Introduction
Lung cancer is the most common cause of
cancer-related death worldwide (1),
and the number of elderly patients aged 75 years or older with lung
cancer is increasing. Previous studies have demonstrated that the
incidence of chemotherapy-related adverse events is higher in
elderly patients with lung cancer than in non-elderly patients
(2,3). Discontinuing chemotherapy due to the
adverse events would shorten the prognosis of the elderly patients
with lung cancer. Thus, a chemotherapy drug with limited adverse
effects should be selected for such patients.
Kinase inhibitors are the standard first-line
treatment for elderly patients with advanced non-small-cell lung
cancer (NSCLC) and driver mutations. Moreover, a programmed death-1
antibody is considered in patients with high programmed
death-ligand 1 expression (tumor proportion score of ≥50%) and also
without driver mutation (4).
Meanwhile, in patients without these conditions, monotherapy using
third-generation chemotherapy drugs, such as docetaxel (DTX),
gemcitabine, and vinorelbine, is recommended (5–7).
S-1 is an oral fluoropyrimidine agent containing the
5-fluorouracil prodrug tegafur and 2 enzyme inhibitors, namely,
5-chloro-2, 4-dihydroxypyridine and potassium oxonate, which can
reduce the adverse effect of tegafur. S-1 is approved for patients
with gastric cancer in 7 Asian countries and 15 European countries.
It is also approved for patients with 8 type of cancers including
NSCLC in Japan. A phase-3 study compared the efficacy of S-1
monotherapy with that of DTX for NSCLC patients previously treated
with platinum-based chemotherapy. The results revealed the
non-inferiority of S-1 to DTX in terms of overall survival (OS)
(8). Therefore, S-1 monotherapy can
be considered for first-line chemotherapy of elderly patients with
NSCLC.
S-1 monotherapy has also showed lesser hematotoxic
adverse events than other third-generation chemotherapy drugs.
Among NSCLC patients, the incidence of febrile neutropenia and
grade 3 or higher neutropenia was lower in those administered with
S-1 than those who received DTX monotherapy (18.2% vs. 37.2%)
(8). Thus, S-1 can be safely
administered to elderly patients. However, digestive toxicity was
frequently observed (8,9). Diarrhea and oral mucositis have been
shown to occur more frequently in patients receiving S-1 than those
receiving DTX (23.9% vs. 14.5%) (8),
indicating that the digestive toxicity of S-1 should be reduced. As
such, several studies have been conducted to this end.
The standard regimen for S-1 monotherapy is 4 weeks
of continuous oral administration followed by a 2-week off period.
A clinical study comprising previously treated NSCLC patients who
underwent at least one chemotherapy regimen showed that the rates
of severe diarrhea and appetite loss were significantly lower in
the alternate-day administration than the standard S-1
administration (9.7% vs. 0% and 19.4% vs. 0%, respectively). By
contrast, no significant difference in the chemotherapeutic effect
for NSCLC was observed. The median progression-free survival (PFS)
for the alternate-day and standard administration groups was 2.1
vs. 2.7 months (log-rank test: P=0.49), and the median OS was 11
vs. 12 months (log-rank test: P=0.35) (10). In addition, alternate-day
administration of S-1 showed lesser adverse events than did the
standard administration in Japanese patients with unresectable
advanced pancreatic cancer in a multi-center, randomized, phase II
study (11).
In this study, we hypothesized that alternate-day
administration of S-1 to elderly patients aged 75 years or older
with previously treated advanced NSCLC will have fewer adverse
events than the standard administration. To investigate this
hypothesis, we conducted a multi-center prospective feasibility
study to evaluate the rate of adverse events in elderly patients
with NSCLC treated with alternate-day S-1 administration.
Materials and methods
Study design and eligibility
criteria
The present study was designed as a multi-center and
prospective feasibility study. We enrolled the patients from
Hiroshima Prefectural Hospital, Hiroshima City Asa Citizens'
Hospital, and Hiroshima University Hospital between January 2014
and January 2016 according to the following eligibility criteria:
Histologically or cytologically confirmed diagnosis of NSCLC; Stage
IIIB and IV disease according to the Union for International Cancer
Control TNM Classification of Malignant Tumors 7th edition
(12), an Eastern Cooperative
Oncology Group, performance status (ECOG PS) of 0–1, an age of ≥75
years, ability to take drugs orally, and an estimated life
expectancy of at least 3 months. The patients had received one or
two regimens of chemotherapy and two or three regimens if epidermal
growth factor receptor (EGFR) tyrosine kinase inhibitors were used.
