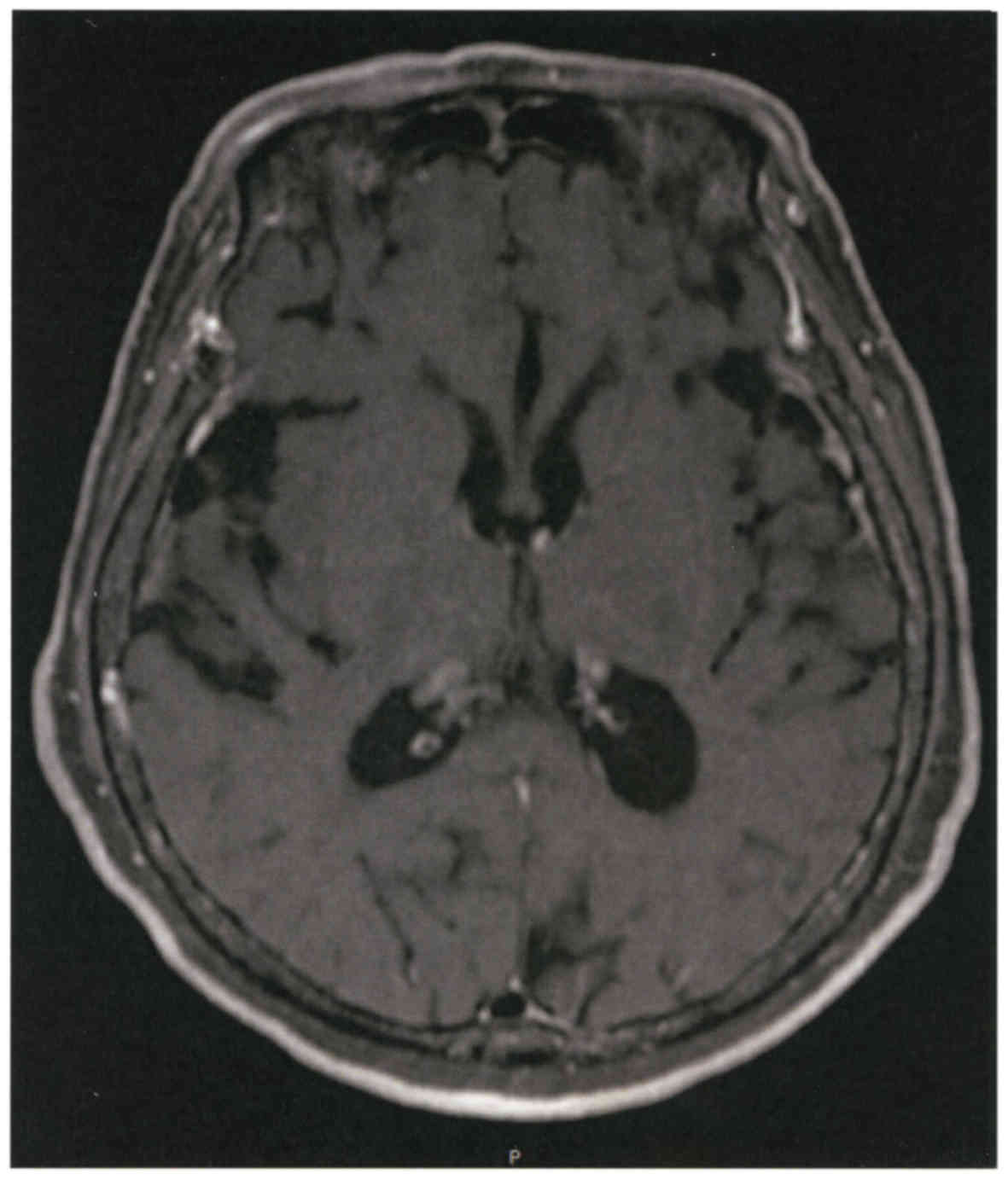
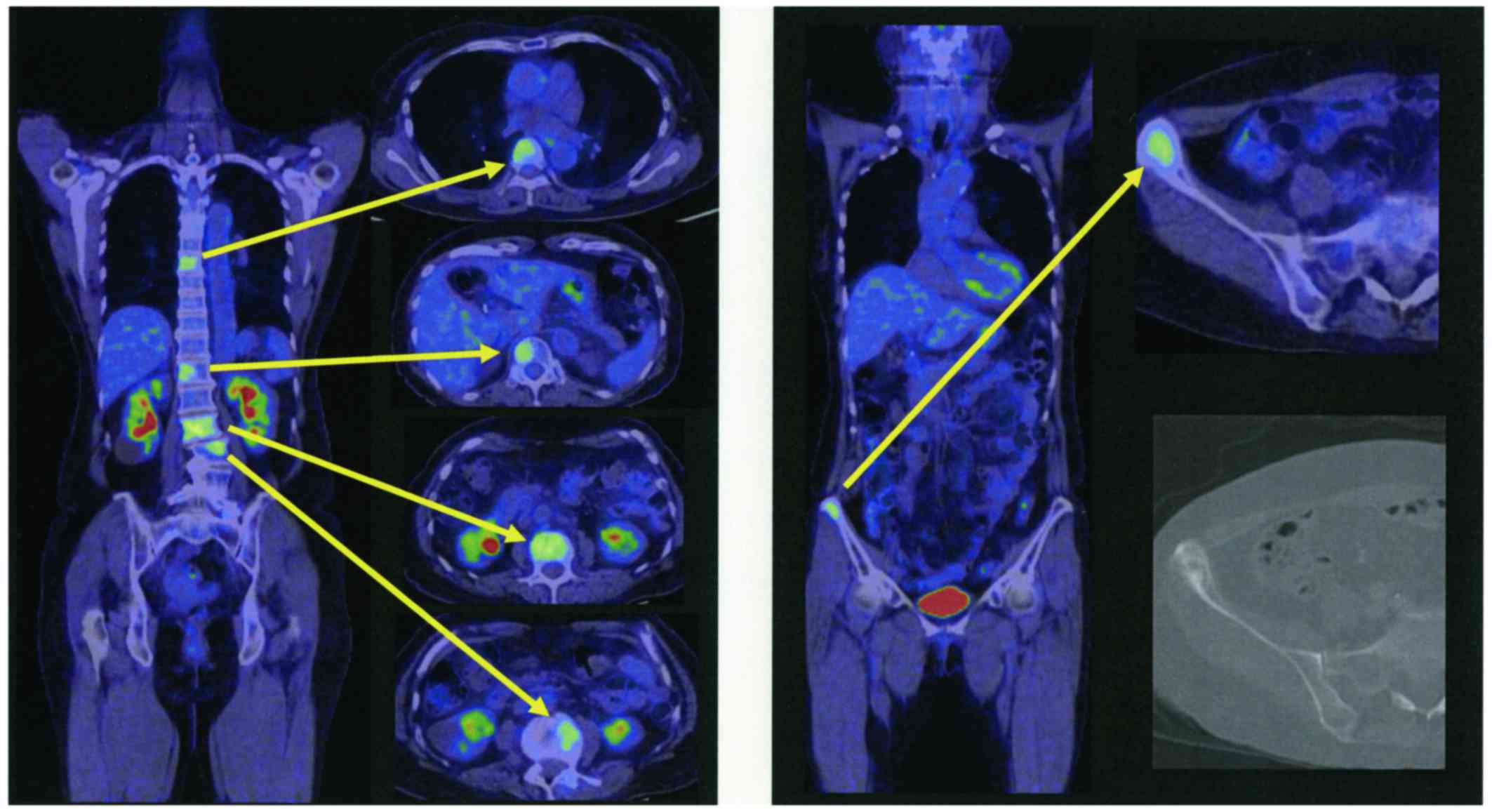
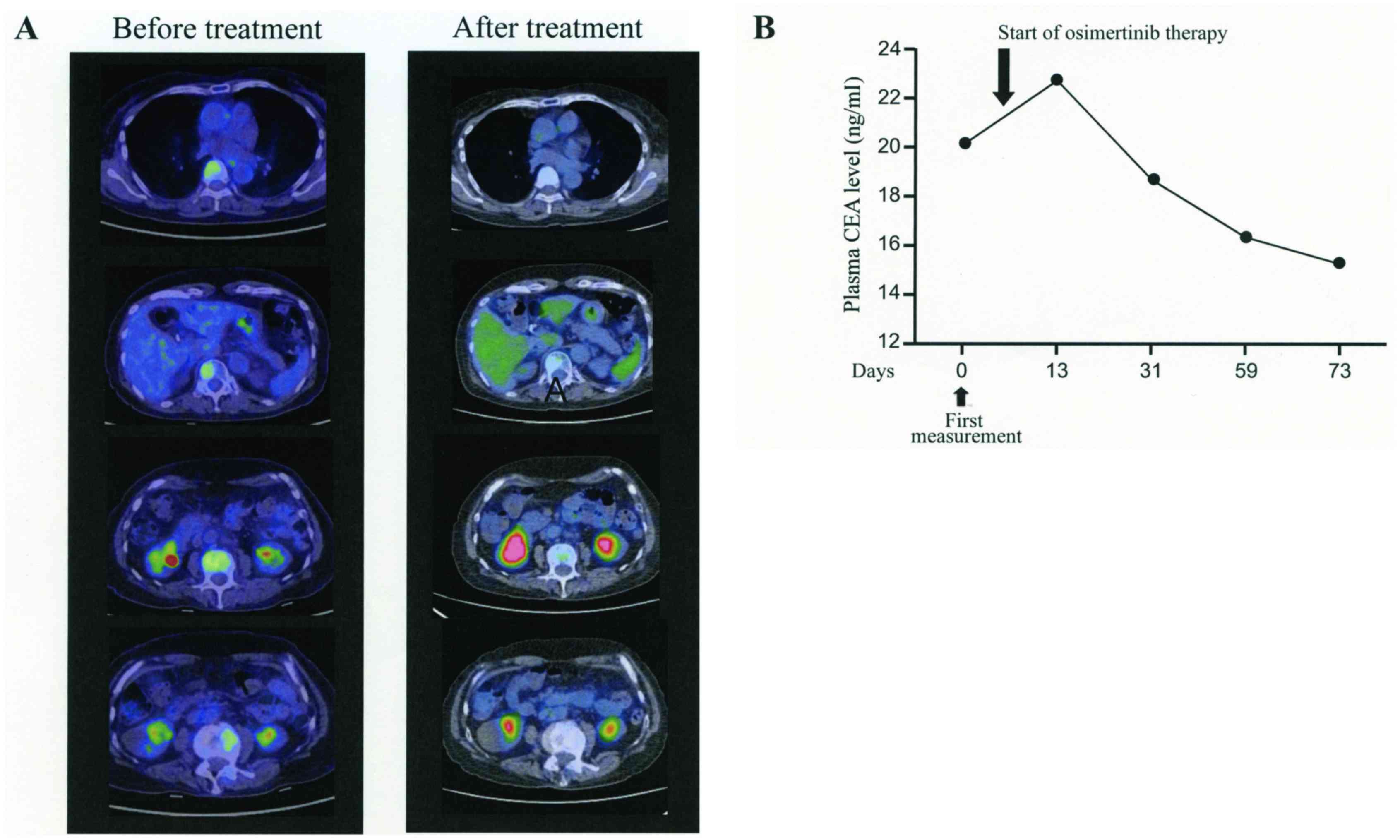
Spandidos Publications style
Nishii Y, Hataji O, Ito K, Watanabe F, Kobayashi T, D'Alessandro‑Gabazza C, Toda M, Taguchi O, Yamamoto N, Gabazza EC, Gabazza EC, et al: Efficacy of osimertinib in a patient with non‑small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status. Mol Clin Oncol 8: 246-249, 2018.
APA
Nishii, Y., Hataji, O., Ito, K., Watanabe, F., Kobayashi, T., D'Alessandro‑Gabazza, C. ... Gabazza, E.C. (2018). Efficacy of osimertinib in a patient with non‑small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status. Molecular and Clinical Oncology, 8, 246-249. https://doi.org/10.3892/mco.2017.1522
MLA
Nishii, Y., Hataji, O., Ito, K., Watanabe, F., Kobayashi, T., D'Alessandro‑Gabazza, C., Toda, M., Taguchi, O., Yamamoto, N., Gabazza, E. C."Efficacy of osimertinib in a patient with non‑small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status". Molecular and Clinical Oncology 8.2 (2018): 246-249.
Chicago
Nishii, Y., Hataji, O., Ito, K., Watanabe, F., Kobayashi, T., D'Alessandro‑Gabazza, C., Toda, M., Taguchi, O., Yamamoto, N., Gabazza, E. C."Efficacy of osimertinib in a patient with non‑small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status". Molecular and Clinical Oncology 8, no. 2 (2018): 246-249. https://doi.org/10.3892/mco.2017.1522