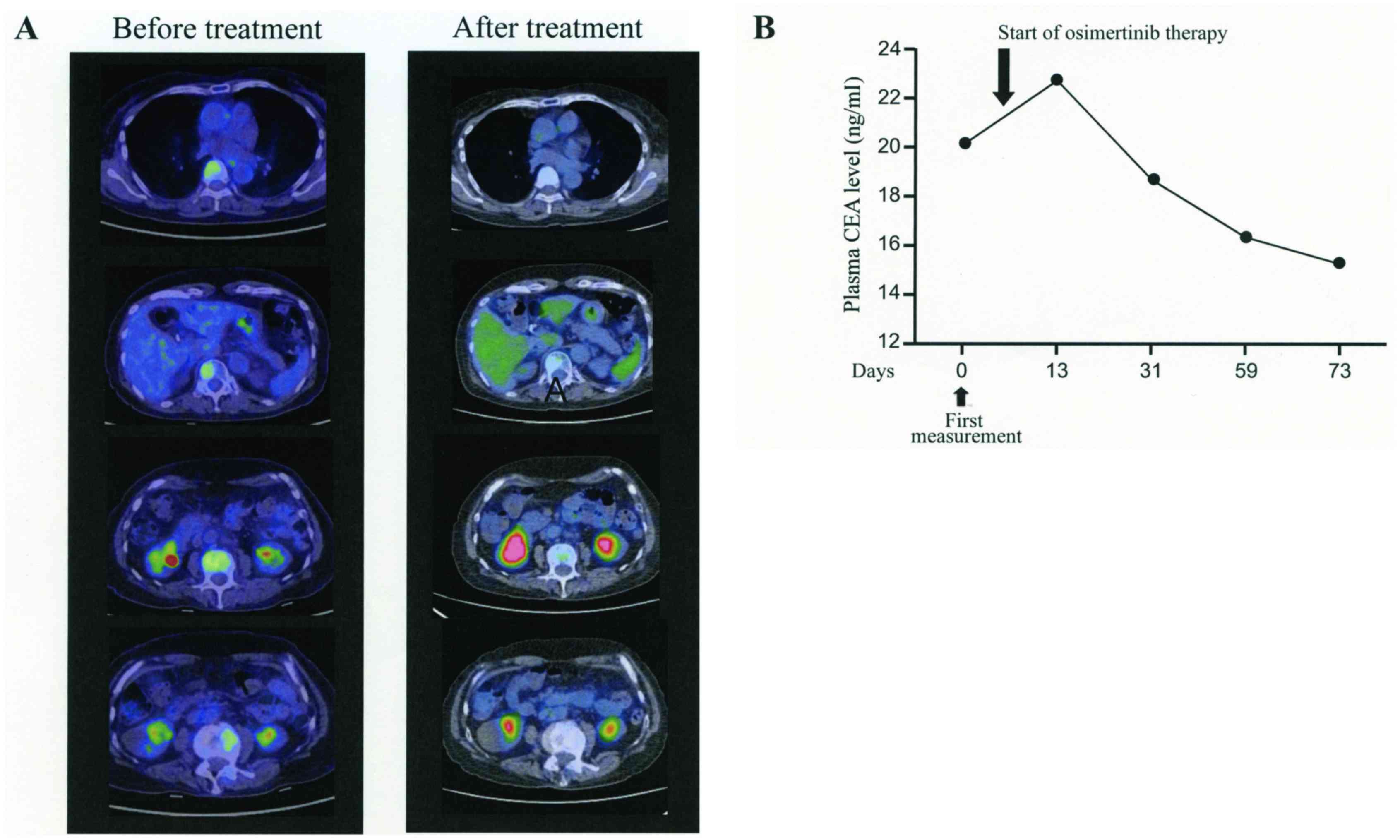
therapy, the PET/CT examination revealed remission of bone
metastasis with undetectable lesions (Fig. 3). Oral administration of osimertinib
was continued and the patient clinical course was followed up in
the outpatient department. After 2 months of therapy, MRI and CT
images showed no disease lesions and the plasma CEA was gradually
decreasing.
Discussion
EGFR-TKIs are currently the standard first-line
therapy for NSCLC patients with EGFR mutations, but most patients
eventually acquire resistance within one year of starting treatment
(2). Recurrence of NSCLC tumors
after EGFR-TKI therapy has been previously treated with cytotoxic
drugs or with different EGFR-TKIs (5). However, cytotoxic drugs are generally
ineffective and the clinical efficacy of EGFR-TKIs in patients with
poor performance status is very limited. Gefitinib, a first
generation EGFR TKI, has shown clinical benefits in terms of
response rate and progression-free survival in NSCLC patients with
poor performance status harboring EGFR mutations (deletion, L858R,
L861Q) other than the T790M mutation (6).
