Introduction
Capecitabine is a fluorinated pyrimidine anticancer
drug demonstrated to be non-inferior to 5-fluorouracil/leucovorin
treatment in the X-ACT study (1).
Capecitabine is also considered a standard postoperative adjuvant
chemotherapy for colorectal cancer, according to the Colorectal
Cancer Treatment Guidelines (2).
However, hand-foot syndrome (HFS) is a frequent adverse reaction to
capecitabine, and the underlying mechanisms of the onset of HFS are
unclear. Histologically, the inhibition of the proliferation of
epidermal basal cells and drug secretion from exocrine sweat glands
are suspected mechanisms of the onset of HFS (3). Major symptoms of HFS are erythema,
dysesthesia, pain, skin cracks, and desquamation of the palms and
soles of the feet (4). The clinical
manifestations of HFS are reversible and non-life-threatening, but
they often markedly affect a patient's quality of life. Because
these manifestations necessitate a modification or discontinuation
of treatment, a greater understanding of their prevention and
treatment is necessary. Currently, there are no established
techniques to prevent or treat HFS, and research has pointed to the
benefits of symptomatic treatment with urea ointments,
moisturizers, topical steroids, or by wearing gloves at night to
retain moisture (5–9). In light of these recommendations,
medical personnel typically use brochures or manuals prepared by
pharmaceutical companies to actively inform patients taking
capecitabine of the various items they can take to prevent and
treat HFS. These efforts and approaches have been described at
relevant academic conferences (10,11).
However, medical personnel cannot ascertain the extent to which
patients have (or have not) taken the items to prevent and treat
HFS. Thus, we hypothesized that ascertaining the extent to which
patients have (or have not) taken these items in a cross-sectional
study may lead to more efficient patient interventions. Improved
forms of intervention by medical personnel based on the current
results should reduce the incidence of HFS through the qualitative
improvement of patient management, and improve the effectiveness of
treatment in the event that HFS develops.
Accordingly, the aim of the current study was to
ascertain the relationship between the extent to which patients
have taken (or not taken) the items to prevent and treat HFS and
the development of HFS.
Subjects and methods
Subjects
Subjects consisted of 90 patients who underwent at
least one course of treatment with a drug regimen that included
capecitabine. Patients were treated at one of four facilities
(Kanagawa Prefectural Keiyukai Keiyu Hospital, Showa University
Northern Yokohama Hospital, Yokohama Rosai Hospital or Fujisawa
City Hospital) from July 2015 to January 2017.
Potential subjects were given a detailed explanation
of the study. The included patients voluntarily consented to
participate in this study and were ≥20 years of age when they
provided consent. Individuals were excluded if they were deemed
unable to provide informed consent or ineligible for other
reasons.
Methodology and items studied
The present study was conducted only one time per
patient using patient survey and pharmacist survey forms. The main
items studied were the extent to which items to prevent and treat
HFS were (or were not) taken, the relationship between the number
of items taken to prevent and treat HFS and the development of
symptoms of HFS, and the differences between the patients'
subjective symptoms of HFS and those assessed by a pharmacist using
the Common Terminology Criteria for Adverse Events (CTCAE) Version
4.0. If HFS was grade 0, the patient was deemed to not have
developed HFS; if HFS was grade 1 or 2, the patient was deemed to
have developed HFS. The items that were taken for preventing and
treating HFS were routine items listed in the HFS Atlas (Chugai
Pharmaceutical Co. Ltd, Tokyo, Japan) (12), which is a brochure prepared by a
pharmaceutical company. This brochure is widely disseminated
domestically, and it is cited in the HFS volume of the Manuals for
Management of Serious Adverse Drug Reactions issued by the Ministry
of Health, Labour and Welfare of Japan. There are no quantitative
criteria for each routine item; hence, the extent to which an item
has been taken is subjectively rated by the patient.