Physical examinations within 14 days after the registration showed
all included patients had adequate organ functions according to the
following parameters: Leukocyte count ≥3,500/mm3;
neutrophil count ≥1,500/mm3; platelet count
≥100,000/mm3; hemoglobin ≥9.0 g/dl; total bilirubin ≤1.5
mg/dl; aspartate aminotransferase and alanine aminotransferase ≤2.5
× upper limit of normal; serum creatinine <1.5 mg/dl; and
creatinine clearance (CCr) ≥50 ml/min. Although the eligibility
criteria included stage IIIB and IV, incidentally only patients
with stage IV were enrolled in this study.
Patients were excluded if they had radiographically
confirmed interstitial pneumonia or pulmonary fibrosis, a massive
pleural or pericardial effusion or ascites requiring drainage,
active double cancer, severe complications, ileus, poorly
controlled diabetes, poorly controlled myocardial infraction within
6 months, symptomatic brain metastasis, concomitant treatment with
flucytosine, psychiatric disorder, previous severe drug allergy
(≥grade 3), previous treatment with fluoropyrimidine, pregnant or
possibly pregnant, active hepatitis B virus infection, and if the
physician concluded that the patient's participation in this trial
was inappropriate.
Treatment schedule
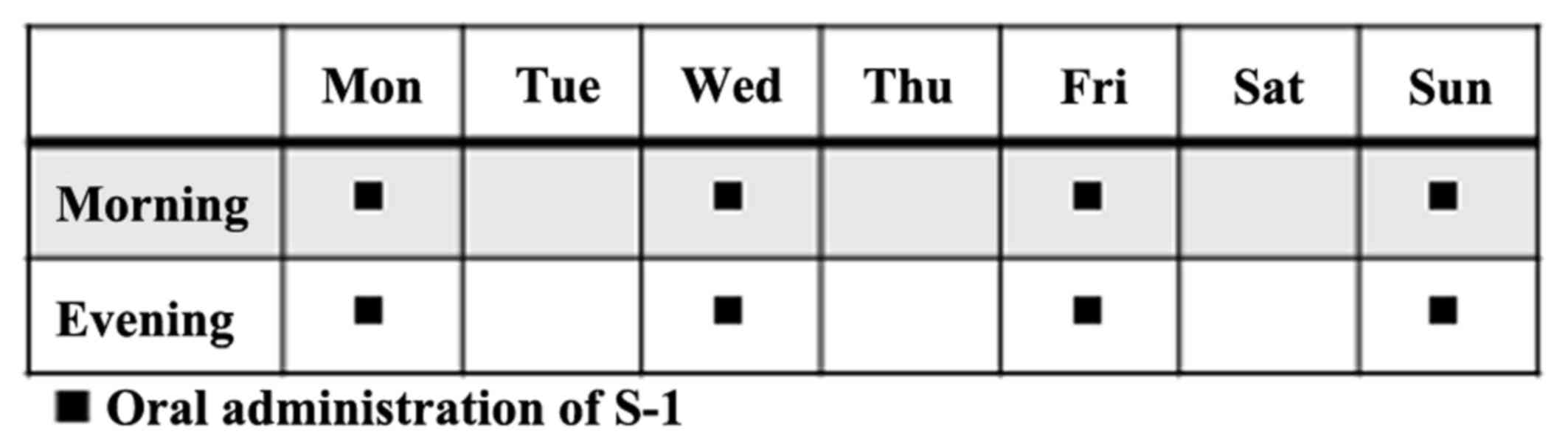
The patients received S-1 orally twice daily for 4
days (Monday, Wednesday, Friday, and Sunday) a week (Fig. 1). The dose of S-1 administered per
day was based on the patient's body surface area as follows:
<1.25 m2, 40×2 mg; 1.25–1.5 m2, 50×2 mg;
>1.5 m2, 60×2 mg. The regimen was continued until
progressive disease (PD) or unacceptable toxicity was observed. The
criteria of dose reduction were as follows: Leukocyte count
≤1,000/mm3; neutrophil count ≤500/mm3;
platelet count ≤25,000/mm3; total bilirubin ≥2.0 mg/dl;
serum creatinine ≥ upper limit of normal; and grade 3 or higher
non-hematologic toxicity. Meanwhile, the criteria to continue
administration were as follows: Leukocyte count
≥2,000/mm3; neutrophil count ≥1,000/mm3;
platelet count ≥50,000/mm3; total bilirubin ≤2.0 mg/dl;
CCr ≥50 ml/min and grade1 or lower diarrhea or stomatitis, grade 2
or lower other non-hematologic toxicity.
