Introduction
Actinic cheilitis (AC), a potentially malignant
lesion (1), is the most frequently
occurring, and among the most important, of the pathological
processes that affect the lips. At the same time, oral and lip
malignancy is the eighth most prevalent neoplastic disorder
worldwide (2), with men being at far
greater risk compared with women (1.3% men vs. 0.3% women)
(3–6). Although risk factors for carcinoma
development are numerous [these include ultraviolet (UV) radiation
exposure, smoking, premalignant lesions, several viruses,
immunosuppression, and chronic trauma] (6,7), AC has
been directly associated with chronic exposure to UV radiation,
notably UVB radiation, due to its greater penetration potential
when compared with UVA (8,9). Albeit that occupational UV radiation
exposure is a major risk factor for AC in men, its association with
smoking and unhealthy eating habits may produce synergistic effects
(10). Clinically, AC encompasses a
broad spectrum of presentation, including pale, flaking or scaly
lips, chronic ulcerations and erosions, white plaques, blurring of
the demarcation between the lip vermillion border and the skin and
vermillion atrophy, areas of erythema, and potentially other
lesions (11–18). Palpation of the lesional surface
produces a fine, ‘sandpaper-like’ feeling (18). While AC is usually asymptomatic,
occasionally the affected person complains of lip dryness, a
stinging or burning sensation, persistent scaling and impaired lip
mobility (16), described as an
inelastic or tight sensation in the lip (18). AC differential diagnosis includes
several inflammatory lip disorders, such as lip eczema, cheilitis
granulomatosa, benign leukoplakia, lichen planus, or
straightforward dry skin and chronic irritation (19).
Squamous cell carcinoma (SCC) constitutes ~90% of
all cases of oral malignancy (20),
and it is estimated that 95% of SCCs of the lip originate from AC
(21,22). Although the reported risk of actinic
keratoses (AKs) progressing to SCC varies from <1 to 20%
(16), the malignant transformation
rate of AC into lip SCC is higher, and ranges from 10 to 30%
(23). Clinically, the keratotic
patches of AC may progress to thickening and induration,
nodularity, and rapid growth, and eventually one or more of them
ulcerate, causing bleeding and pain. Such changes are suggestive of
AC progression to SCC of the lip (9,10,18,24).
Although cutaneous SCC originating from AKs has a relatively low
rate of metastasis (~0.53%) (25),
SCC of the lip is much more prone to metastasis (3–20%) (26), and the risk of cervical lymph node
metastatic spread is higher in lip SCC compared with cutaneous SCC,
with lymph node disease detected 1–3 years after initial diagnosis
and treatment in the case of SCC of the lips (10).
The difficulty of discriminating between mere
dysplasias or invasive carcinomas is a constant therapeutic
struggle, additionally complicated by the tendency towards field
cancerization, inducing multicentric lesion sites (27). Several diagnostic techniques hold
future promise for early detection, risk assessment and monitoring
of skin cancer. Molecular analysis of the differences between the
normal, inflammatory and malignant keratinocyte proteomes are
likely to discover new biomarkers for SCC diagnosis, follow-up, and
development of individualized targeted therapies for SCC and other
cutaneous malignancies (28–30). Furthermore, more relevant for
everyday clinical practice, dermoscopy and in vivo
reflectance confocal microscopy (RCM) help further define the
diagnostic and prognostic criteria of AC and SCC. RCM, a
noninvasive imaging technology proving useful in the diagnosis of
several skin diseases, has helped to bridge the gap between
dermoscopy and histology (31–38). As
with dermoscopy, RCM allows for in vivo horizontal enface
examination of lesions, generating images of the epidermis and
superficial dermis at resolutions close to those of optical light
microscopy (32,33). In epithelium, a resolution of 1 μm
with a field of view of 200–400 µm and a penetration depth of ~500
µm have been achieved (31).
Early lesion detection and prompt, effective therapy
still remain the most important determining factors of long-term
patient survival and quality of life (39). Among the main reasons for using this
non-invasive imaging technique is its ability to detect
premalignant disorders such as AC, as well as skin and mucosal
cancers at their earliest stage.
Patients and methods
After having given written informed consent, the two
patients included in the present case report were subjected to the
evaluation and treatment protocol described below.
Clinical evaluation
Patient examination for the presence of AC and/or
SCC was based on clinical evaluations following guidelines for the
visual inspection and diagnosis of skin cancer. Clinical
photographs of skin and mucosal lesional sites were taken using a
digital camera (Nikon D3300; Nikon Corporation, Tokyo, Japan).
