Introduction
Laryngeal carcinoma (LC) is a common tumor of the
head and neck. LC patients at early clinical stages (I and II) are
traditionally treated with surgery or radiotherapy, whereas
patients with advanced-stage disease (III and IV) may require
comprehensive sequential treatment combining surgery, radiotherapy
and chemotherapy (1). However,
despite aggressive treatment, no major improvement has been
achieved in terms of prognosis, with a 5-year survival rate of
50–60%, which is even lower in patients at advanced clinical stages
(2).
Induction chemotherapy (IC) is considered as a
reliable approach to controlling locally advanced LC, increasing
the rate of laryngeal preservation and decreasing the risk of local
spread or distant metastasis, thereby increasing the therapeutic
efficacy. However, locoregional control must be performed in
accordance with the tumor borders following IC, which should be
marked at the start of the therapy (3), regardless of the response to IC. The
clinical value of IC remains a matter of debate, particularly in
cases with resectable advanced LC. Randomized controlled trials
(RCTs) have reported conflicting results, whereas earlier
systematic reviews have not demonstrated an obvious benefit of IC
in terms of overall survival (OS) (4–7). There
is currently no definitive evidence favoring IC with locoregional
control over locoregional control alone for locally advanced and
resectable LC. Accordingly, a meta-analysis of OS rate, local
control, metastases and laryngeal preservation was conducted.
Materials and methods
Inclusion criteria
RCTs were considered eligible when they included
formerly untreated patients with resectable non-metastatic LC,
performing a comparison between IC followed by locoregional
treatment (laryngectomy, or radiotherapy, or concomitant
radiotherapy and chemotherapy, or laryngectomy combined with
radiotherapy or chemoradiotherapy) and locoregional treatment
alone. RCTs on laryngeal preservation were also considered as
eligible if they performed a comparison between radical surgery
together with radiotherapy vs. IC followed by radiotherapy or
chemoradiotherapy in responders, or radical surgery with radiation
therapy or chemoradiotherapy in non-responders. The included
studies were articles published in English and they included
patients treated between January 1, 1965 and December 31, 2017.
Exclusion criteria
The exclusion criteria were the following: i)
Abstracts, letters, or meeting proceedings; ii) incomplete studies,
studies with data duplication or not reporting outcomes of
interest; iii) a case number of <40.
Search strategy
A systematic search was performed through Medline,
EMBASE and The Cochrane Library for studies published up to June
1985 using a combination of the following search terms: Neoplasms,
laryngeal/laryngeal neoplasm/neoplasm/laryngeal/larynx
neoplasms/larynx neoplasm/neoplasm, larynx/neoplasms, larynx/cancer
of larynx/larynx cancers/laryngeal cancer/cancer,
laryngeal/cancers, laryngeal/laryngeal cancers/larynx
cancer/cancer, larynx/cancers, larynx/cancer of the larynx,
chemotherapies, induction/chemotherapy, induction/induction
chemotherapies, laryngectomies/laryngectomy/radiation
oncology/oncology, radiation/therapeutic radiology/radiology. The
reference lists of the retrieved articles were checked for other
relevant publications.
Data collection and analysis
Independent extraction of the data was performed by
two authors. The extracted information included name of first
author, publication date, number of patients, patient
characteristics, study design/risk of bias, TNM stage and outcome.
The data were entered into a standardized Excel file. Disagreements
were resolved through discussion and consensus.
Time-to-event data from each study were summarized
using the log hazard ratio (HR) and variance. When this information
was not reported by the trials, it was estimated from data such as
the log-rank test P-value (8),
whereas time-to-event data were calculated from the Kaplan-Meier
survival curves. Kaplan-Meier curves were interpreted with the
Engauge Digitizer software, version 4.1. Data were combined using
RevMan software, version 5.3. Pooling of the log HR and its
variance was performed with the use of an inverse-variance weighted
mean, and the findings are presented as HR and 95% confidence
interval (95% CI).
The DerSimonian-Laird random effects analysis was
used for estimating the differences in survival (9) by generating a collective survival
difference with a 95% CI using a heterogeneity assay at each
endpoint. The survival rate was calculated from the survival curves
where it was not readily available in the text or tables. Subjects
censored before each endpoint were subtracted from the denominators
(patient no. during follow-up), providing a conservative CI for the
summary statistic. Counting of the censored cases was performed via
placement of tick marks on survival curves (10).
Heterogeneity across studies was evaluated with the
use of the I2 statistic, which is considered as a
quantitative measure of irregularity across studies. Studies with
I2 25–50% were considered as having low heterogeneity,
whereas those with I2 50–75% were considered as having
reasonable heterogeneity and those with I2>75% as
having high heterogeneity (11).
I2>50% reflected significant heterogeneity (12). A fixed-effects model was applied
unless there was significant unexplained heterogeneity, in which
case a random-effects model was employed.
Results
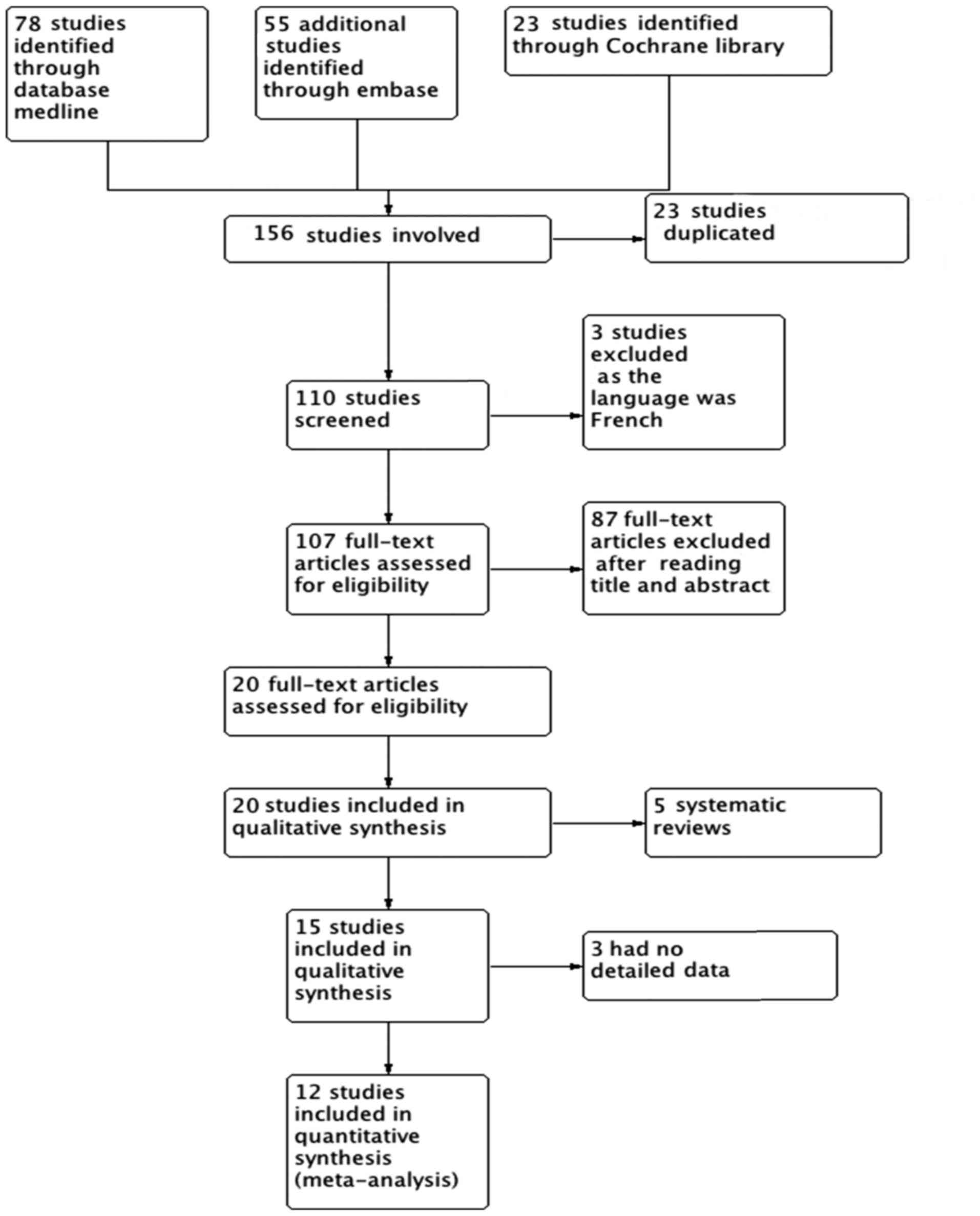
A total of 156 citations were identified from the
database search: 23 RCTs were excluded due to data duplication and
87 RCTs were excluded after reading the titles and abstracts. A
full-text review of the remaining articles was performed, and 3
studies did not include relevant data in detail (13–15), 3
were published in French (16–19), and
5 more were excluded as they were systematic reviews (20–24).