Statistical analysis and ethical
considerations
The responses to the individual survey items were
simply tallied, and the factors affecting the presence or absence
of HFS symptoms were examined using univariate analysis
(significance level: 5%). In order to examine the relationship
between the extent to which items to prevent and treat HFS were (or
were not) taken and the development of symptoms of HFS, the
Wilcoxon rank-sum test was performed with ‘the presence or absence
of symptoms of HFS as determined by a pharmacist’ serving as the
dependent variable and ‘the number of items (out of a 15 total) in
the HFS Atlas (12) that were (or
were not) taken to prevent and treat HFS’ serving as the
explanatory variable. To examine the relationship between the
individual items taken to prevent and treat HFS and the development
of symptoms of HFS, Pearson's chi-squared test or Fisher's exact
test was performed with ‘the presence or absence of symptoms of HFS
as determined by a pharmacist’ serving as the dependent variable
and ‘the number of individual items taken (out of a 15 total in the
HFS Atlas)’ (12) serving as the
explanatory variable. In order to examine the differences between a
patient's subjective symptoms of HFS and the assessment of HFS by a
pharmacist, Fisher's exact test was performed with ‘the presence or
absence of symptoms of HFS as determined by a pharmacist’ serving
as the dependent variable and ‘the patient's symptoms of HFS’
serving as the explanatory variable.
The statistical software JMP® 12.2 (SAS
Institute, Cary, NC, USA) was used for analysis. This study was
ethically reviewed and approved by the Ethics Committee of Keiyu
Hospital (Yokohama, Japan), Showa University Northern Yokohama
Hospital (Yokohama, Japan), Yokohama Rosai Hospital (Yokohama,
Japan), Fujisawa City Hospital (Fujisawa, Japan), and Hoshi
University (Tokyo, Japan).
Results
Patient characteristics
Subjects consisted of 90 patients; 54 males and 36
females. Patients had a median age of 66 years, and the median
cumulative dose of capecitabine administered was 105,000 mg. The
patient characteristics are shown in Table I.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristics | Patients (n =90) | % |
|---|
| Sex |
|
|
| Male | 54 | 60.0 |
|
Female | 36 | 40.0 |
| Age (years) |
|
|
|
Median | 66 |
|
|
Range | 36–83 |
|
| Body surface area
(m2) |
|
|
|
Median | 1.61 |
|
|
Range | 1.16–2.09 |
|
| Primary tumor site
and therapy regimen |
|
|
|
Colorectal | 79 | 87.8 |
|
capecitabine, oxaliplatin | 46 |
|
|
capecitabine, oxaliplatin,
bevacizumab | 25 |
|
|
capecitabine, bevacizumab | 5 |
|
|
capecitabine, irinotecan,
bevacizumab | 3 |
|
|
Stomach | 10 | 11.1 |
|
capecitabine, oxaliplatin | 6 |
|
|
capecitabine, cisplatin,
herceptin | 2 |
|
|
capecitabine, herceptin | 1 |
|
|
capecitabine, oxaliplatin,
herceptin | 1 |
|
|
Breast | 1 | 1.1 |
|
capecitabine | 1 |
|
| Capecitabine
cumulative dose (mg) |
|
|
|
Median | 105,000 |
|
|
Range | 25,200-1,764,000 |
|
| Survey date
(days)a |
|
|
|
Median | 85 |
|
|
Range | 14–988 |
|
| Type of
treatment |
|
|
| Adjuvant
therapy | 46 | 51.1 |
|
Palliative therapy | 44 | 48.9 |
Extent to which items to prevent and
treat HFS were (or were not) taken
Of the 90 patients, 78 (88.9%) were prophylactically
prescribed an ointment for HFS. All 78 patients were prescribed an
ointment containing heparin or an analogue. Two of the 78 patients
also used an ointment containing urea. Of the 90 patients, 49 were
prescribed vitamin B6. The extent to which the 15 items in the HFS
Atlas (12) were (or were not)
followed is shown in Table II.
Eighty-five of the 90 patients had followed at least some of the 15
items in the HFS Atlas (12). The
items those 85 patients most often took were ‘applying a
moisturizer’ (74.1%), ‘keeping one's skin clean (e.g., washing
one's hands and feet)’ (64.7%), and ‘avoiding strenuous exercise
(e.g., long walks, jogging, or aerobics)’ (55.3%). The items that
the 85 patients least often took were ‘using sunscreen on exposed
areas’ (14.1%) and ‘using soft insoles’ (11.8%).
 | Table II.Association between the extent to
which 15 items in the HFS Atlas were (or were not) taken and the
development of symptoms of HFS. |
Table II.
