Introduction
In recent years, endoscopic mucosal resection (EMR)
has been indicated for the treatment of superficial, early-stage
colorectal cancer due to its minimal invasiveness and excellent
results in terms of clinical outcome (1,2). In
addition, taking into consideration the possibility of incomplete
tumor removal due to the difficulty in resecting lesions sized
>2 cm by EMR, endoscopic submucosal dissection has been adapted
for use in this procedure (1,3). Cases
of local recurrence following radical EMR are extremely rare. We
herein report an extremely rare case of recurrence at 26 months
following lesion removal by a curative endoscopic procedure
performed according to the 2016 Colon Cancer Treatment Guidelines
of the Japanese Society for Cancer of the Colon and Rectum (JSCCR
guidelines) for the treatment of colorectal cancer.
Case report
A 63-year-old man underwent EMR for a 0-IIa lesion
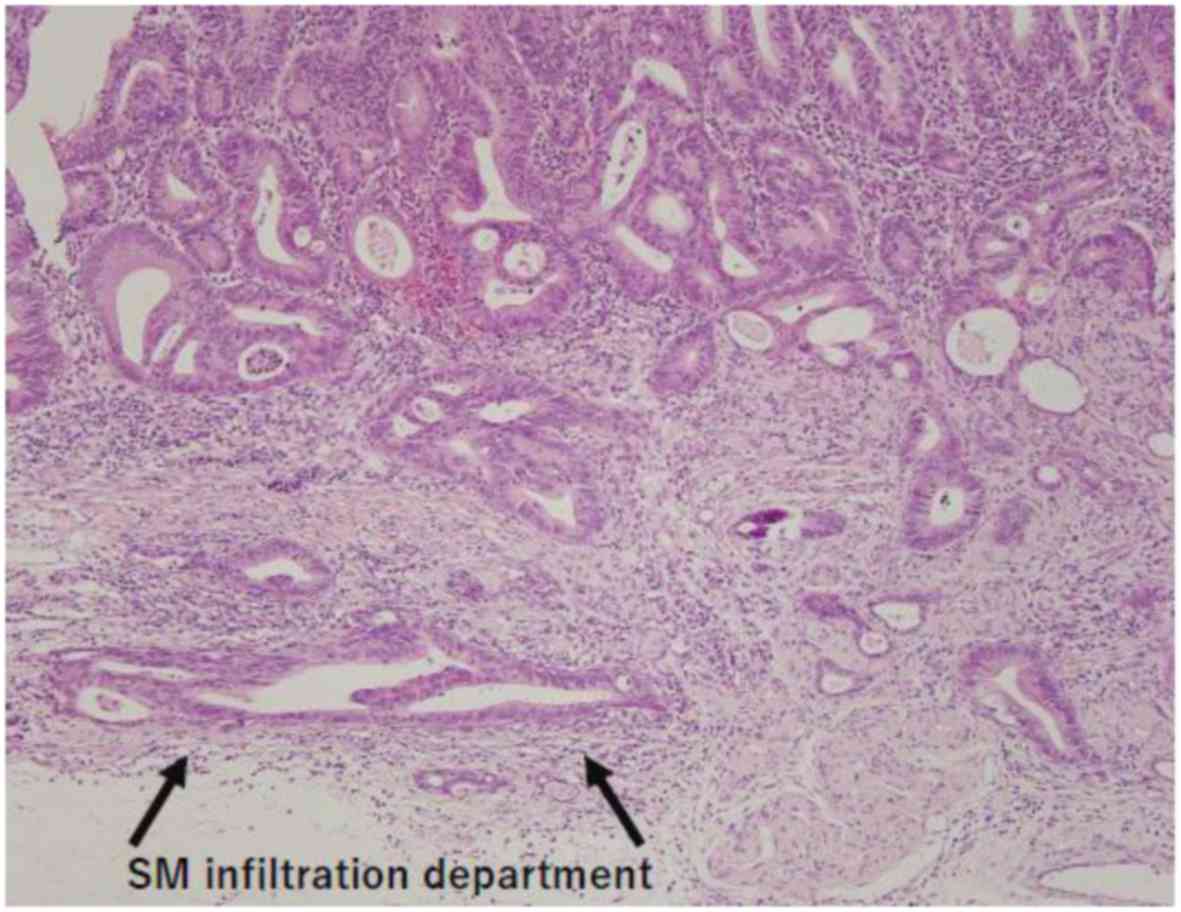
in the Ra portion of the rectum in October, 2014. The pathological
findings (Fig. 1) were tub1, T1a
(SM1, 420 µm), ly0 and v0, and the EMR was considered to be a
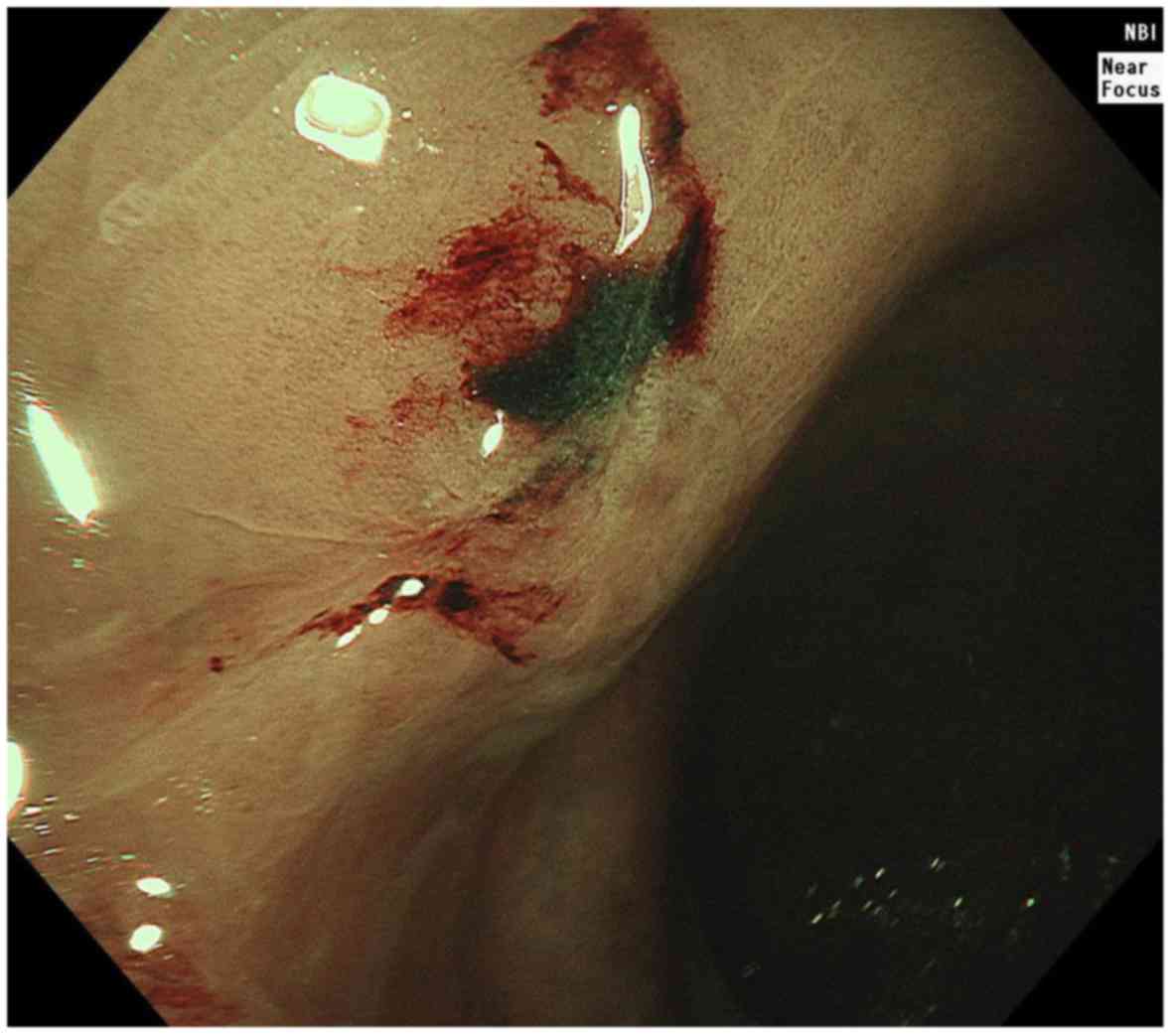
transitional procedure. A colonoscopy (Fig. 2) performed 26 months after the
radical EMR revealed a submucosal tumor (SMT) near the EMR scar in
the rectum. The patient was referred for treatment according to the
recommendations of our hospital in December, 2016.