Finally, 12 RCTs (25–36) fulfilled our inclusion criteria and
were entered in the present meta-analysis. The flowchart of the
study inclusion process is shown in Fig.
1.
Of the 12 RCTs, 7 were on LC and 5 on hypopharyngeal
cancer. All the RCTs compared patients receiving IC followed by
locoregional therapy (laryngectomy and/or radiotherapy or
concomitant radiotherapy and chemotherapy), vs. those undergoing
locoregional therapy alone (laryngectomy and/or radiotherapy or
chemoradiotherapy). Despite the differences among these trials,
such as duration of the study and/or follow-up, the effect of study
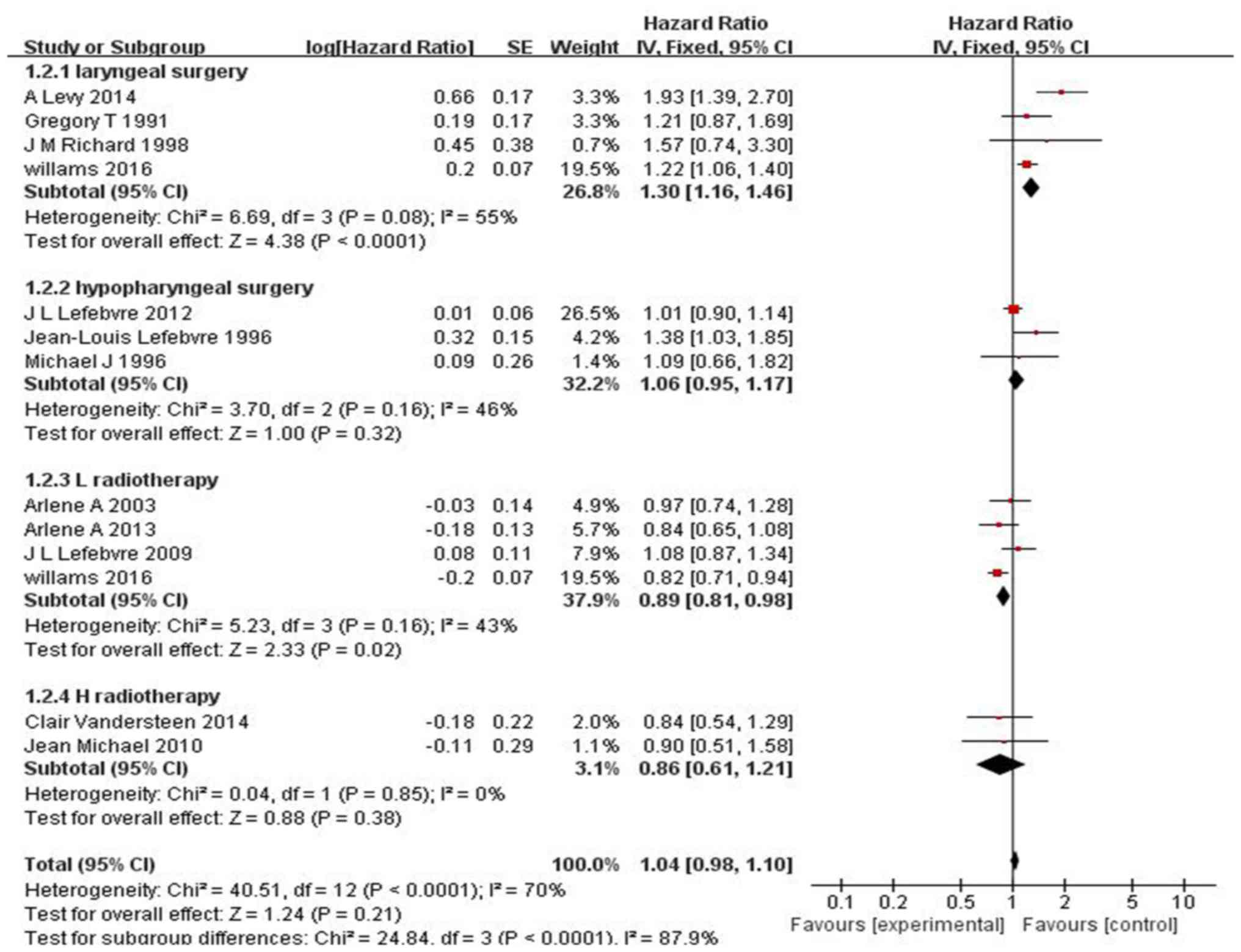
heterogeneity on the OS for LC as well as for hypopharyngeal cancer
was not statistically significant (I2=21%, P=0.28; s 2);
moreover, no significant difference in OS was observed between
patients who received and those who did not receive IC for
hypopharyngeal cancer or laryngeal cancer (HR=1.04, 95% CI:
0.98–1.10, P=0.21; Fig. 2). However,
IC had better treatment outcomes for patients with LC compared with
surgery (HR=1.30, 95% CI: 1.16–1.46, P<0.0001; Fig. 2), and IC may have result in more
adverse side effects in laryngeal cancer compared with radiotherapy
(HR=0.89, 95% CI: 0.81–0.98, P=0.02; Fig. 2), However, as the number of cases in
the present study was limited, these results require confirmation
through further studies and large multicenter clinical trials.