Evaluation of efficacy and
toxicity
Tumor response was evaluated every 8 weeks according
to the Response Evaluation Criteria in Solid Tumors guideline
version 1.1. PFS was defined as the period from enrollment until
the date of confirmation of PD or death as a result of any cause.
Toxicity was evaluated based on the National Cancer Institute
Common Terminology Criteria for Adverse Events, version 4.0
Japanese edition, Japan Clinical Oncology Group version (13). Disease control rate (DCR) was defined
as the percentage of patients who obtained complete response,
partial response, and stable disease (SD) from the treatment.
Study endpoints and statistical
analysis
The primary endpoint was safety and was evaluated by
calculating the proportion of ≥grade 3 adverse events. The
secondary endpoints were PFS, DCR, and 1-year survival rate. This
was a pilot feasibility study to estimate only the safety of
alternate-day administration, and the number of enrolled patients
was inadequate for evaluating the efficacy of such method of
administration. Kaplan-Meier method was used to draw the survival
curve. A P-value of <0.05 was considered significant. All data
analyses were performed using JMP PRO statistical software version
12.2.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 10 patients were enrolled, but 2 patients
failed to initiate the treatment protocol due to renal dysfunction
or intolerance of oral ingestion after enrollment. Thus, 8 patients
were observed until March 2017. Table
I shows the patients' background characteristics. The cohort
comprised 6 men and 2 women, and the median age was 79 years. Of
the 8 patients, 2 had a PS score of 0, while the other 6 had a PS
score of 1. All patients were diagnosed with Stage IV disease and
receiving second-line therapy. EGFR gene mutations and ALK gene
translocation were negative in all patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | n=8 |
|---|
| Sex |
|
| Male | 6 |
|
Female | 2 |
| Age, years |
|
| Range
(median) | 75–85 (79) |
| ECOG PS |
|
| 0 | 2 |
| 1 | 6 |
| Histology |
|
| Ad | 5 |
| Sq | 2 |
| NOS | 1 |
| Clinical stage |
|
| IIIB | 0 |
| IV | 8 |
| EGFR mutation |
|
|
Positive | 0 |
|
Negative | 8 |
| ALK
translocation |
|
|
Positive | 0 |
|
Negative | 8 |
| The number of prior
chemotherapy regimen |
|
| 1 | 8 |
| 2 | 0 |
| First-line
regimen |
|
|
CBDCA+PEM | 2 |
|
CBDCA+PTX | 2 |
| PEM (+
BEV) | 3 (2) |
| DTX | 1 |
Toxicity and treatment delivery
No grade 3 or higher adverse events were observed.
There were 4 cases of ≤grade 2 digestive symptoms such as anorexia,
diarrhea, or stomatitis, and grade 1 lacrimation was observed in 1
case. In addition, grade 2 renal dysfunction was observed in 2
cases, and ileus and elevated total bilirubin were observed in 1
case each (Table II).
 | Table II.Hematologic and non-hematologic
toxicities. |
Table II.
Hematologic and non-hematologic
toxicities.
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any (%) | Grade 3/4 |
|---|
| All | 4 | 5 | 0 | 0 |
| 0 |
| Hematologic |
|
|
|
|
|
|
|
Neutropenia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
|
Anemia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
|
Thrombocytopenia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
| Febrile
neutropenia | 0 | 0 | 0 | 0 | 0 (0) | 0 |
|
Non-hematologic |
|
|
|
|
|
|
|
Anorexia | 1 | 1 | 0 | 0 | 2 (25) | 0 |
|
Diarrhea | 1 | 0 | 0 | 0 | 1 (12.5) | 0 |
|
Stomatitis | 1 | 0 | 0 | 0 | 1 (12.5) | 0 |
|
Lacrimation | 1 | 0 | 0 | 0 | 1 (12.5) | 0 |
|
Ileus | 0 | 1 | 0 | 0 | 1 (12.5) | 0 |
|
Increased serum
creatinine | 0 | 2 | 0 | 0 | 2 (25) | 0 |
|
Increased total bilirubin | 0 | 1 | 0 | 0 | 1 (12.5) | 0 |
The S-1 administration period ranged from 0.7 to 1.5
months (median: 1.1 months). Treatment was suspended in 2 patients
due to grade 2 renal dysfunction. In 1 patient, treatment was also
suspended due to grade 2 ileus. The median duration of treatment
suspension in these 3 patients was 11 days. Table III shows a summary of the baseline
characteristics of these 3 patients.
 | Table III.Baseline characteristics of the
patients in whom S-1 administration was discontinued. |
Table III.