Hematological examination revealed mild anemia. The
tumor marker levels had been normal since the initial EMR
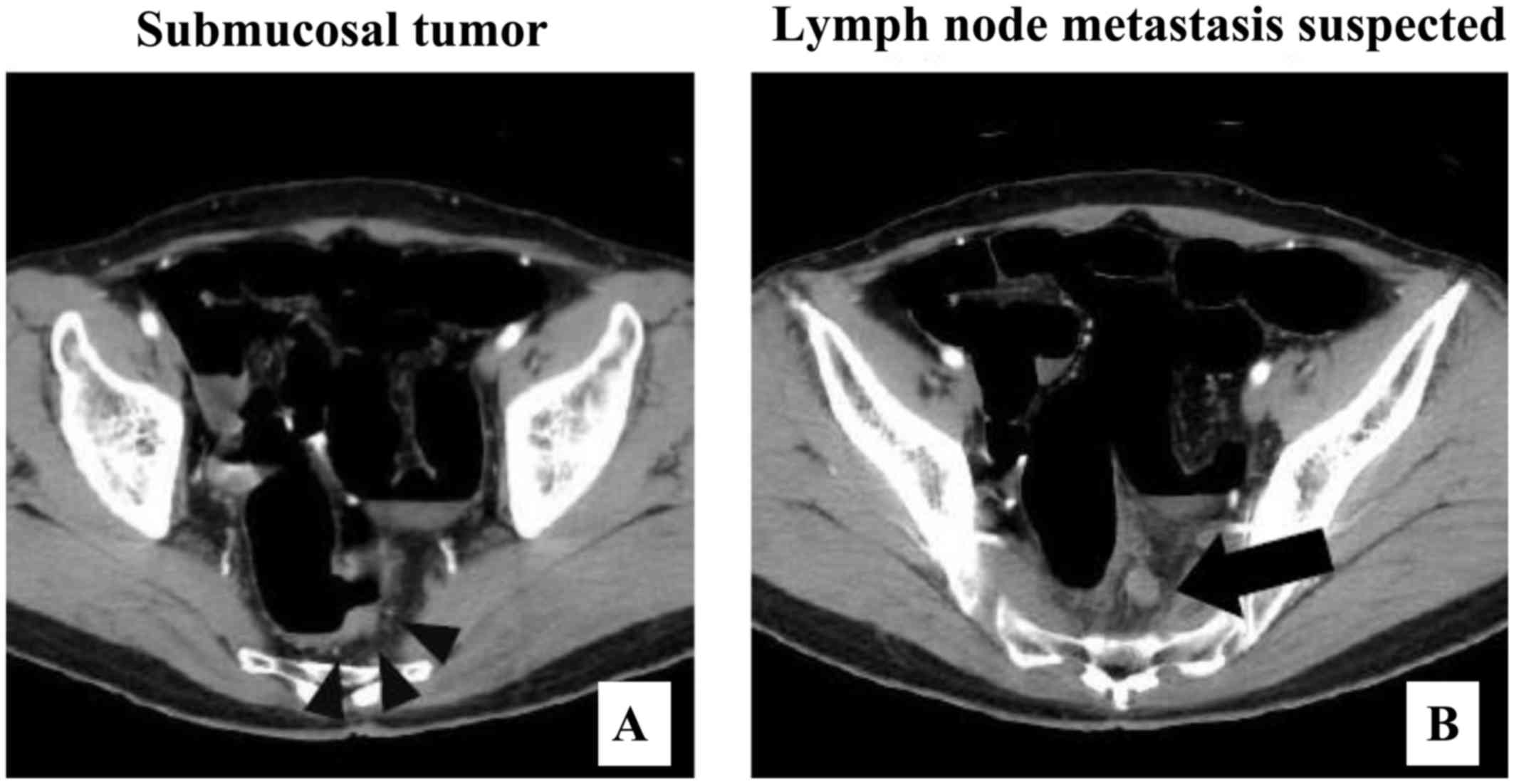
treatment. An abdominal enhanced computed tomography scan (Fig. 3A and B) revealed infiltration of the
thickness of the left wall of the Ra portion of the rectum with
limited extramural extension, and a lymph node 10 mm in diameter.