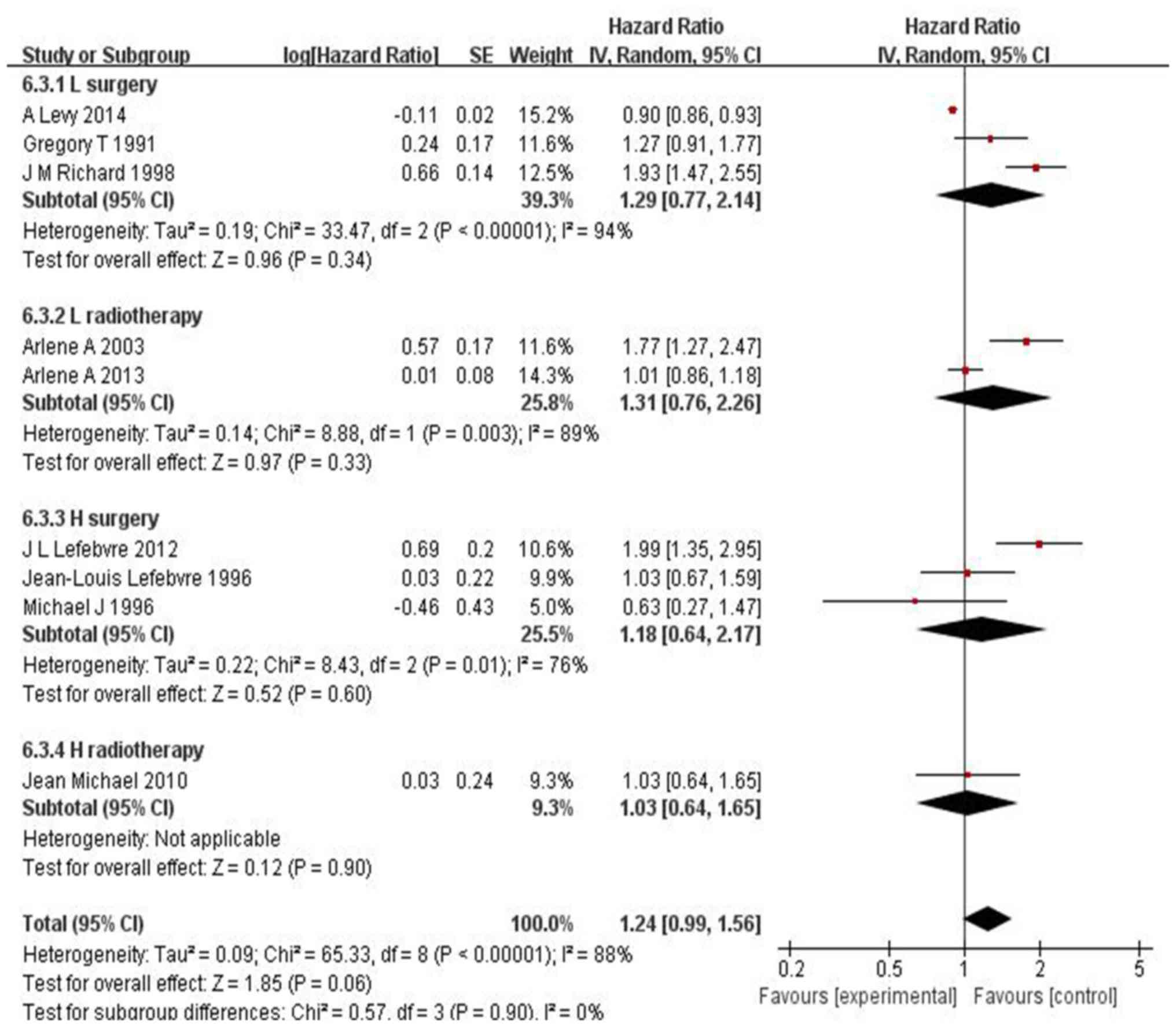
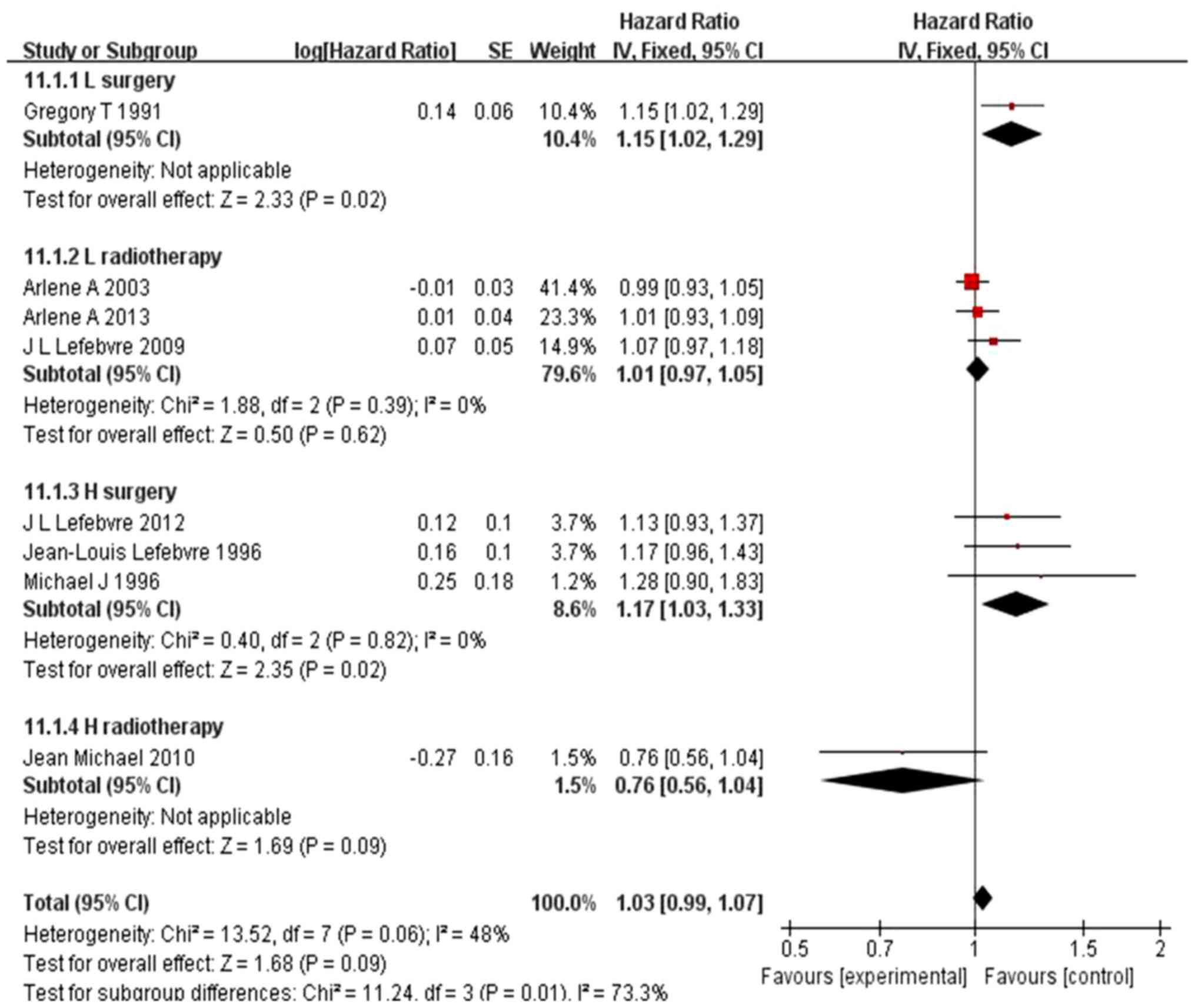
There was obvious heterogeneity regarding
disease-free survival (DFS) (I2=88%, P<0.00001) and
laryngeal preservation (LP) (I2=93%, P<0.00001).