Baseline characteristics of the
patients in whom S-1 administration was discontinued.
| Case | Cause of
discontinuation | Sex | Age | ECOG PS | Histology | Clinical stage | 1st line
regimen | Ccr |
|---|
| 1 | Renal
dysfunction | Female | 84 | 1 | Ad | IV | PEM | 53 |
| 2 | Renal
dysfunction | Male | 81 | 1 | Ad | IV | PEM + BEV | 63 |
| 3 | Ileus | Male | 75 | 1 | Sq | IV | DTX | 82 |
Efficacy
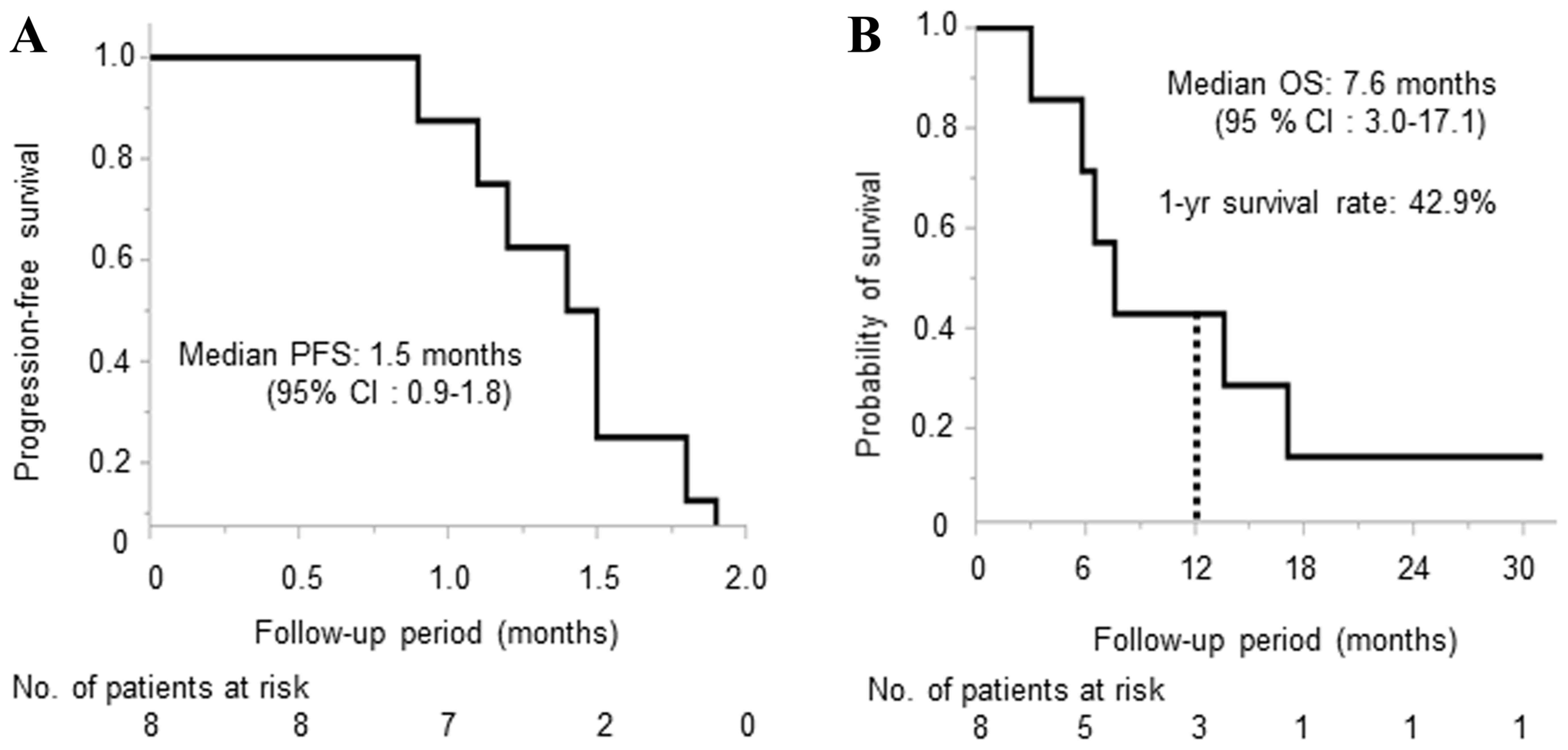
The median PFS and OS was 1.5 months (95% confidence
interval: 0.9–1.8) and 7.6 months (95% confidence interval:
3.0–17.1), respectively (Fig. 2),
and the 1-year survival rate was 42.9%. Regarding antitumor effect,
1 case reached stable disease, and 7 cases reached PD. DCR was
12.5% (Table IV). The individual
characteristics of the 8 patients including the treatment
administered after this study are shown in Table V.
 | Table IV.Overall response. |
Table IV.