Dermoscopy
In each case, dermoscopic images were acquired using
a digital videodermoscopy system (FotoFinder, Bad Birnbach,
Germany) and a VivaScope® 1500 VivaCam macro-camera
[Caliber Imaging & Diagnostics, Inc. (formerly, Lucid Inc.),
Rochester, NY, USA].
In vivo RCM imaging
A commercially available reflectance confocal
microscope (VivaScope® 1500; Caliber Imaging &
Diagnostics, Inc.) was used for confocal imaging. A detailed
description of this technique and the device used has been
previously published (35,40).
The two patients discussed in the present case
report underwent classic vermilionectomy under local anesthesia in
the Department of Oral and Maxillofacial Surgery of ‘Dr Carol
Davila’ Central Military Hospital in Bucharest, with clear excision
margins on histopathology. There were no post-operative
complications, and sutures were removed in 10 days in both
cases.
Histology
For the two patients featured in this case report,
the excised tissues were subjected to histopathological examination
using standard hematoxylin-eosin staining.
Case reports
Case 1
A 71-year-old Caucasian male was referred in 2015
from the Oral and Maxillofacial Surgery Department of ‘Dr Carol
Davila’ Central Military Hospital in Bucharest to the Dermatology
Department of ‘Prof. N. C. Paulescu’ National Institute of
Diabetes, Nutrition and Metabolic Diseases, Bucharest, for the
evaluation of multiple, asymptomatic milky-white keratotic areas on
the surface of his lower lip. The patient reported significant
occupational sun exposure throughout his lifetime, consistent with
the presence of areas of mottled, telangiectatic, and lentiginous
skin changes. A clinical examination revealed multiple white
keratotic areas on an atrophic lower lip surface in conjunction
with a blurred vermillion-skin contour (Fig. 1A). Dermoscopy revealed a milky-white
plaque with well-defined borders, equivalent to hyperkeratosis,
surrounded by telangiectatic and tortuous vessels (Fig. 1B). An RCM examination revealed areas
of uneven tissular architecture with enlarged intercellular spaces
and loss of the normal ‘honeycomb’ appearance (Fig. 1C and D). Dilated and tortuous
vessels, and perivascular inflammatory cells with shiny appearance,
were also observed (Fig. 1C and E).
The papillary dermis exhibited large dark areas, representing blood
vessels containing white central elements corresponding to
erythrocytes, and bright perivascular elements of inflammatory
infiltrate (Fig. 1E). Staining with
hematoxylin-eosin disclosed features highly suggestive for the
diagnosis of AC (Fig. 1F). The
postoperative results were excellent, visible in the 7-month
follow-up clinical photograph (Fig.
1G), without any indication of local recurrence. The patient