Osimertinib is a third-generation EGFR-TKI that is
able to inactivate EGFR harboring T790M mutation. The AURA1/2
clinical study has demonstrated effectiveness of osimertinib in
EGFR-T790M mutation-positive NSCLC patients with 66% response rate
and a median PFS of 11 months (7).
In addition, osimertinib was significantly superior to
platinum-pemetrexed treatment in the AURA3 study (8). Osimertinib may also suppress brain
metastases from NSCLC tumors compared to gefitinib, afatinib and
rociletinib (9). It is worthy to
note that most patients that were included in previous clinical
trials had performance status of grade 0 to 1. The clinical
efficacy of osimertinib in patients with EGFR harboring T790M
mutation and with poor performance status has been previously
reported in only two cases (10,11).
Both previous cases had also advanced lung adenocarcinoma with
brain metastasis, received therapy with combined chemotherapy or
with first-line TKI (gefitinib, erlotinib or afatinib), were
diagnosed as having tumor positive for T790M mutation during
disease progression, and showed dramatic response to osimertinib
therapy despite poor performance status (10,11).
Osimertinib-related adverse effect (leukopenia) was reported only
in one of the two previously reported cases (11). Our current patient showed no adverse
effect during osimertinib administration.
T790M mutation was detected in samples taken from a
metastatic pleural tumor or primary lung tumor but results from
liquid biopsy was unavailable in the two previous case reports
(10,11). We detected T790M mutation in
metastatic bone tumor but the result of liquid biopsy was negative.
Although liquid biopsy of the spinal fluid was negative for T790M
mutation, the detection rate of T790M in patients with brain
metastases has been shown to be as low as 17%. In addition, the
AURA study has demonstrated that osimertinib is effective in cases
with CNS metastasis and in T790M-positive NSCL tumors with good
performance status (9,12). The present report further
demonstrates the clinical efficacy of osimertinib in NSCLC patients
with brain lesion harboring EGFR T790M mutation and with poor
performance status.
This report provides evidence on the safety and
efficacy of osimertinib for treating NSCLC patients with CNS lesion
and poor performance status.
Written informed consent was obtained from the
patient and the report was approved by the Ethical Committee of
Matsusaka Municipal Hospital.
References
|
1
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goss G, Tsai CM, Shepherd FA, Bazhenova L,
Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, et al:
Osimertinib for pretreated EGFR Thr790Met-positive advanced
non-small-cell lung cancer (AURA2): A multicentre, open-label,
single-arm, phase 2 study. Lancet Oncol. 17:1643–1652. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in Advanced non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oxnard GR, Arcila ME, Chmielecki J,
Ladanyi M, Miller VA and Pao W: New strategies in overcoming
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors in lung cancer. Clin Cancer Res. 17:5530–5537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue A, Kobayashi K, Usui K, Maemondo M,
Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A, et al:
First-line gefitinib for patients with advanced non-small-cell lung
cancer harboring epidermal growth factor receptor mutations without
indication for chemotherapy. J Clin Oncol. 27:1394–1400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Ramalingam SS, Jänne PA, Cantarini
M and Mitsudomi T: LBA2_PR: Osimertinib (AZD9291) in pre-treated
pts with T790M-positive advanced NSCLC: Updated Phase 1 (P1) and
pooled Phase 2 (P2) results. J Thorac Oncol. 11(4 Suppl):
S152–S153. 2016. View Article : Google Scholar
|
|
8
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ballard P, Yates JW, Yang Z, Kim DW, Yang
JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, et al:
Preclinical comparison of osimertinib with other EGFR-TKIs in
EGFR-Mutant NSCLC brain metastases models, and early evidence of
clinical brain metastases activity. Clin Cancer Res. 22:5130–5140.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai H, Hayashi H, Iwasa T, Hasegawa Y,
Takeda M and Nakagawa K: Successful osimertinib treatment for
leptomeningeal carcinomatosis from lung adenocarcinoma with the
T790M mutation of EGFR. ESMO Open. 2 Suppl 1:e0001042017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uemura T, Oguri T, Okayama M, Furuta H,
Kanemitsu Y, Takakuwa O, Ohkubo H, Takemura M, Maeno K, Ito Y and
Niimi A: Dramatic intracranial response to osimertinib in a poor
performance status patient with lung adenocarcinoma harboring the
epidermal growth factor receptor T790M mutation: A case report. Mol
Clin Oncol. 6:525–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hata A, Katakami N, Yoshioka H, Kaji R,
Masago K, Fujita S, Imai Y, Nishiyama A, Ishida T, Nishimura Y and
Yatabe Y: Spatiotemporal T790M heterogeneity in individual patients
with EGFR-mutant non-small-cell lung cancer after acquired
resistance to EGFR-TKI. J Thorac Oncol. 10:1553–1559. 2015.
View Article : Google Scholar : PubMed/NCBI
|