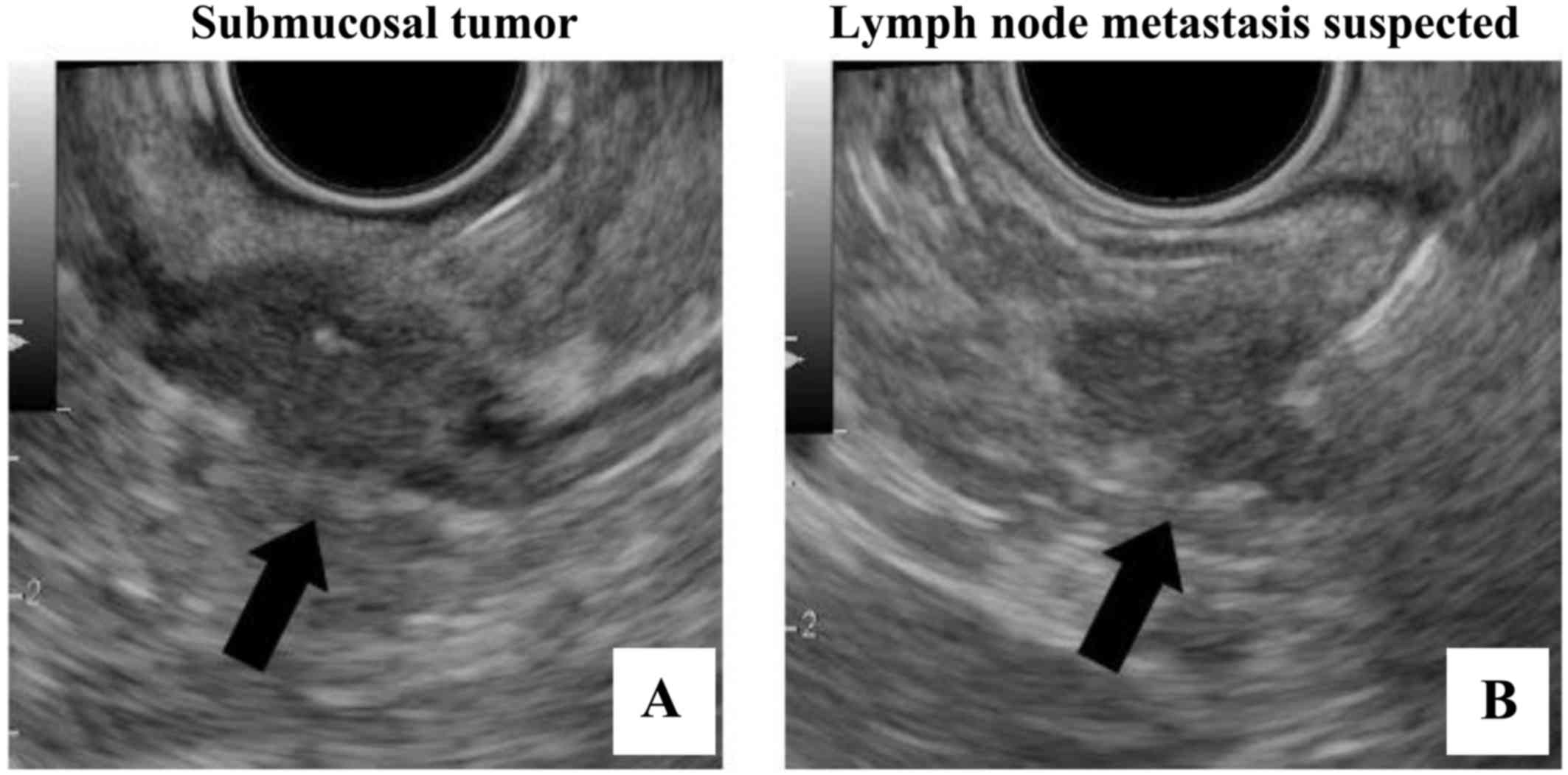
Endoscopic ultrasound-guided fine-needle aspiration (Fig. 4A and B) also revealed an SMT on the
left side of the Ra portion of the rectum that extended from the
submucosal layer beyond the serosal layer, and a lymph node sized
17×11 mm to the left of the Ra portion near the oral side 2 cm from
the SMT. The pathological findings confirmed the SMT as
adenocarcinoma with a metastatic lymph node. Local recurrence and
lymph node recurrence of rectal cancer following radical EMR was
diagnosed, as the SMT and lymph node were in proximity to the EMR
scar and the pathological findings revealed adenocarcinoma in both
the SMT and the lymph node. Laparoscopic ultra-low anterior
resection, D3 lymph node dissection and a diverting ileostomy were
performed. The operative time was 3 h and 41 min, and the blood
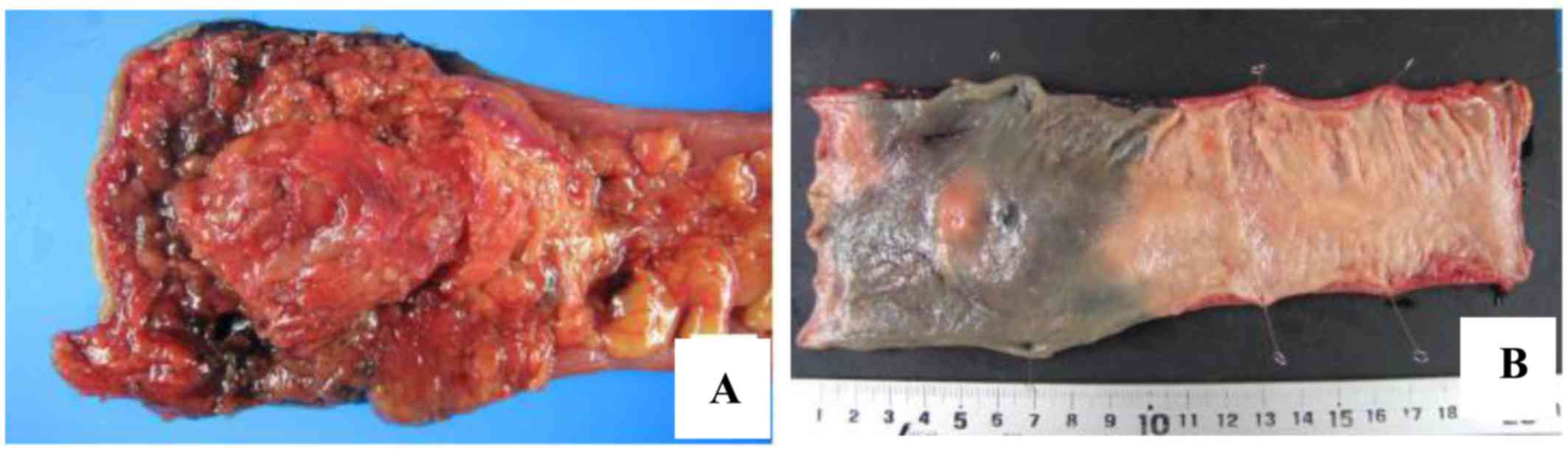
loss was 5 ml. The resected specimens (Fig. 5A and B) included an SMT lesion at the
Ra portion of the rectum, and the periphery of the tumor exhibited
a hard consistency from the submucosal layer extending beyond the
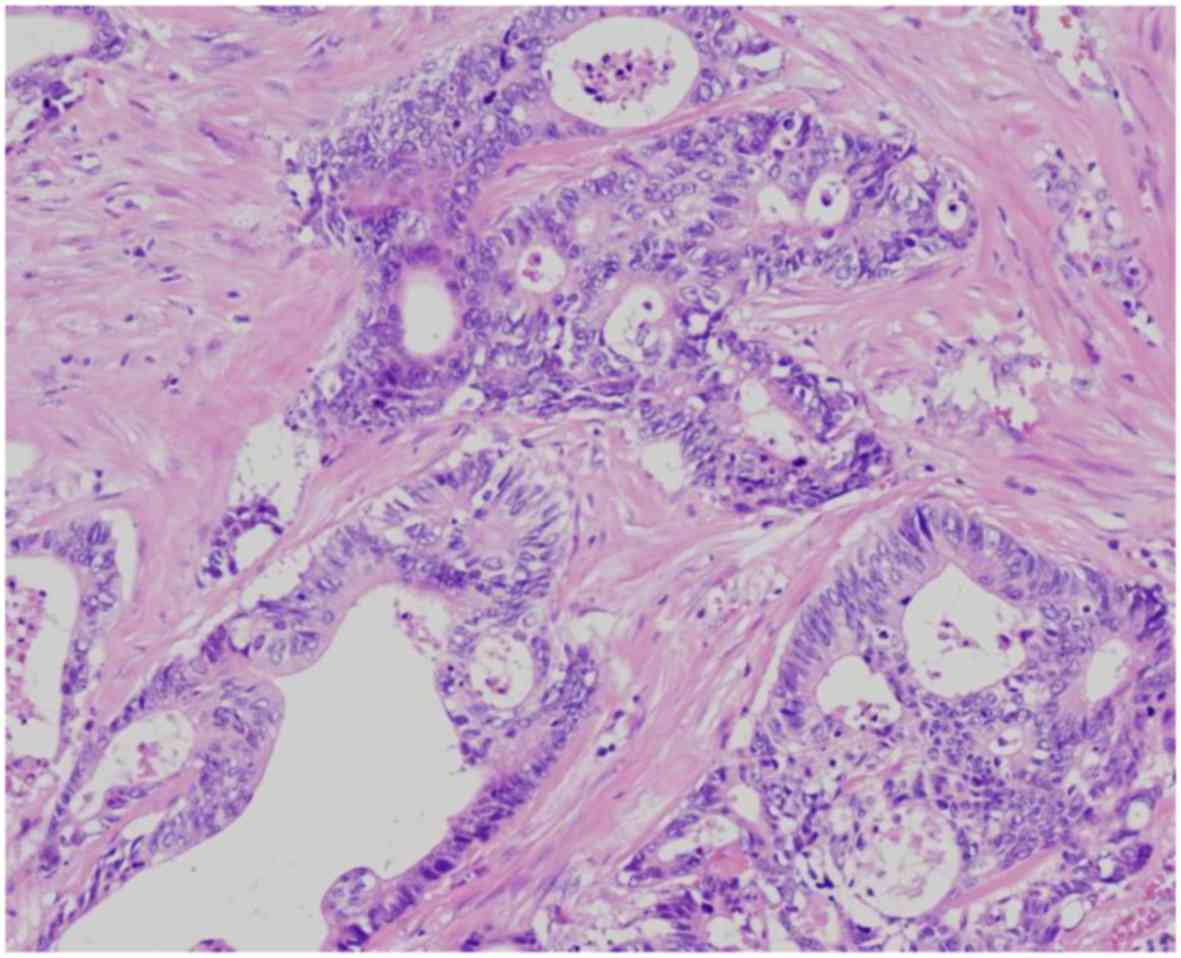
serosal layer. The pathological findings (Fig. 6) were T3 (A/SS), ly0, v3, PN1b, pPM0,
pDM0, pRM0 (100 µm) and pN0 (0/15). XELOX therapy was administered
for 6 months as adjuvant chemotherapy, and this was followed by
chest and abdominal enhanced computed tomography scans every 6
months. The final follow-up appointment was performed during