was instructed to continue rigorous photoprotection, and further
follow-up visits were scheduled.
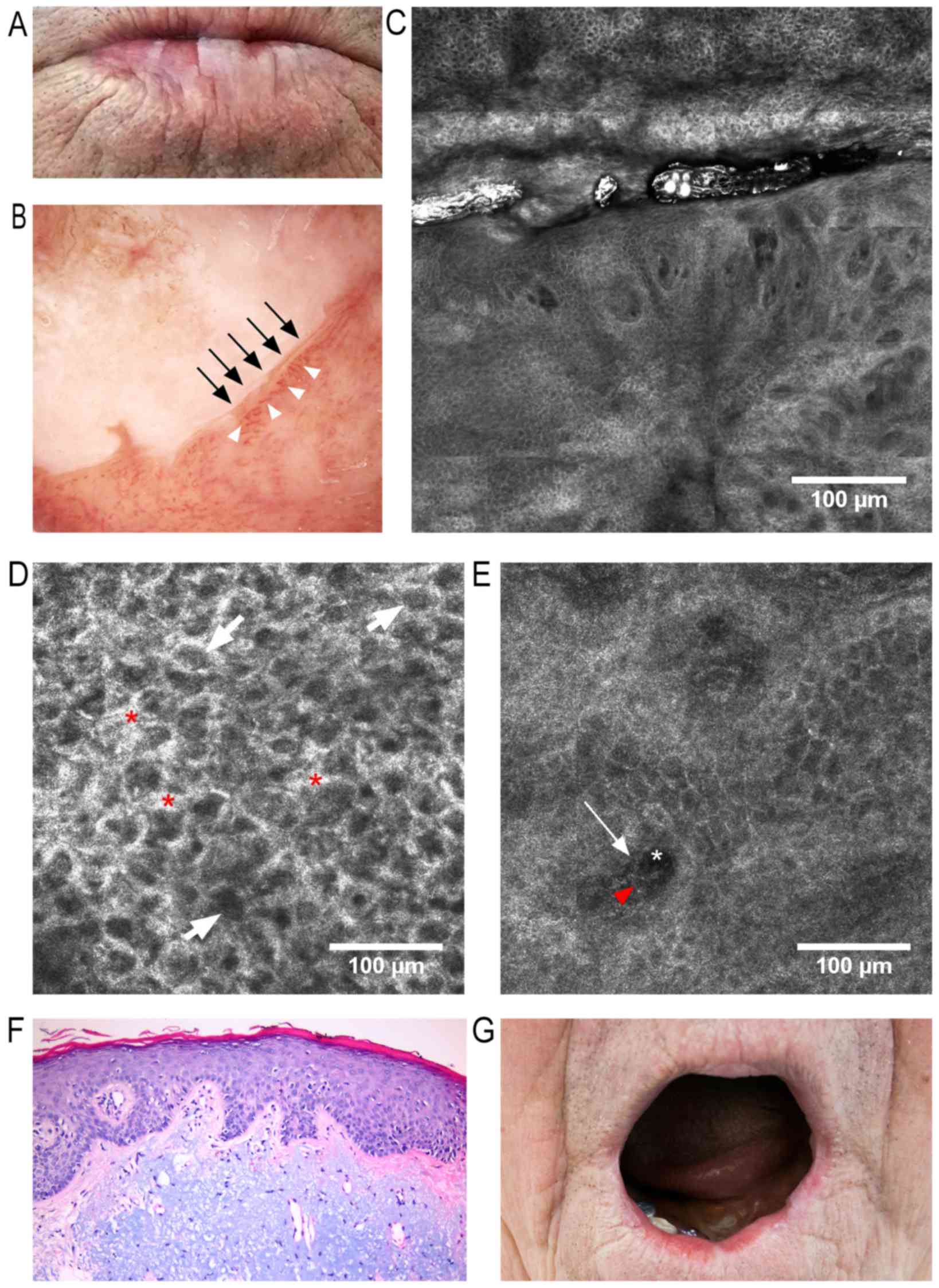 | Figure 1.f(A) Clinical image: White keratotic
areas on the lower lip surface with blurring of skin-vermillion
contour. (B) Dermoscopic image: Milky-white plaque with
well-defined borders (black arrows) surrounded by dilated and
tortuous vessels (white arrowheads). (C) RCM block (dimensions:
2.5×2.5 mm) showing, in the upper third, a keratotic area with
impaired keratinocyte architecture, enlarged intercellular spaces
and atypical honeycomb appearance, and in the lower two-thirds,
dilated blood vessels with tortuous aspect and bright perivascular
inflammatory cells. (D) RCM image (500×500 µm) at the stratum
spinosum, showing atypical honeycombed pattern, enlarged
intercellular spaces (red asterisks), and dark central elements
representing nuclei surrounded by bright cytoplasm (white arrows).
(E) RCM image (500×500 µm) at the dermo-epidermal junction
(DEJ)/papillary dermis, revealing dark areas representing papillary
blood vessels (white asterisk) containing central elements
corresponding to blood cells (red arrowhead) and bright
perivascular structures equivalent to perivascular inflammatory
cells (white arrow). (F) Histopathological image (hematoxylin and
eosin staining; magnification, ×200) of AC, showing hyperkeratosis
and irregular acanthosis, mildly atypical keratinocytes within the
lower third of epithelium, and marked solar elastosis and vascular
ectasia within the dermis. (G) Clinical image captured 7 months
after classic vermilionectomy, with minimal scarring and deformity,
and with no signs of recurrence. RCM, reflectance confocal
microscopy. |
Case 2
A 65-year-old Caucasian male, without any remarkable
personal or family medical history, was referred in 2017 from the
Department of Oral and Maxillofacial Surgery of ‘Dr Carol Davila’
Central Military Hospital in Bucharest to the Dermatology
Department of ‘Prof. N.C. Paulescu’ National Institute of Diabetes,
Nutrition and Metabolic Diseases, Bucharest, for the evaluation of
two adjacent tumors growing on his lower lip. A clinical
examination revealed an increased lower lip volume, and two
central, asymptomatic, well-demarcated, round-to-oval tumors, with
diameters of 20 mm and 5 mm, respectively. The larger lesion was
brown-to-dark red in color, whereas both tumors presented keratotic
surfaces and several ulcerations and crusts. Areas of ulceration
and a significant amount of crusting on the remaining lower lip
surface could be observed, with approximately two-thirds of the lip
being affected, all on a background of visible vermillion atrophy