Accordingly, the random-effects model was applied. The results for
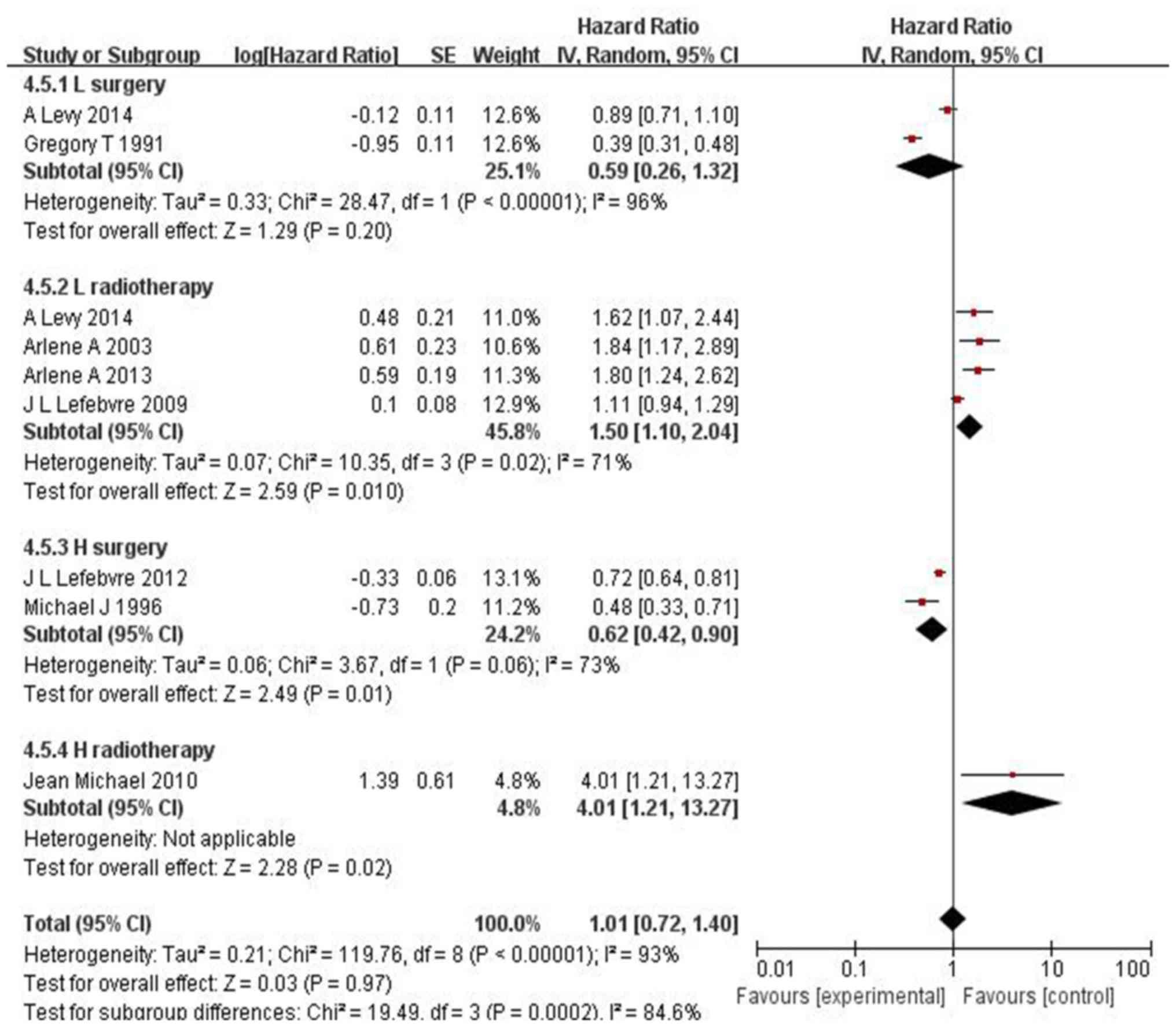
DFS were HR=1.24, 95% CI: 0.99–1.56 and P=0.06 (Fig. 3) and for LP HR=1.01, 95% CI:
0.72–1.40 and P=0.97(Fig. 4). Thus,
IC exhibited higher efficacy vs. surgery in LC and vs. radiotherapy
in hypopharyngeal cancer.