December 2017, and there has been no recurrence in the first 12
months after surgery.
Discussion
In recent years, EMR has been indicated for the
treatment of superficial, early-stage colorectal cancer due to its
minimal invasiveness and excellent results in terms of clinical
outcome. The decision on the use of endoscopic resection is made in
accordance with the JSCCR guidelines (1). Endoscopic resection is recommended in
tumors with little possibility of lymph node metastasis and in
tumors with a size and site (M or SM of <1,000 µm infiltration
degree) permitting excision in bulk. The size and the visual system
used to assess the tumor is not considered in the use of endoscopic
resection. In addition, the criteria for additional resection of
pT1 (SM) colon cancer that has already been resected (3) are also being investigated. It is
desirable to add surgical resection in cases of a positive vertical
stump. Furthermore, following histological examination of the
obtained specimens, if the pathological findings reveal i) SM
infiltration degree of ≥1,000 µm; ii) positive lymphovascular
invasion; iii) poorly differentiated adenocarcinoma, signet ring
cell carcinoma, or mucinous adenocarcinoma; or iv) conglobate
(budding) lesion grade 2/3 in cases of advanced infiltration,
intestinal resection with lymph node dissection should be
considered as an additional treatment. These criteria were applied
to the 0-IIa lesion in the Ra portion of the rectum in the present
case. The pathological findings at the time of EMR were