(Fig. 2A). Dermoscopy of the tumors
revealed marked vascular polymorphism, including telangiectatic,
branching blood vessels, as well as hairpin, serpiginous, truncated
and dotted vessels. Brown and white structureless areas were also
visible. The surrounding lip surface presented slight-to-moderate
vascular polymorphism, with dotted and short, anastomosing vessels
(Fig. 2B). Dermoscopy of the
perilesional area revealed ulcerations of the mucosa, surrounded by
milky-white areas, telangiectatic and tortuous vessels, suggestive
of AC (Fig. 2C). Upon RCM
examination, an RCM block of the larger lower lip lesion (Fig. 2D) uncovered a disorganized
keratinocyte architecture and loss of the normal honeycombed
pattern with extensive keratinocyte atypia, markedly dilated blood
vessels (Fig. 2E), and bright
inflammatory cells (Fig. 2F). Aside
from keratinocyte pleomorphisms and enlarged intercellular spaces,
a particular ‘swirl’ was noticed, most likely due to incomplete
keratinization (Fig. 2G). RCM images
of the perilesional area revealed erosions surrounded by an
appreciable amount of bright inflammatory cells (Fig. 2H). The tumors were completely excised
with oncologically safe surgical margins and a histopathological
examination (using hematoxylin-eosin staining) revealed, in the
perilesional area, features highly suggestive for the diagnosis of
AC (Fig. 2I), whereas an examination
of the excised tumors confirmed the diagnosis of moderately
differentiated invasive SCC (Fig.
2J). On the second day following vermilionectomy, the wound was
healing well, with a minimal amount of crusting (Fig. 2K). At 5 weeks following surgery,
there was minimal scarring and deformity of the lower lip (Fig. 2L). Due to the surgical technique
employed, there was significant sparing of healthy tissue, which
allowed for the avoidance of microstomia and its cosmetic and
functional consequences in this patient.
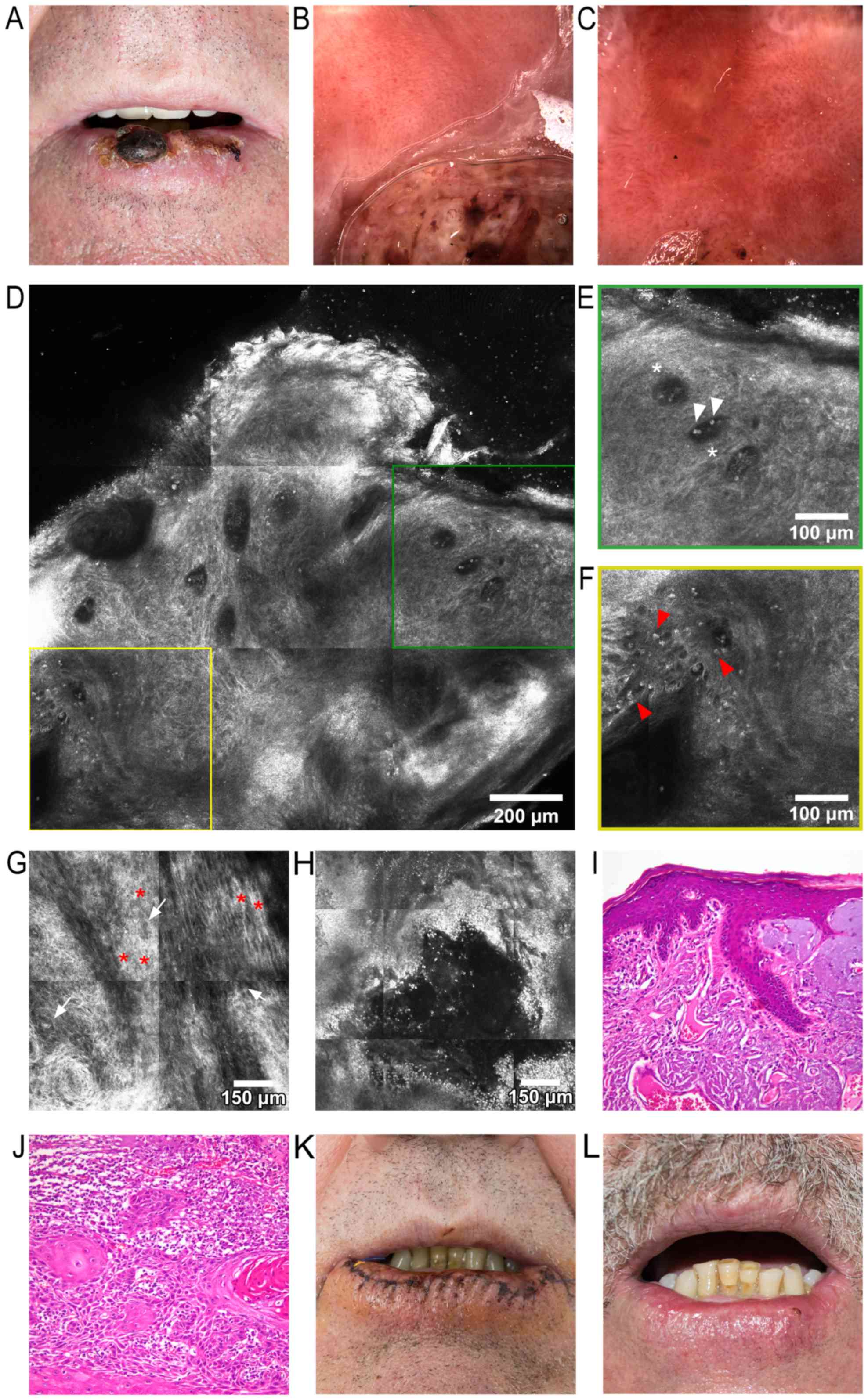 | Figure 2.(A) Clinical image, showing the two
tumors on the lower lip, with approximately two thirds of the lower
lip exhibiting clinical signs of actinic cheilitis. (B) Dermoscopic
image of the larger lower lip tumor: Part of the tumor is observed
in the lower half, showing marked vascular polymorphism, including
telangiectatic, branching blood vessels, as well as hairpin,
serpiginous, truncated and dotted blood vessels. (C) Dermoscopic
image of the perilesional area, revealing ulcerations of the mucosa
surrounded by milky-white areas, and telangiectatic and tortuous
vessels. (D) RCM block (dimensions: 1.5×1.5 mm), showing a
disorganized keratinocyte architecture and loss of the normal
honeycomb pattern, markedly dilated blood vessels, and scattered