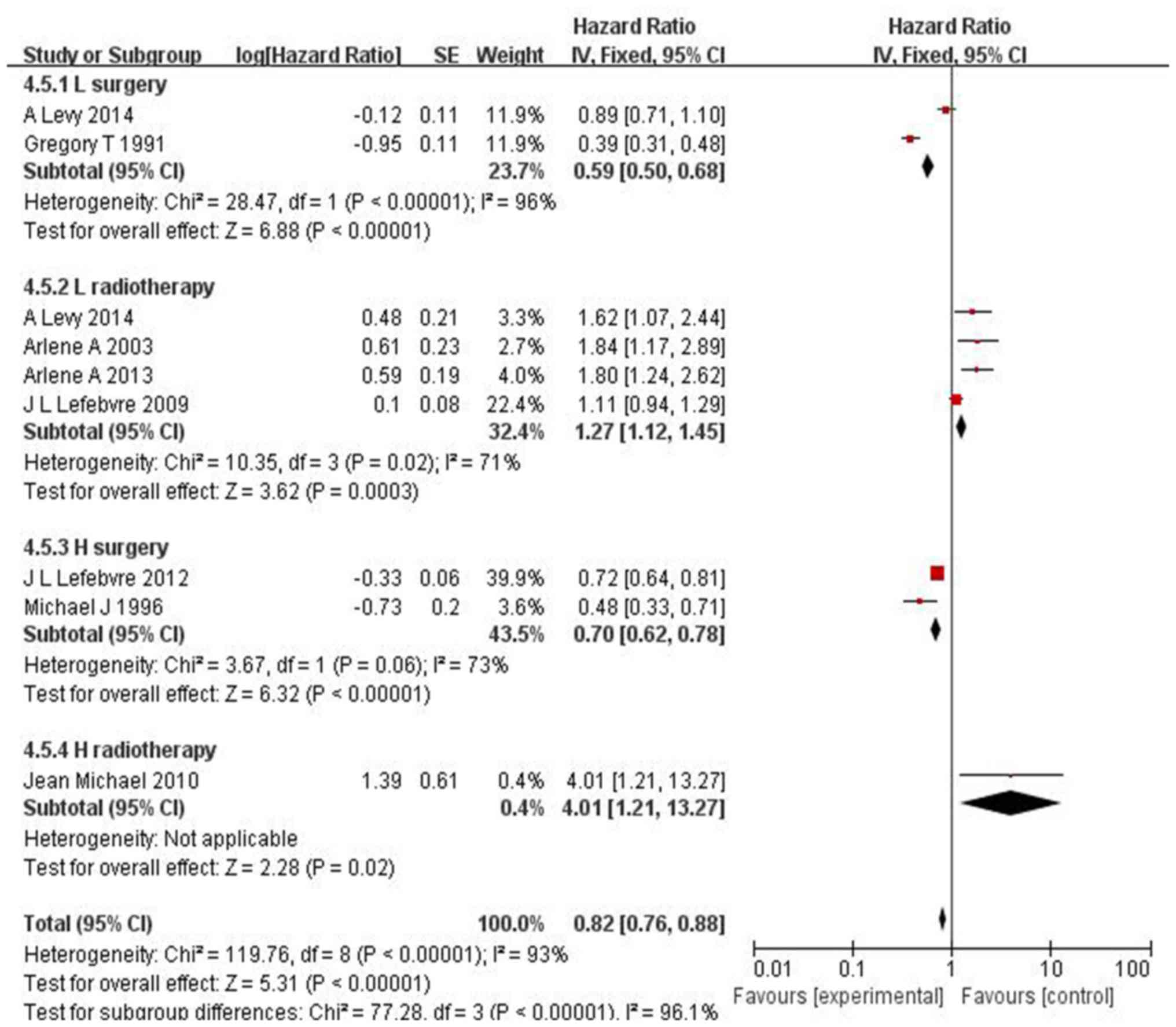
In the 12 RCTs on LC and hypopharyngeal cancer
(including 4,320 patients) that focused on locoregional control,
laryngeal preservation was not possible (HR=0.82, 95% CI:
0.76–0.88, P<0.00001) following IC in responders, without a
decrease in OS (Fig. 5).
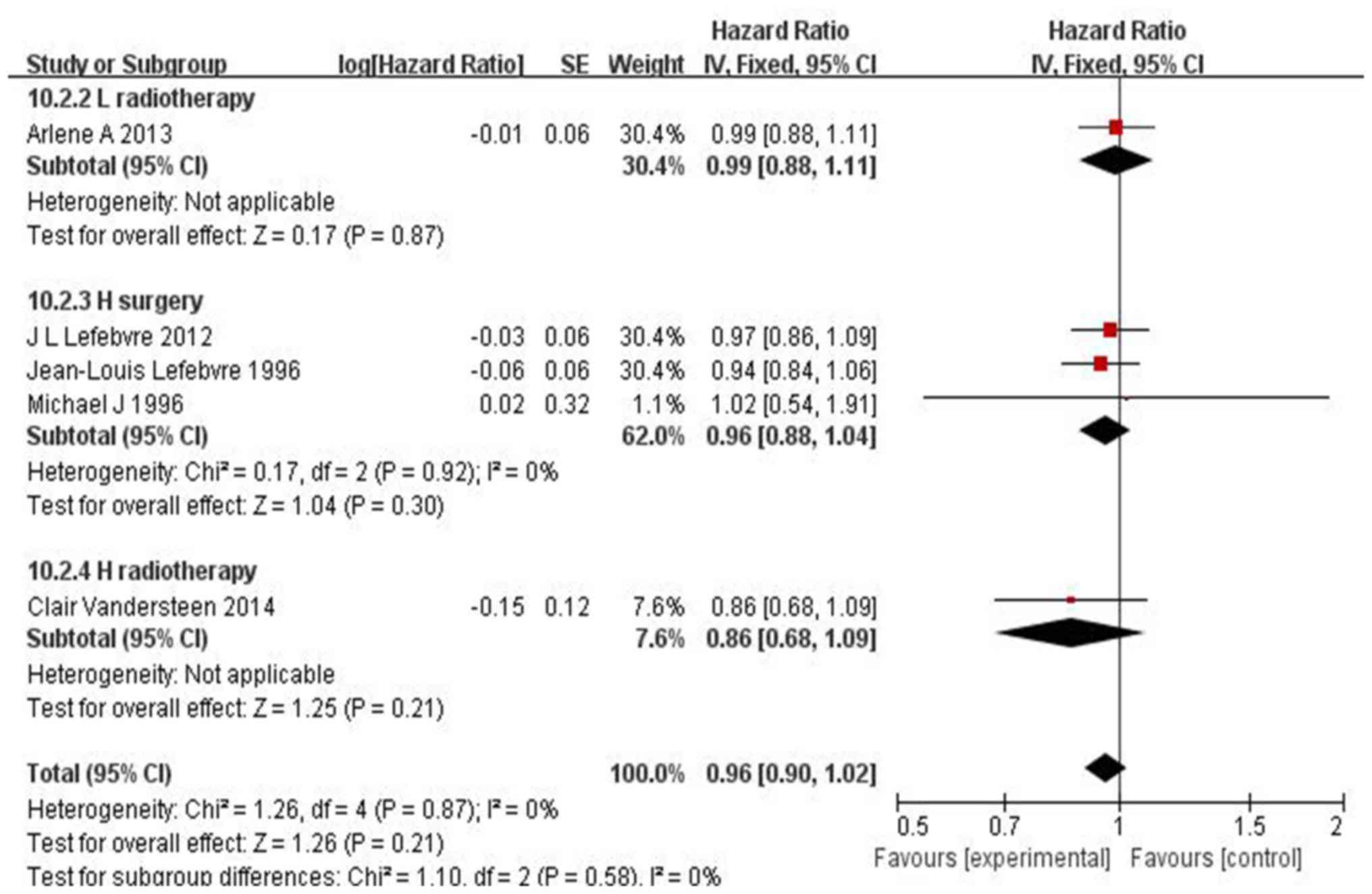
No substantial difference was observed in the local
recurrence rate between patients who did and those who did not
receive IC (HR=0.96, 95% CI: 0.90–1.02, P=0.21) (Fig. 6). However, in hypopharyngeal
carcinoma, the IC group exhibited a significantly lower long-term
(5-year) rate of distant recurrence (difference of 11.7%; 95% CI:
10.3–13.3%, P=0.02) vs. surgery (Fig.