tub1>tub2, the depth of invasion was SM1 (420 µm), lymphatic
invasion and venous invasion on hematoxylin and eosin
(H&E)-stained sections were both negative, and the resection
margin was also negative. The tumor was resected in bulk. For these
reasons, the JSCCR guidelines were followed, but local recurrence
was discovered at 26 months after radical EMR. The pathological
findings of the initial treating physician at our hospital were
reviewed. Although the physician had based the diagnosis on H&E
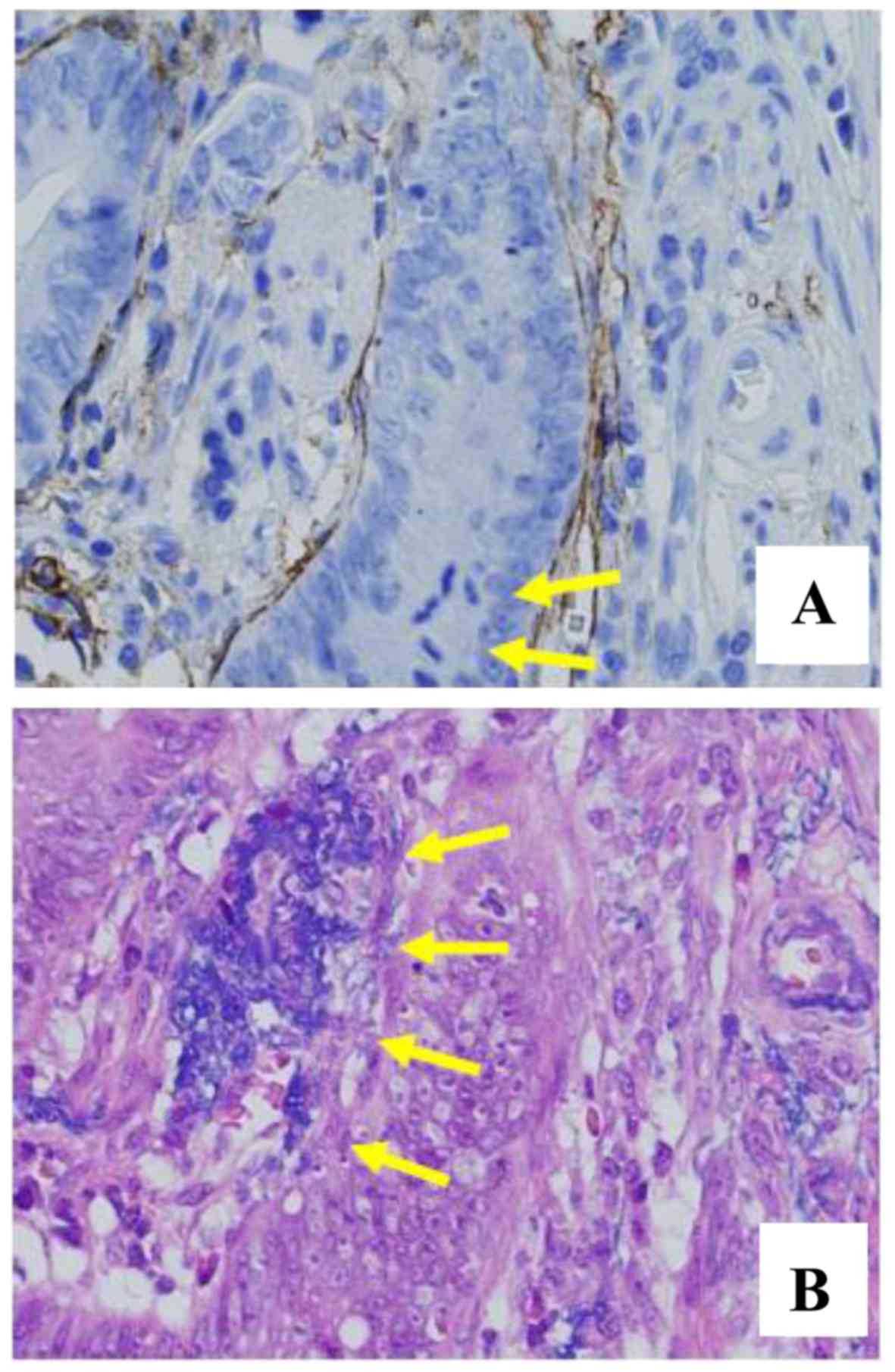
staining alone, the presence of lymphatic invasion and venous
invasion was diagnosed based on D2-40 staining and Victoria blue
(VB)-H&E staining (Fig. 7A and
B) and the recurrence was diagnosed as ly1 and v1. Therefore,
the accurate preoperative diagnosis was tub1>tub2, T1a (SM1, 420
µm), ly1 (D2-40) and v1 (VB-H&E).
Cases of local recurrence following tumor removal by
an endoscopic curative procedure according to the JSCCR guidelines
are extremely rare. This is a valuable case: It is the 3rd reported
case, to our knowledge, of local recurrence of T1a rectal cancer
following radical EMR in Japan, with the other two cases reported
by Tanaka et al (4) and
Kuroda et al (5). In
addition, the case reported by Tanaka et al (4) suggested that, if the mucosal muscle
layer is disrupted by the diffuse spread of cancer tissue, the risk
of metastasis is high. The case reported by Kuroda et al
(5) was considered on a