bright elements representing inflammatory cells. (E) RCM detail
image (500×500 mm) from (D), showing dark areas corresponding to
dilated blood vessels (white asterisks) containing moderately
refractile elements (white arrowheads). (F) RCM detailed image
(500×500 mm) from (D), depicting white dots and plump refractile
elements (red arrowheads) corresponding to inflammatory cells. (G)
RCM block (1×1 mm) at the stratum spinosum, demonstrating
keratinocitary pleomorphisms of shape and size (white arrows);
thickened intercellular spaces, equivalent to spongiosis, can be
observed (red asterisks), and a particular ‘swirl’ effect in the
lower-left corner of the image. (H) RCM block (1×1 mm) of the
perilesional area, revealing an ulceration and multiple bright
elements corresponding to inflammatory cells. (I) Histopathological
image (hematoxylin-eosin staining; magnification, ×200) of actinic
keilitis, showing epidermis thickened by hyperkeratosis, acanthosis
and irregular elongation of rete ridges, several atypical
keratinocytes present in the lower third of the epithelium,
subjacent dermis with fibrosis, several ectatic blood vessels and
marked solar elastosis. (J). Histopathological image
(hematoxylin-eosin staining; magnification, ×200) of moderately
differentiated invasive squamous cell carcinoma: Trabeculi, and
nests of moderately pleomorphic atypical squamoid cells with
occasional parakeratotic pearl-like structures. (K) Clinical
photograph captured 2 days after vermilionectomy. (L) Clinical
image of the patient 5 weeks after surgery; there is minimal
scarring and deformity of the lower lip visible at this time. RCM,
reflectance confocal microscopy. |
Discussion
Due to the subtle nature of the initial clinical
manifestations of AC, confirming the diagnosis is of overwhelming
importance. Furthermore, no correlations between the clinical
aspect and histopathological changes have been established thus far
(24), and there are no clear-cut
clinical characteristics that separate AC from early SCC of the
lips (12,18).
Dermoscopic criteria for AC diagnosis include
ill-demarcated lesion borders, white-coloured projections,
‘island’-like structures and radially arranged vascular
telangiectasia surrounding ulcerated areas (41). In the present case report, a
milky-white plaque with well-defined borders surrounded by
telangiectatic and tortuous vessels was identified in our patient
(Case 1). Classically described dermoscopic features of SCC are
glomerular or dotted vessels against a red, scaly background
(32,42). In our other patient (Case 2),
dermoscopy revealed marked vascular polymorphism (telangiectatic,
branching, hairpin, serpiginous, truncated and dotted blood
vessels), and white, structureless areas.