Association between the extent to
which 15 items in the HFS Atlas were (or were not) taken and the
development of symptoms of HFS.
|
| HFS (pharmacist's
assessment) |
|---|
|
|
|
|---|
| Items | + (n=60) | − (n=25) | Total (%) | Odds ratio | 95% CI | P-value |
|---|
| Applying a
moisturizer |
|
|
| 0.63 | 0.20–1.96 | 0.424a |
| + | 43 | 20 | 63 (74.1) |
|
|
|
| − | 17 | 5 | 22 |
|
|
|
| Keeping one's skin
clean, e.g., washing one's hands and feet |
|
|
| 0.47 | 0.17–1.36 | 0.160a |
| + | 36 | 19 | 55 (64.7) |
|
|
|
| − | 24 | 6 | 30 |
|
|
|
| Avoiding strenuous
exercise (e.g., long walks, jogging or aerobics) |
|
|
| 0.47 | 0.18–1.26 | 0.128a |
| + | 30 | 17 | 47 (55.3) |
|
|
|
| − | 30 | 8 | 38 |
|
|
|
| Avoiding activities
that place pressure on the fingers or palms (e.g., acupressure and
writing for long periods) |
|
|
| 0.36 | 0.14–0.96 | 0.038a,b |
| + | 26 | 17 | 43 (50.6) |
|
|
|
| − | 34 | 8 | 42 |
|
|
|
| Wearing soft,
comfortable footwear |
|
|
| 0.51 | 0.20–1.32 | 0.161a |
| + | 26 | 15 | 41 (48.2) |
|
|
|
| − | 34 | 10 | 44 |
|
|
|
| Not wearing
tight-fitting footwear or socks |
|
|
| 0.29 | 0.11–0.79 | 0.013 a,b |
| + | 23 | 17 | 40
(47.1) |
|
|
|
| − | 37 | 8 | 45 |
|
|
|
| Refraining from
taking hot baths or showers |
|
|
| 0.35 | 0.13–0.92 | 0.031a,b |
| + | 23 | 16 | 39 (45.9) |
|
|
|
| − | 37 | 9 | 46 |
|
|
|
| Avoiding wearing
high-heeled shoes since they concentrate pressure on the toes |
|
|
| 0.62 | 0.24–1.57 | 0.309a |
| + | 24 | 13 | 37 (43.5) |
|
|
|
| − | 36 | 12 | 48 |
|
|
|
| Being mindful of
hot water when doing laundry by hand |
|
|
| 0.46 | 0.18–1.17 | 0.100a |
| + | 22 | 14 | 36 (42.4) |
|
|
|
| − | 38 | 11 | 49 |
|
|
|
| Avoid direct
sunlight (using an umbrella/hat/gloves/long sleeves) |
|
|
| 0.72 | 0.28–1.85 | 0.496a |
| + | 24 | 12 | 36 (42.4) |
|
|
|
| − | 36 | 13 | 49 |
|
|
|
| Refraining from