representative level, and the total percentage of cancer in the
specimens was not examined. If the infiltrated portion is somewhat
close to the resection margin, it may be possible for cross-end
positivity or lymphovascular invasion positivity to be diagnosed if
other sections of the total sample are considered.
The usefulness and limitations of using special
staining methods, such as D2-40 and VB-H&E, to determine
lymphovascular invasion has been frequently reported in colon
cancer. Lymphovascular invasion is the most important risk factor
of metastasis (6), but diagnosis may
be subjective depending on the pathologist (7). Matsubara et al (8) reported that reproducibility of the
diagnosis of lymphovascular invasion was problematic in specimens
stained with H&E, and it is better to consider the use of
special staining methods to improve the diagnostic accuracy. Nikami
et al (9) reported on the
results of special staining in 139 lesions in patients with SM
cancer, and found that the diagnosis of lymph node metastasis by
evaluating lymphovascular invasion based on H&E staining
exhibited a sensitivity of 75.0% and a specificity of 50.3%,
whereas the use of special staining exhibited a sensitivity of
93.8%, a specificity of 46.0%, a positive predictive value of 12.9%
and a negative predictive value of 98.9%. Thus, the improved
sensitivity of special staining may be meaningful, as the absence
of lymph node metastasis may be considered almost certain if the
specimen is negative for lymphovascular invasion.