RCM criteria for AC include disruption of the
stratum corneum, single detached corneocytes and parakeratosis, an
atypical honeycomb pattern with variation in cell size and
morphology in the stratum granulosum and spinosum, dermal solar
elastosis described as bright dense bundles with lace-like
appearance, and dilated and tortuous dermal blood vessels with
increased blood flow. It is also considered that keratinocyte
atypia and an atypical honeycomb pattern serve as the most
important features for AC diagnosis (19). The findings of the present case
studies support the observation (19) that keratinocyte atypia and an
atypical honeycomb pattern represent the most important diagnostic
criteria of AC. True atypia as observed in AC displays cells and
nuclei of different shapes and sizes, creating an atypical
honeycomb pattern on RCM that must be distinguished from
spongiosis.
Regarding RCM evaluation of lip SCC, Rishpon et
al (32) reported certain
distinct features, such as extensive atypia and disarrangement of
the spinous and granular layer, round bright nucleated cells
(corresponding to atypical and dyskeratotic keratinocytes observed
on histopathological slides), and atypical nucleated cells in the
superficial dermis (32). In our
case of lip SCC, severe disarrangement of the spinous layer,
extensive keratinocyte atypia, round, dilated and tortuous blood
vessels accompanied by perivascular inflammatory infiltrates,
dermal solar elastosis, and an abundant inflammatory infiltrate
were observed.
In clinical practice, troubles associated with
visual detection of carcinoma and dysplasia margins could be
mediated by analysis of frozen sections from the lesion edge, but
this is costly, time-consuming, conditional on the experience of
the pathologist, and it is not widely available (43). Hence, non-invasive imaging
technologies, such as RCM and dermoscopy, may allow an accurate
identification of tumor margins in real time, adding substantial
benefits for patients. RCM clearly has the potential to evaluate
features of normal mucosa, dysplasia and lip SCC, and may provide a
very appealing alternative for mucosal margins assessment due to
its ability to resolve cellular morphology and tissue architecture
in real time (44).
Numerous surgical and non-surgical strategies have
been devised for the management of AC. Common treatment options of
AC include cryosurgery, photodynamic therapy, 50% trichloroacetic
acid chemical peel, CO2 laser ablation,
electrodessication, and vermilionectomy. Topical treatments include
applications of 5-fluorouracil, imiquimod, and 3% Diclofenac in
2.5% hyaluronic acid (45–47). One of the classical approaches for
the management of lip SCC is considered to be surgical excision
(3–5,48). In
the present case report, we opted for surgical excision via
vermilionectomy, given the fact that it provides a safe, quick, and
economically efficient therapeutic option, particularly in the case
of lower lip SCC. The two patients have been scheduled for regular
follow-up visits.
In conclusion, due to the high rate of malignant
transformation of AC into SCC of the lips, because of its great
clinical variability, and since, for the majority of patients, it
appears to be innocuous, the gravity of AC may, in a large number
of cases, be underestimated, and all methods contributing to early
diagnosis and dynamic monitoring of lesions must be employed.
Considering how the majority of skin lesions are effortlessly
assessed by clinical examination and dermoscopy, what role does RCM
serve in patient care? Even though RCM might be considered
time-consuming, in our experience image acquisition only required
~10 min per lesion, but markedly increased our diagnostic
confidence, thereby limiting medical errors.
Taking all our findings together, the present
authors stress the importance of a correct diagnosis, proper
treatment and longterm patient follow-up as indispensable for
impeding the development of SCC of the lips, or for its early
diagnosis.
Acknowledgements
Not applicable.
Funding
This study was partly supported by grant no.
PN-III-P1-1.2-PCCDI-2017-0341, financed by Executive Agency for
Higher Education, Research, Development and Innovation.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
All authors have equally contributed to writing and
editing the manuscript.
Ethics approval and consent to
participate
The two patients provided their informed consent for
this study, which also included their consent to publish the
results from their treatment.
Consent for publication
All authors have read and approved this
manuscript.
Competing of interests
The authors declare they have no competing
interests.
References
|
1
|
de Souza Lucena EE, Costa DCB, da Silveira
EJD and Lima KC: Prevalence and factors associated to actinic
cheilitis in beach workers. Oral Diseases. 18:575–579. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strengthening the prevention of oral
cancer: the WHO perspective. Community Dent Oral Epidemiol.
33:397–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baker S: Current management of cancer of
the lip. Oncology (Williston Park, NY). 4:107–120; discussion
122–104. 1990.
|
|
4
|
Stucker FJ and Lian TS: Management of
cancer of the lip. Operative Techniques in Otolaryngology Head and
Neck Surgery. 15:226–233. 2004. View Article : Google Scholar
|
|
5
|
Zitsch RP 3rd: Carcinoma of the lip.