wringing out a wet cloth by hand |
|
|
| 0.78 | 0.27–2.26 | 0.649a |
| + | 14 | 7 | 21 (24.7) |
|
|
|
| − | 46 | 18 | 64 |
|
|
|
| Taking care to
gently hold a kitchen knife during use |
|
|
| 0.48 | 0.16–1.38 | 0.168a |
| + | 11 | 8 | 19 (22.4) |
|
|
|
| − | 49 | 17 | 66 |
|
|
|
| Wearing thick
cotton socks |
|
|
| 1.75 | 0.52–5.92 | 0.364a |
| + | 15 | 4 | 19 (22.4) |
|
|
|
| − | 45 | 21 | 66 |
|
|
|
| Using sunscreen on
exposed areas |
|
|
| 0.81 | 0.22–2.97 | 0.742c |
| + | 8 | 4 | 12 (14.1) |
|
|
|
| − | 52 | 21 | 73 |
|
|
|
| Using soft
insoles |
|
|
| 0.36 | 0.10–1.39 | 0.150c |
| + | 5 | 5 | 10 (11.8) |
|
|
|
| − | 55 | 20 | 75 |
|
|
|
Association between the number and
extent of items taken to prevent and treat HFS and the development
of HFS symptoms
The relationship between the number of items taken
to prevent and treat HFS and the development of HFS symptoms was
also examined. Our results indicated that patients who developed
symptoms of HFS had taken fewer items to prevent and treat HFS,
while patients who did not develop symptoms of HFS had taken more
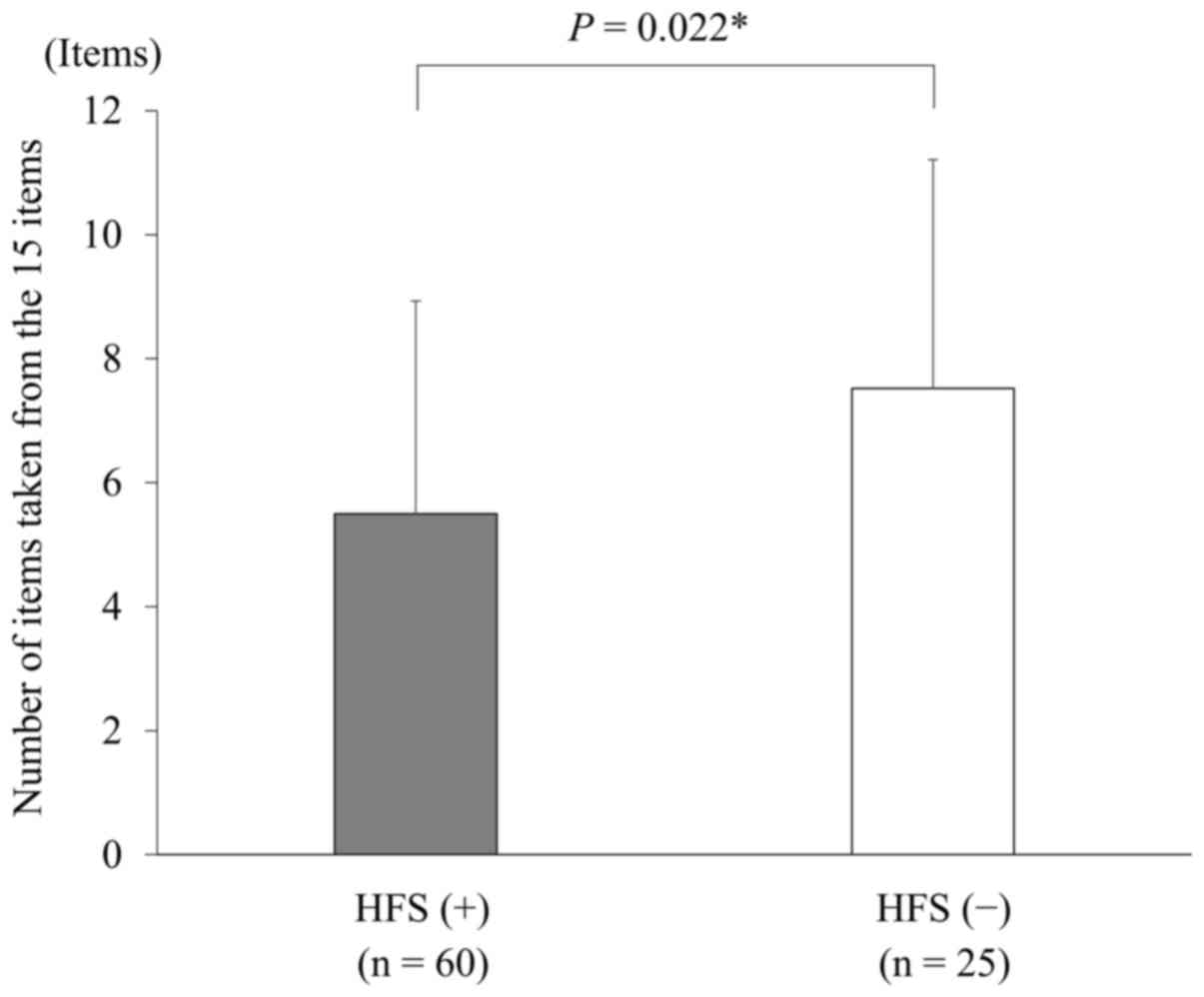
items to prevent and treat HFS (P=0.022) (Fig. 1). The relationship between the extent
to which individual items in the HFS Atlas (12) were (or were not) taken and the
development of symptoms of HFS was also examined. Our results
indicated that patients differed significantly in terms of three
items: ‘avoiding activities that place pressure on the fingers or
palms (e.g., acupressure and writing for long periods)’ [odds ratio
(OR)=0.36; 95% confidence interval (CI) = 0.14–0.96; P=0.038], ‘not
wearing tight-fitting footwear or socks’ (OR=0.29; 95%
CI=0.11–0.79; P=0.013), and ‘refraining from taking hot baths or
showers’ (OR=0.35; 95% CI=0.13–0.92; P=0.031). Patients who took
these three items had a significantly lower incidence of symptoms
of HFS (Table II).