7).
Discussion
In the present study, it was demonstrated that IC
was beneficial for patients with locally advanced and resectable
LC, with a 11.7% lower rate of distant metastasis. However, this
conclusion may differ among different studies. Jie et al
(3) also reported that the IC group
had a lower rate of distant metastasis by 8% (95% CI: 1–16,
P=0.02). Furthermore, a randomized phase 3 trial (37) divided patients into two groups, one
receiving IC followed by concurrent chemoradiotherapy (n=70), and
the other receiving concurrent chemoradiotherapy alone (n=75);
finally, 5 (7%) patients in the induction group and 8 (11%) in the
concurrent chemoradiotherapy group developed distant metastasis.
Their findings demonstrated that adding IC may be superior to
concurrent chemoradiotherapy alone in the treatment of locally
advanced head and neck squamous cell carcinoma (HNSCC). However, Su
et al (38) conducted a
meta-analysis in 2008, and included 4 RCTs reporting that the
difference in distant metastasis between the treatment group and
the control group was not significant, while 1 study reported that
the difference was statistically significant. Thus, more
large-scale RCTs and/or extensive meta-analyses are required.
However, IC was not found to be associated with any major
differences regarding local recurrence. Furthermore, the TAX324
study (34) reported that there was
no significant difference in local and distant recurrence between
the IC and control groups.
The combination of cisplatin and 5-fluorouracil (PF)
was applied as IC. The use of docetaxel has been shown to improve
OS rate, but this may due to the patients exhibiting different
responses to IC. Human papillomavirus (HPV) infection, smoking,
drinking, epidermal growth factor receptor (EGFR) expression and
sex may act as prognostic factors in HNSCC (39). Among patients with oropharyngeal
carcinoma, 64% were HPV-16 positive, and the age range was 55–63
years, with positive subjects being younger compared with negative
subjects. Men are more susceptible compared with women (73.3 vs.
41.6%, respectively). The degree of HPV infection and virus subtype
was obviously associated with the response to IC and better OS and
disease-specific survival. High EGFR expression was also associated
with poor response to IC and poor OS. In the present study, smoking
appeared to be significantly associated with higher EGFR expression
and lower HPV load. The abovementioned factors may affect the OS
rate of patients with LC and the extent of response to IC. The
additive effect of lower EGFR expression and higher HPV titer was
associated with better OS and disease-specific survival.
HPV-negative tumors or those with higher EGFR expression had the
worst OS and disease-specific survival, as all patients (10/10)
succumbed to the disease within 2.5 years. However, IC appears to
be beneficial in terms of DFS. The impact of IC is likely to differ
according to the location of the tumor. Currently, in patients with
resectable locally advanced hypopharyngeal cancer, surgery,
radiotherapy or chemoradiotherapy are considered as the standard
treatments. As shown in Fig. 2, in
patients with hypopharyngeal cancer, IC is likely to favorably
affect OS rate compared with surgery. However, the result does not
appear to be consistent with the conventional belief in respect of
the sequential or concurrent chemoradiotherapy, as it appears that
IC is more effective in LC compared with hypopharyngeal cancer. As
regards LC, IC may be beneficial in terms of OS rate in patients
with resectable disease, which has also been suggested by other
meta-analyses (4,6,7). This
may be due to a number of factors, such as the heterogeneity of the
patients and the location of the tumor, and the data of the present
study may not suffice. Therefore, it is necessary to analyze the
factors associated with the response to IC in patients with
advanced LC.
In addition, PF + docetaxel (TPF) is hypothesized to
be the optimal IC choice for the control of LC patients, which is
likely to be due to the fact that squamous cell carcinoma is
sensitive to docetaxel and 95% of LC cases are squamous cell
carcinomas. However, it has not been elucidated whether induction
TPF enhances resectability on provision of priority to surgery in
patients with locally advanced and resectable LC, which has been
reported previously. The TAX324 study suggested that IC with TPF
confers a long-term survival benefit compared with PF in locally
advanced head and neck cancer. Therefore, it is recommended that
patients who are candidates for IC ARE treated with TPF. In 2010,
Calais (40) added the taxane
docetaxel to PF, creating the TPF triplet regimen, which achieved
significantly higher laryngeal preservation and laryngectomy-free
survival rates compared with the PF doublet regimen. TPF is
currently the accepted standard IC regimen in clinical trials
including patients with resectable disease. However, Levy et
al (32) reported that the
addition of taxanes did not improve outcome in their series. Levy
et al questioned the validity of the results of certain
studies, such as the TAX324 trial (34), as it only included a total of 35
patients; thus, the interpretation of these results should be
performed with caution and more studies are required to clearly
determine the role of taxanes in this setting.
A number of laboratory trials validated the benefits
associated with EGFR-targeted agents for the treatment of locally
advanced and resectable LC. Traditional Chinese medicines, such as
curcumin and resveratrol, are also currently applied in the
treatment of head and neck cancer (41–43), in
addition to being reported to be effective in tumors in other
locations (44). Predictive
biomarkers that reflect the efficacy and safety of IC are expected
to assist with treatment selection, or used to determine whether IC
must be performed, particularly in resectable lesions. In cases
where the biomarkers predict disadvantage to IC, it should not be
performed; otherwise, IC may be beneficial in terms of survival
rate. Potential biomarkers may include DNA gene mutations,
epigenetic variations, as well as levels of mRNA or protein
expression (45). It was discovered
that the mechanism underlying the antitumor effects of liriodenine
is likely mediated via upregulation of p53 expression, which
eventually stimulates cell apoptosis (45). p53 gene changes are strongly
associated with low risk outcomes in PF-based IC, which suggests
that patients with LC must first undergo screening for p53 changes
prior to the selection of the most suitable treatment protocol. An
international team (46) conducted a
genome-wide association study (GWAS) on 993 patients with squamous
cell LC and 1,995 cancer-free controls from Chinese communities,
and identified three novel susceptibility loci at 11q12 (rs174549),
6p21 (rs2857595) and 12q24 (rs10492336). This was the first global
cancer research applying GWAS, the results of which are expected to
further advance the research on the mechanism of LC, aiding early
identification, timely diagnosis and molecular targeted therapy of
LC. In addition, Liang et al (47) conducted a study on dendritic cell
(DC) fusion vaccine that acts on human laryngeal carcinoma HEp-2
cells. In the present study, it was revealed that that SOCS1 siRNA
and IL-12 gene modified DCs together, which may provide novel
strategies for polygenic therapy in LC. To the best of our
knowledge, this is the first study on this subject available in the
literature to date. Therefore, additional investigation is required
to elucidate whether IC can enable organ preservation in
non-laryngeal locations.