Otolaryngol Clin North Am. 26:265–277. 1993.PubMed/NCBI
|
|
6
|
Galyon SW and Frodel JL: Lip and perioral
defects. Otolaryngol Clin North Am. 34(647): 6662001.
|
|
7
|
Boda D, Neagu M, Constantin C, et al: HPV
strain distribution in patients with genital warts in a female
population sample. Oncol Let. 12:1779–1782. 2016. View Article : Google Scholar
|
|
8
|
Neagu M, Caruntu C, Constantin C, et al:
Chemically induced skin carcinogenesis: Updates in experimental
models (Review). Oncol Rep. 35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wood NH, Khammissa R, Meyerov R, Lemmer J
and Feller L: Actinic cheilitis: a case report and a review of the
literature. Eur J Dent. 5:101–106. 2011.PubMed/NCBI
|
|
10
|
Kwon NH, Kim SY and Kim GM: A case of
metastatic squamous cell carcinoma arising from actinic cheilitis.
Ann Dermatol. 23:101–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miranda AM, Soares LG, Ferrari TM, Silva
DG, Falabella ME and Tinoco E: Prevalence of actinic cheilitis in a
population of agricultural sugarcane workers. Acta Odontol
Latinoam. 25:201–207. 2012.PubMed/NCBI
|
|
12
|
Miranda AM, Ferrari T, Leite T, Domingos
T, Cunha K and Dias E: Value of videoroscopy in the detection of
alterations of actinic cheilitis and the selection of biopsy areas.
Med Oral Patol Oral Cir Bucal. 20:e292–e297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaugars GE, Pillion T, Svirsky JA, Page
DG, Burns JC and Abbey LM: Actinic cheilitis: A review of 152
cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
88:181–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vieira RAMAR, Minicucci EM, Marques MEA
and Marques SA: Actinic cheilitis and squamous cell carcinoma of
the lip: clinical, histopathological and immunogenetic aspects. An
Bras Dermatol. 87:105–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savage NW, McKay C and Faulkner C: Actinic
cheilitis in dental practice. Australian dental journal 55 Suppl.
1:78–84. 2010. View Article : Google Scholar
|
|
16
|
Cavalcante ASR, Anbinder AL and Carvalho
YR: Actinic Cheilitis: Clinical and Histological Features. J. Oral
Maxillofac. Surg. 66:498–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Santana Sarmento DJ, da Costa Miguel
MC, Queiroz LM, Godoy GP and da Silveira EJ: Actinic cheilitis:
clinicopathologic profile and association with degree of dysplasia.
Int J Dermatol. 53:466–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markopoulos A, Farmaki Albanidou E and
Kayavis I: Actinic cheilitis: clinical and pathologic
characteristics in 65 cases. Oral Diseases. 10:212–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ulrich M, Gonzalez S, Asschenfeldt Lange
B, et al: Non invasive diagnosis and monitoring of actinic
cheilitis with reflectance confocal microscopy. Journal of the
European Academy of Dermatology and Venereology : JEADV.
25:276–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rivera C and Venegas B: Histological and
molecular aspects of oral squamous cell carcinoma (Review). Oncol.
Lett. 8:7–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miranda AMO, Ferrari TM and Calandro TLL:
Actinic cheilitis: Clinical aspects and prevalence found in a rural
population of the interior of Brazil. Saúde Pesqui. 4:67–72.
2011.(In Portuguese).
|
|
22
|
Lopes ML, Silva Junior FL, Lima KC,
Oliveira PT and Silveira EJ: Clinicopathological profile and
management of 161 cases of actinic cheilitis. An Bras Dermatol.
90:505–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marques Piñera K, Lorenço SV, Silva LF,
Sotto MN and Carneiro PC: Actinic lesions in fishermen's lower lip:
clinical, cytopathological and histopathologic analysis. Clinics.
65:363–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nico Menta Simonsen M, Rivitti EA and
Lourenço SV: Actinic cheilitis: histologic study of the entire
vermilion and comparison with previous biopsy. J Cutan Pathol.
34:309–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Abreu MAMM, da Silva OMP, Pimentel DRN,
et al: Actinic cheilitis adjacent to squamous carcinoma of the lips
as an indicator of prognosis. Braz J Otorhinolaryngol. 72:767–771.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glogau RG: The risk of progression to
invasive disease. J Am Acad Dermatol. 42:S23–S24. 2000. View Article : Google Scholar
|
|
27
|
Slaughter DP, Southwick HW and Smejkal W:
‘Field cancerization’ in oral stratified squamous epithelium.