Differences in a patient's subjective
symptoms of HFS and a pharmacist's assessment of HFS
The differences in the patient's subjective symptoms
of HFS and a pharmacist's assessment of HFS were examined. Results
indicated significant differences in ‘the presence or absence of
symptoms of HFS as determined by a pharmacist’ and ‘the patient's
subjective symptoms of HFS’ (P=0.0002). In 18.9% of patients, the
patient reported symptoms related to HFS, but the pharmacist
determined that the patient had no symptoms of HFS (Table III).
 | Table III.Differences in a patient's subjective
symptoms of HFS and a pharmacist's assessment of HFS. |
Table III.
Differences in a patient's subjective
symptoms of HFS and a pharmacist's assessment of HFS.
|
| HFS |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Patient's
subjective symptoms | + (n=63) | − (n=27) | Odds ratio | 95% CI | P-value |
|---|
|
|
|
| 11.76 | 2.91–47.62 | 0.0002a |
| + | 60 | 17 |
|
|
|
| − | 3 | 10 |
|
|
|
Discussion
The current study surveyed patients who were being
treated with a drug regimen that included capecitabine, and it
ascertained the extent to which items to prevent and treat HFS were
(or were not) taken. Results of the present study reveal that
patients who took more items for preventing and treating HFS (as
listed in the HFS Atlas) (12) had a
lower incidence of symptoms of HFS than patients who did not take
those items. There are no guaranteed items to prevent and treat
HFS, and definitive treatment of HFS is achieved via a respite from
the drug causing HFS (13). In the
event of a superficial disturbance of skin sensation, i.e., grade 1
HFS with no evident pain, a respite is presumably unnecessary.
Patients are typically instructed by medical personnel to modify
their routine, e.g., to avoid physical or thermal irritants,
protect the skin, prevent a secondary infection, and avoid direct
sunlight. The HFS Atlas (12) is a
manual prepared by the manufacturer/distributor of capecitabine
that broadly summarizes routine instructions to prevent and treat
HFS into 15 items. The current study examined the percentage of
items in the HFS Atlas (12) that
were (or were not) taken to prevent and treat HFS. Our results
revealed that the percentage of items taken differed widely from
11.8 to 74.1%.
The items that patients took most often were
‘applying a moisturizer’, ‘keeping one's skin clean, e.g., washing
one's hands and feet’ and ‘avoiding strenuous exercise (e.g., long
walks, jogging, or aerobics)’. The items that patients took least
often were ‘using sunscreen on exposed areas’ and ‘using soft
insoles’. The two major reasons why patients often applied a
moisturizer were because multiple medical personnel clearly
explained to patients that the moisturizer was prescribed and
because patients were aware of how to specifically use the
moisturizer. The rate at which patients took certain items was
presumably affected by differences in how easy those items were for
patients to take and in the manner in which medical personnel
(which included pharmacists) gave instructions.
The relationship between the number of items taken
to prevent and treat HFS and the development of symptoms of HFS was
also examined. Our results indicated that patients who had
developed symptoms of HFS had taken fewer items to prevent and
treat HFS, while patients who did not develop symptoms of HFS had
taken more items to prevent and treat HFS. In order to avoid the
exacerbation of HFS, HFS must be prevented or the capecitabine dose
must be reduced or halted in accordance with the symptoms. The
current study covered a wide range of regimens, so the relationship
between the development of HFS and a capecitabine respite or dose
reduction was not examined. When examining a dose reduction or
respite, a crucial aspect is whether numerous items have been taken
to prevent HFS. The current study examined the relationship between
the extent to which individual items in the HFS Atlas (12) were (or were not) taken and the
development of symptoms of HFS.