The number of related studies on LC is limited, and
the sample size of the present study is small; therefore, the
results must be interpreted with caution. In the present study, 3
articles reported patient withdrawals. Due to non-standardized
cases with loss to follow-up and incomplete records, the actual and
long-term curative efficacy of IC should be interpreted with
caution, pending further research. The sample size of each RCT
included in this study was satisfactory, and all RCTs reported
adverse reactions in terms of mucosal damage caused by IC, with
salivary gland, pharyngeal, esophageal and laryngeal toxicity being
the most common serious events. These complications were associated
with fatalities in all groups. In addition, attention must be paid
to the possibility of IC-related kidney injury with ensuing renal
functional changes. In the late-stage clinical studies, attention
should be paid to the following: Applying the randomization
principle, ensuring balance between groups, thereby improving the
credibility of the research results; using blinding methods to
reduce information bias; correct estimation of the sample size to
reduce the sampling error; and censoring of withdrawals and cases
lost to follow-up. In addition, the number of cases and the reasons
for analysis should be provided; rigorous scientific inclusion and
exclusion criteria should be established to ensure high quality of
the RCTs; attention must be paid to the monitoring and recording of
adverse reactions to better evaluate the safety of the
interventions. In conclusion, IC confers an advantage in terms of
lowering the rate of distant metastases, in addition to prolonging
DFS, enabling laryngeal preservation and increasing the OS rate in
the patients with locally advanced and resectable LC. However,
there is no sufficient evidence to support its superiority in terms
of locoregional control and local recurrence. More studies on
laryngeal preservation are required to optimize IC protocols;
moreover, additional molecular biomarkers are required to identify
patients that are likely to respond to IC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
All patients provided consent for publication.
Authors' information
XFW made substantial contributions to the conception
and design of the study, as well as the acquisition, analysis and
interpretation of data. LG gave final approval of the version to be
published and made substantial contributions to the conception and
design of the study. PG contributed in drafting the manuscript and
revising it critically for important intellectual content, as well
as the collection of data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
IC
|
induction chemotherapy
|
|
LC
|
laryngeal carcinoma
|
|
RCTs
|
randomized controlled trials
|
|
HR
|
hazard ratio
|
|
95% CI
|
95% confidence interval
|
|
LP
|
laryngeal preservation
|
|
DCs
|
dendritic cells
|
|
PF
|
cisplatin with 5-fluorouracil
|
|
EGFR
|
epidermal growth factor receptor
|
|
DFS
|
disease-free survival
|
|
GWAS
|
genome-wide association study
|
References
|
1
|
Forastiere AA: Head and neck cancer:
Overview of recent developments and future directions. Semin Oncol.
27(Suppl 8): 1–4. 2000.PubMed/NCBI
|
|
2
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: TAX 324 Study Group: Cisplatin and
fluorouracil alone or with docetaxel in head and neck cancer. N
Engl J Med. 357:1705–1715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jie M, Ying L, Xi Y, et al: Induction
chemotherapy inpatients with resectable head and neck squamous cell
carcinoma: A Meta-analysis. World J Surg Oncol. 11:61–67.
2013.PubMed/NCBI
|
|
4
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC Collaborative Group. Meta-Analysis of
Chemotherapy on Head and Neck Cancer. Lancet. 355:949–955. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monnerat C, Faivre S, Temam S, Bourhis J
and Raymond E: End points for new agents in induction chemotherapy
for locally advanced head and neck cancers. Ann Oncol. 13:995–1006.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pignon JP, le Maître A, Maillard E and
Bourhis J; MACH-NC Collaborative Group: Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glenny AM, Furness S, Worthington HV,
Conway DI, Oliver R, Clarkson JE, Macluskey M, Pavitt S, Chan KK
and Brocklehurst P; CSROC Expert Panel: Interventions for the
treatment of oral cavity and oropharyngeal cancer: Radiotherapy.
Cochrane Database Syst Rev. 4:CD0063872010.
|
|
8
|
Parmar MKB, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitsudomi T, Hamajima N, Ogawa M and
Takahashi T: Prognostic significance of p53 alterations in patients
with non-small cell lung cancer: a meta-analysis. Clin Cancer Res.
6:4055–4063. 2000.PubMed/NCBI
|
|
11
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Armitage P, Berry G and Matthews JNS:
Analysing means and proportions. Statistical Methods in Medical
Research. 4th edition. Blackwell Science Ltd.; Hoboken, NJ; pp.
83–146. 2008
|
|
13
|
Olsen KD: Reexamining the treatment of
advanced laryngeal cancer. Head Neck. 32:1–7. 2010.PubMed/NCBI
|
|
14
|
Jarząb A, Grabarska A, Kiełbus M,
Jeleniewicz W, Dmoszyńska-Graniczka M, Skalicka-Woźniak K,
Sieniawska E, Polberg K and Stepulak A: Osthole induces apoptosis,
suppresses cell-cycle progression and proliferation of cancer
cells. Anticancer Res. 34:6473–6480. 2014.PubMed/NCBI
|
|
15
|
Dewyer NA, Wolf GT, Light E, Worden F,
Urba S, Eisbruch A, Bradford CR, Chepeha DB, Prince ME, Moyer J, et
al: Circulating CD4-positive lymphocyte levels as predictor of
response to induction chemotherapy in patients with advanced
laryngeal cancer. Head Neck. 36:9–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bourhis J, Lefebvre JL, Temam S, Lusinchi
A, Janot F, Wibault P and Pignon JP: Laryngeal preservation:
nonsurgical approaches. Cancer Radiother. 8:S24–S28. 2004.(In
French). PubMed/NCBI
|
|
17
|
Pointreau Y, Lafond C, Legouté F,
Trémolières P, Servagi-Vernat S, Giraud P, Maingon P, Calais G and
Lapeyre M: Radiotherapy of larynx cancers. Cancer Radiother.
20:(Suppl):. S131–S135. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calais G, Chapet S, Ruffier-Loubière A and
Bernadou G: Induction chemotherapy for locally advanced head and
neck cancer. Cancer Radiother. 17:498–501. 2013.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lévy A, Blanchard P, Janot F, Temam S,
Bourhis J, Daly-Schveitzer N and Tao Y: Results of definitive
radiotherapy for squamous cell carcinomas of the larynx patients
with subglottic extension. Cancer Radiother. 18:1–6. 2014.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forastiere AA, Weber RS and Trotti A:
Organ Preservation for Advanced Larynx Cancer: Issues and Outcomes.
J Clin Oncol. 33:3262–3268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toohill RJ, Duncavage JA, Grossmam TW,
Malin TC, Teplin RW, Wilson JF, Byhardt RW, Haas JS, Cox JD,
Anderson T, et al: The effects of delay in standard treatment due
to induction chemotherapy in two randomized prospective studies.
Laryngoscope. 97:407–412. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carew JF and Shah JP: Advances in
multimodality therapy for laryngeal cancer. CA Cancer J Clin.
48:211–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gilbert J and Forastiere A: Organ
preservation trials for laryngeal cancer. Otolaryng Clin N Am.