Clinical implications of multicentric origin. Cancer. 6:963–968.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lupu M, Caruntu C, Ghita MA, et al: Gene
Expression and Proteome Analysis as Sources of Biomarkers in Basal
Cell Carcinoma Dis Markers. 2016:1–9. 2016.
|
|
29
|
Ion A, Popa IM, Papagheorghe LML, et al:
Proteomic Approaches to Biomarker Discovery in Cutaneous T Cell
Lymphoma. Dis Markers. 2016:1–8. 2016. View Article : Google Scholar
|
|
30
|
Voiculescu V, Calenic B, Ghita M, et al:
From Normal Skin to Squamous Cell Carcinoma: A Quest for Novel
Biomarkers. Dis Markers. 2016:1–14. 2016. View Article : Google Scholar
|
|
31
|
White WM, Rajadhyaksha M, González S,
Fabian RL and Anderson RR: Non-invasive Imaging of Human Oral
Mucosa in vivo by Confocal Reflectance Microscopy. Laryngoscope.
109:1709–1717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rishpon A, Kim N, Scope A, et al:
Reflectance confocal microscopy criteria for squamous cell
carcinomas and actinic keratoses. Archives of dermatology.
145:766–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rajadhyaksha M, González S, Zavislan JM,
Anderson Rox R and Webb RH: In Vivo Confocal Scanning Laser
Microscopy of Human Skin II: Advances in Instrumentation and
Comparison With Histology11The authors have declared conflict of
interest. J Invest Dermatol. 113:293–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghita MA, Caruntu C, Rosca AE, et al:
Reflectance confocal microscopy and dermoscopy for in vivo, non
invasive skin imaging of superficial basal cell carcinoma. Oncol
Lett. 11:3019–3024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Căruntu C1, Boda D, Guţu DE and Căruntu A:
In vivo reflectance confocal microscopy of basal cell carcinoma
with cystic degeneration. Rom J Morphol Embryol. 55:1437–1441.
2014.PubMed/NCBI
|
|
36
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55:1191–1196. 2014.PubMed/NCBI
|
|
37
|
Diaconeasa A, Boda D, Neagu M, Constantin
C, Căruntu C, Vlădău L and Guţu D: The role of confocal microscopy
in the dermato-oncology practice. J Med Life. 4:63–74.
2011.PubMed/NCBI
|
|
38
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ridgway JM, Armstrong WB, Guo S, Mahmood
U, Su J, Jackson RP, Shibuya T, Crumley RL, Gu M, Chen Z, et al: In
vivo optical coherence tomography of the human oral cavity and
oropharynx. Arch Otolaryngol Head Neck Surg. 132:1074–1081. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
García-Hernández A, Roldán-Marín R,
Iglesias-Garcia P and Malvehy J: In vivo noninvasive imaging of
healthy lower lip mucosa: a correlation study between high
definition optical coherence tomography, reflectance confocal
microscopy, and histology. Dermatol Res Pract. 2013:2052562013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ito T, Natsuga K, Tanimura S, Aoyagi S and
Shimizu H: Dermoscopic features of plasma cell cheilitis and
actinic cheilitis. Acta Derm Venereol. 94:593–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zalaudek I, Argenziano G, Leinweber B, et
al: Dermoscopy of Bowen's disease. Br J Dermatol. 150:1112–1116.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nicoletti G, Brenta F, Malovini A,
Musumarra G, Scevola S and Faga A: Study to determine whether
intraoperative frozen section biopsy improves surgical treatment of
non melanoma skin cancer. Mol Clin Oncol. 1:390–394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clark AL, Gillenwater AM, Collier TG,
Naderi Alizadeh R, El Naggar AK and Kortum Richards RR: Confocal
microscopy for real time detection of oral cavity neoplasia. Clin
Cancer Res. 9:4714–4721. 2003.PubMed/NCBI
|
|
45
|
Shah AY, Doherty SD and Rosen T: Actinic
cheilitis: a treatment review. Int J Dermatol. 49:1225–1234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ulrich C, Forschner T, Ulrich M,
Stockfleth E, Sterry W and Termeer C: Management of actinic
cheilitis using diclofenac 3% gel: a report of six cases. Br J
Dermatol. 156:43–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matei C, Tampa M, Caruntu C, Ion RM,
Georgescu SR, Dumitrascu GR, Constantin C and Neagu M: Protein
microarray for complex apoptosis monitoring of dysplastic oral
keratinocytes in experimental photodynamic therapy. Biol Res.
47:332014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Calabrese L, Ionna F, Tradati N, et al:
Squamous cell carcinoma of the upper lip analysis of 123 cases. Int
J Oncol. 3:667–669. 1993.PubMed/NCBI
|
