Our results indicated that patients differed
significantly in terms of three important items: ‘avoiding
activities that place pressure on the fingers or palms (e.g.,
acupressure and writing for long periods)’ (the item taken fourth
most often), ‘not wearing tight-fitting footwear or socks’ (the
item taken sixth most often), and ‘refraining from taking hot baths
or showers’ (the item taken seventh most often). Items that
differed significantly included instructions to avoid external
irritants such as physical and thermal irritants. Regarding other
instructions, such as protecting the skin, preventing secondary
infection, and avoiding direct sunlight, there were no significant
differences in the development of HFS. Research has indicated that
the hands are constantly exposed to external irritants and that
symptoms are readily exacerbated in areas of the feet where
pressure is concentrated or where shoes cause friction. These
findings corroborate the importance of keeping the hands and feet
still (14). The item that patients
most often took to prevent and treat HFS was ‘applying a
moisturizer’. The application of an ointment containing heparin or
an analogue, and urea cream or ointment, an ointment containing
vitamin A, or petroleum jelly has been recommended, but the current
results revealed no significant differences between whether these
recommendations were followed and the development of HFS were
observed.
The differences in a patient's subjective symptoms
of HFS and a pharmacist's assessment of HFS were also examined. Our
results indicated that ~20% of the patients reported symptoms of
HFS even though a pharmacist found no such symptoms. Based on this
finding, patients may notice symptoms of HFS earlier than the
assessment with CTCAE Version 4.0 by medical personnel (including
pharmacists) indicates. This reveals the need for improved
assessment criteria as well as a more concerted effort to obtain
detailed information from patients.
The current study has several limitations. First,
this study was a cross-sectional survey; therefore, the cumulative
dose of capecitabine was not uniform at the time of the study. This
calls into question the reliability of the cumulative dose of
capecitabine at which symptoms of HFS developed or did not develop.
HFS due to capecitabine is considered to result from a cumulative
toxicity. One study reported that a median cumulative dose of
capecitabine of 56,000 mg/m2 results in an incidence of
symptoms of grade 1 HFS of 80% or higher, while another study
reported that a median cumulative dose of capecitabine of 100,000
or 200,000 mg/m2 results in an incidence of symptoms of
grade 1 HFS of 90% or higher (15).
When a study is conducted early on in treatment, assessing whether
items to treat and prevent HFS are effective or not is difficult,
as is determining whether symptoms of HFS have yet to develop. This
is true regardless of whether or not items to treat and prevent HFS
are actually effective. Second, the risk of developing HFS will
presumably differ as a result of differences in patient
characteristics, and the current analysis did not take that into
account. Studies have reported that the elderly, patients with
renal dysfunction, and patients with anemia have a high incidence
of grade 2 or more severe HFS (15).
Studies have also reported that diabetes mellitus, concomitant
administration of bevacizumab and capecitabine, history of
receiving a fluoropyrimidine drug, and early onset of symptoms
(within 21 days) are potential risk factors for the development of
HFS (14). Third, the number of
patients analyzed was as small as 90 patients. Our sample size was
the number of patients recruited at 4 facilities (key hospitals for
cancer care). The sample size needs to be increased, and the
current findings need to be verified in future studies. In light of
these points, the current finding of a relationship between the
extent to which items to prevent and treat HFS were (or were not)
taken and the development of symptoms of HFS is not generalizable.
However, the current study is significant in the sense that a
cross-sectional survey conducted at multiple facilities revealed a
relationship between the rates at which patient education to
prevent HFS was implemented in routine practice and the development
of HFS. The novelty of this study is not only from the drugs but
also from other 15 aspects including patient behaviors, which
suggests the importance of the management is patient's behavior
which patients tend to ignore.
In the present study, medical personnel specifically
checked which items were taken by patients. Based on our findings,
instructions emphasizing that patients take all necessary items to
prevent and treat HFS and that they avoid external irritants, which
are highly likely to influence the development of symptoms of HFS,
lead to a qualitative improvement in the management of HFS.