In summary, we herein reported an extremely rare
case of cancer recurrence 26 months after the removal of a rectal
tumor by EMR performed according to the JSCCR guidelines. The use
of H&E staining alone may make it difficult to diagnose
lymphovascular invasion; therefore, the addition of special
staining must be considered when the extent of lymphovascular
invasion is unclear on H&E staining.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NM and KM conceived and designed the study. NM, KM,
TaT, ToT, SM, TS, JT, TI, HI, YT, KYa and KYo acquired the data.
NM, KM, TaT, NS and TM analyzed and interpreted the data. NM and KM
drafted the manuscript. NM, KM, TaT and KYo critically revised the
manuscript for important intellectual content. KYo supervised the
study and acquired the data. All the authors have read and approved
the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case details and accompanying
images.
Competing interests
KYo has received grants, personal fees and
non-financial support from Chugai Pharmaceutical Co., Ltd., during
the care of the reported patient; grants and personal fees from
Taiho Pharmaceutical Co., Ltd., Pfizer Inc., Yakult Honsha Co.,
Ltd., and grants from Bristol-Myers Squibb and Kyowa Hakko Kirin
Co., Ltd., outside the submitted work; honoraria from Taiho
Pharmaceutical Co., Ltd., Pfizer Inc., Chugai Pharmaceutical Co.,
Ltd., Kyowa Hakko Kirin Co., Ltd., and Yakult Honsha Co., Ltd.; and
had a consultant or advisory relationship with Taiho Pharmaceutical
Co., Ltd. and La Roche, Ltd. TaT has received honoraria for
lectures from Takeda Pharmaceutical Co., Ltd. All remaining authors
declare that they have no competing interests to disclose.
References
|
1
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
Kanemitsu Y, et al Japanese Society for Cancer of the Colon and
Rectum, : Japanese Society for Cancer of the Colon and Rectum
(JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int
J Clin Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tamegai Y, Fukunaga Y, Chino A, Taniguchi
C, Suzuki S, Morishige K, et al: Progress of Endoscopic Treatment
for Early Colorectal Cancers. J Jpn Soc Coloproctol. 65:800–807.
2012. View Article : Google Scholar
|
|
3
|
Tajika M, Niwa Y, Bhatia V, Kondo S,
Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Ogura T, et al:
Comparison of endoscopic submucosal dissection and endoscopic
mucosal resection for large colorectal tumors. Eur J Gastroenterol
Hepatol. 23:1042–1049. 2011.PubMed/NCBI
|
|
4
|
Tanaka N, Igarashi M, Kobayashi K, Sano Y,
Saito Y, Yamamoto H, et al: Surveillance of colorectal cancer after
endoscopic treatment. Colon Dis. 2007:112–120. 2007.(In
Japanese).
|
|
5
|
Kuroda Y, Takayama Y, Matsumoto S, Teraji
T, Tahara K, Saeki K, et al: A resected case of local progressive
sigmoid colon carcinoma after radial endoscopic mucosal resection
for SM1 carcinoma. Clin Res. 90:127–305. 2013.(In Japanese).
|
|
6
|
Kobayashi H, Ikegami M, Mitobe J and
Urashima M: Risk Factor for Lymph Node Metastasis of Colorectal
Carcinomas with Submucosal Invasion. Tokyo Jikeikai Med J.
124:113–126. 2009.
|
|
7
|
Taniguchi H, Fukazawa Y, Sekine S, Shimoda
T and Kushima R: Vascular Infiltration of Submucosal Invasive
Colorectal Cancer. Stomach Intestine Tokyo. 44:1241–1248. 2009.
|
|
8
|
Matsubara A, Kushima R, Taniguchi H and
Sekine S: Special Stains Useful for Diagnosis of Colon Tumors.
Stomach Intestine Tokyo. 45:699–704. 2010.
|
|
9
|
Nikami T, Saito S, Ishii H, Kobayashi H,
Mitobe J, Aihara H, et al: Risk Factors for Lymph Node Metastasis
of Submucosal Invasive Colon Cancer-Emphasis on Detection of Vessel
Permeation Using Special Stains. Stomach Intestine Tokyo.
46:1459–1468. 2011.
|