35:1035–1054. 2002. View Article : Google Scholar
|
|
24
|
Gorphe P, Matias M, Even C, Ferte C,
Bidault F, Garcia G, Temam S, Nguyen F, Blanchard P, Tao Y, et al:
Laryngo-esophageal Dysfunction-free Survival in a Preservation
Protocol for T3 Laryngeal Squamous-cell Carcinoma. Anticancer Res.
36:6625–6630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf GT, Fisher SG, Hong WK, Hillman R,
Spaulding M, Laramore GE, Endicott JW, McClatchey K and Henderson
WG; Department of Veterans Affairs Laryngeal Cancer Study Group:
Induction chemotherapy plus radiation compared with surgery plus
radiation in patients with advanced laryngeal cancer. N Engl J Med.
324:1685–1690. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zelefsky MJ, Kraus DH, Pfister DG, Raben
A, Shah JP, Strong EW, Spiro RH, Bosl GJ and Harrison LB: Combined
chemotherapy and radiotherapy versus surgery and postoperative
radiotherapy for advanced hypopharyngeal cancer. Head Neck.
18:405–411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Richard JM, Sancho-Garnier H, Pessey JJ,
Luboinski B, Lefebvre JL, Dehesdin D, Stromboni-Luboinski M and
Hill C: Randomized trial of induction chemotherapy in larynx
carcinoma. Oral Oncol. 34:224–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forastiere A, Goepfert H, Maor M, Pajak T,
Webwe R, Morrison W, et al: Concurrent chemotherapy and
radiotherapy for organ preservation in advanced laryngeal cancer. N
Engl J Med. 349:2091–2098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prades JM, Lallemant B, Garrel R, Reyt E,
Righini C, Schmitt T, Remini N, Saban-Roche L, Timoshenko AP,
Trombert B, et al: Randomized phase III trial comparing induction
chemotherapy followed by radiotherapy to concomitant
chemoradiotherapy for laryngeal preservation in T3M0 pyriform sinus
carcinoma. Acta Otolaryngol. 130:150–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lefebvre JL, Andry G, Chevalier D,
Luboinski B, Collette L, Traissac L, de Raucourt D and Langendijk
JA; EORTC Head and Neck Cancer Group: Laryngeal preservation with
induction chemotherapy for hypopharyngeal squamous cell carcinoma:
10-year results of EORTC trial 24891. Ann Oncol. 23:2708–2714.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA,
Thorstad W, et al: Long-term results of RTOG 91–11: a comparison of
three nonsurgical treatment strategies to preserve the larynx in
patients with locally advanced larynx cancer. J Clin Oncol.
31:8452013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levy A, Blanchard P, Temam S, Maison MM,
Janot F, Mirghani H, Bidault F, Guigay J, Lusinchi A, Bourhis J, et
al: Squamous cell carcinoma of the larynx with subglottic
extension: Is larynx preservation possible? Strahlenther Onkol.
190:654–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vandersteen C, Benezery K, Chamorey E,
Ettaiche M, Dassonville O, Poissonnet G, Riss JC, Pierre CS,
Hannoun-Lévi JM, Chand ME, et al: Contemporary therapeutic
management of locally advanced hypopharyngeal cancer: Oncologic and
functional outcomes - a report on 100 cases. Acta Otolaryngol.
135:193–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lorch JH, Goloubeva O, Haddad RI, Cullen
K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE and Posner
MR; TAX 324 Study Group: Induction chemotherapy with cisplatin and
fluorouracil alone or in combination with docetaxel in locally
advanced squamous-cell cancer of the head and neck: Long-term
results of the TAX 324 randomised phase 3 trial. Lancet Oncol.
12:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lefebvre JL, Chevalier D, Luboinski B,
Kirkpatrick A, Collette L and Sahmoud T: Larynx preservation in
pyriform sinus cancer: preliminary results of a European
Organization for Research and Treatment of Cancer phase III trial.
EORTC Head and Neck Cancer Cooperative Group. Journal of the
National Cancer Institute. 8:890–899. 1996. View Article : Google Scholar
|
|
36
|
Lefebvre JL, Rolland F, Tesselaar M,
Bardet E, Leemans CR, Geoffrois L, Hupperets P, Barzan L, de
Raucourt D, Chevalier D, et al: EORTC Head and Neck Cancer
Cooperative Group; EORTC Radiation Oncology Group: Phase 3
randomized trial on larynx preservation comparing sequential vs
alternating chemotherapy and radiotherapy. J Natl Cancer Inst.
101:142–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su YX, Zheng JW, Zheng GS, Liao GQ and
Zhang ZY: Neoadjuvant chemotherapy of cisplatin and fluorouracil
regimen in head and neck squamous cell carcinoma: A meta-analysis.
Chin Med J (Engl). 121:1939–1944. 2008.PubMed/NCBI
|
|
39
|
Kumar B, Cordell KG, Lee JS, et al:
Response to Therapy and Outcome in Oropharyngeal Cancer are
Associated with Biomarkers Including HPV, EGFR, Gender and Smoking.
Int J Radiat Oncol Biol Phys. 69(Suppl 1): 1092007. View Article : Google Scholar
|
|
40
|
Calais G: TPF: a rational choice for
larynx preservation? Oncologist. 2010.15 Suppl 3 (. (15 Suppl 3):
19–24. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wilken R, Veena MS, Wang MB and Srivatsan
ES: Curcumin: A review of anti-cancer properties and therapeutic
activity in head and neck squamous cell carcinoma. Mol Cancer.
10:122011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baumeister P, Reiter M and Harréus U:
Curcumin and Other Polyphenolic Compounds in Head and Neck Cancer
Chemoprevention. Oxid Med Cell Longev. 2012(902716)2012.PubMed/NCBI
|
|
43
|
Qiao Y, Gao K, Wang Y, Wang X and Cui B:
Resveratrol ameliorates diabetic nephropathy in rats through
negative regulation of the p38 MAPK/TGF-β1 pathway. Exp Ther Med.
13:3223–3230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghalandarlaki N, Alizadeh AM and
Ashkani-Esfahani S: Nanotechnology-applied curcumin for different
diseases therapy. Biomed Res Int. 2014:3942642014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li L, Xu Y and Wang B: Liriodenine induces
the apoptosis of human laryngocarcinoma cells via the upregulation
of p53 expression. Oncol Lett. 9:1121–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei Q, Yu D, Liu M, Wang M, Zhao M, Liu M,
Jia W, Ma H, Fang J, Xu W, et al: Genome-wide association study
identifies three susceptibility loci for laryngeal squamous cell
carcinoma in the Chinese population. Nat Genet. 46:1110–1114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liang W and Wang XF: In vitro induction of
specific anti-tumoral immunity against laryngeal carcinoma by using
human interleukin-12 gene-transfected dendritic cells. Chin Med J
(Engl). 124:1357–1361. 2011.PubMed/NCBI
|