Therefore, our findings are highly applicable in clinical
settings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets during and/or analyzed during the
current study available from the corresponding author on reasonable
request.
Authors' contributions
SS contributed to data collection and
interpretation, and wrote the initial draft of the manuscript. SN,
YI, DS, JK and DI contributed to data collection and
interpretation, and critically reviewed the manuscript. KT and KI
contributed to analysis and interpretation of data, and assisted in
the preparation of the manuscript. FS and HS contributed to
management of data, and critically reviewed the manuscript. TY
designed the study, and contributed to analysis and interpretation
of data, and assisted in the preparation of the manuscript. All
authors prepared the survey sheet. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was ethically reviewed and approved by
the Ethics Committee of Keiyu Hospital, Showa University Northern
Yokohama Hospital, Yokohama Rosai Hospital, Fujisawa City Hospital,
and Hoshi University. Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
Guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsuboi H, Yonemoto K and Katsuoka K: A
case of bleomycin-induced acral erythema (AE) with eccrine squamous
syringometaplasia (ESS) and summary of reports of AE with ESS in
the literature. J Dermatol. 32:921–925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikolaou V, Syrigos K and Saif MW:
Incidence and implications of chemotherapy related hand-foot
syndrome. Expert Opin Drug Saf. 15:1625–1633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mortimer JE, Lauman MK, Tan B, Dempsey CL,
Shillington AC and Hutchins KS: Pyridoxine treatment and prevention
of hand-and-foot syndrome in patients receiving capecitabine. J
Oncol Pharm Pract. 9:161–166. 2003. View Article : Google Scholar
|
|
6
|
Lassere Y and Hoff P: Management of
hand-foot syndrome in patients treated with capecitabine (Xeloda).
Eur J Oncol Nurs. 8:S31–S40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gressett SM, Stanford BL and Hardwicke F:
Management of hand-foot syndrome induced by capecitabine. J Oncol
Pharm Pract. 12:131–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagore E, Insa A and Sanmartín O:
Antineoplastic therapy-induced palmar plantar erythrodysesthesia
(‘hand-foot’) syndrome. Incidence, recognition and management. Am J
Clin Dermatol. 1:225–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakane M: Neurotoxicity and Dermatologic
Toxicity of Cancer Chemotherapy. Jpn J Cancer Chemother. 33:29–33.
2006.
|
|
10
|
Mae Y, Yokokawa T, Kawakami K, Yagi N,
Suenaga M, Shinozaki E, Matsusaka S, Mizunuma N and Hama T:
Usefulness of Pharmaceutical Outpatient Clinic in XELOX Therapy.
Jpn J Pharm Health Care Sci. 37:611–615. 2011. View Article : Google Scholar
|
|
11
|
Morimoto E and Inoue N: Current state of
nursing care for prevention of hand-foot-syndrome from
capecitabine, from the study for certified nurse in cancer
chemotherapy nursing in Japan. J Jpn Soc Cancer Nurs. 28:30–36.
2014.
|
|
12
|
Chugai Pharmaceutical Co. Ltd; Hand-Foot
Syndrome Atlas. 3rd edition. Tokyo: 2011
|
|
13
|
Yamazaki N and Taguchi T: Hand-foot
syndrome. J Clin Exp Med. 216:257–260. 2006.
|
|
14
|
Yokokawa T, Kawakami K, Mae Y, Sugita K,
Watanabe H, Suzuki K, Suenaga M, Mizunuma N, Yamaguchi T and Hama
T: Risk factors exacerbating hand-foot skin reaction induced by
capecitabine plus oxaliplatin with or without bevacizumab therapy.
Ann Pharmacother. 49:1120–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scheithauer W, McKendrick J, Begbie S,
Borner M, Burns WI, Burris HA, Cassidy J, Jodrell D, Koralewski P,
Levine EL, et al: Oral capecitabine as an alternative to i.v.
5-fluorouracil-based adjuvant therapy for colon cancer: safety
results of a randomized, phase III trial. Ann Oncol. 14:1735–1743.
2003. View Article : Google Scholar : PubMed/NCBI
|