Overall response.
| Tumor response | n=8 (%) |
|---|
| CR | 0 (0) |
| PR | 0 (0) |
| SD | 1 (12.5) |
| PD | 7 (87.5) |
| DCR (CR + PR +
SD) | 1 (12.5) |
 | Table V.Individual characteristics of the
patients (n=8). |
Table V.
Individual characteristics of the
patients (n=8).
| Patient number | Sex | Age (years) | ECOG PS | Histology | T | N | M | Overall
response | PFS (months) | Treatment after
S.1 | OS (months) |
|---|
| 1 | Female | 84 | 1 | Ad | 3 | 3 | 1a | SD | 1.2 | GEM→VNR→RT→DTX | 13.6 |
| 2 | Male | 75 | 1 | Ad | 1 | 0 | 1b | PD | 1.8 | PEM+BEV→DTX | 17.1 |
| 3 | Male | 81 | 1 | Ad | 4 | 3 | 1b | PD | 1.9 | DTX+BEV | 7.6 |
| 4 | Male | 75 | 1 | Ad | 1b | 0 | 1a | PD | 1.5 |
DXT→nab-PTX→VNR | 31.1 |
| 5 | Male | 82 | 0 | Sq | 4 | 1 | 1a | PD | 0.9 | WBRT | 5.8 |
| 6 | Male | 76 | 0 | Ad | 4 | 3 | 1b | PD | 1.1 | DTX→VNR→DTX | 6.5 |
| 7 | Male | 75 | 1 | Sq | 4 | 2 | 1a | PD | 1.4 | Erlotinib | 2.8 |
| 8 | Female | 85 | 1 | NOS | 2a | 3 | 1b | PD | 1.5 | RT | 3.0 |
Discussion
We conducted a multi-center and prospective
feasibility study to evaluate the safety of alternate-day S-1
therapy in elderly patients with NSCLC. In this study, no severe
grade 3 or higher adverse events was observed, and the major
adverse events were gastrointestinal toxicity, consistent with
previous reports (10).
The mechanism by which alternate-day S-1
administration reduces toxicity has been studied. Previous studies
showed differences in the cell cycle between normal and malignant
cells. Normal cells regenerate in 0.5–1.5 days, whereas cancer
cells regenerate in 3 to 5 days, with the S-phase lasting more than
24 h (14,15). Five-fluorouracil (5-FU), a metabolite
included in S-1, acts on S-phase cells and suppresses cell
proliferation. Based on this biological mechanism, the
alternate-day S-1 administration would allow growth and
reproduction of normal cells, while maintaining its anticancer
effect. Using gastric cancer cell lines in vitro and in
vivo, a previous study showed that alternate-day S-1
administration had lower toxicity while yielding similar antitumor
effect compared with standard daily administration (16). In addition, a retrospective study
showed that alternate-day S-1 administration decreases
gastrointestinal toxicity in the patients with advanced gastric
cancer (17). These results indicate
that alternate-day administration is reasonable for decreasing the
toxicity while maintaining the efficacy of S-1.
Severe gastrointestinal toxicities were not observed
during this study, while up to grade 2 anorexia, diarrhea, and
stomatitis were observed in 50% of the patients. This rate of
adverse event was similar to that in a previous study in which
alternate-day S-1 was administrated to previously treated NSCLC
patients including 13.3% elderly patients (10). By contrast, in a study of standard
S-1 administration as first-line treatment for elderly NSCLC
patients, severe anorexia and nausea/vomiting were observed in 4.3%
of patients (18). In addition, in a
study of 2-week S-1 monotherapy treatment followed by a 1-week
interval as a first-line treatment of elderly NSCLC patients,
severe neutropenia and anorexia occurred in 5.0 and 7.5% of
patients, respectively (19). These
results indicate that alternate-day S-1 administration can be safer
than the standard S-1 administration and would be a possible
treatment regimen for elderly patients with NSCLC.
Although no hematological toxicities were observed
during this study, grade 2 renal dysfunction was observed in 2
cases, and grade 2 ileus was observed in 1 case. Drug
administration was suspended in these patients in accordance with
the study protocol. The patient who developed ileus had a history
of abdominal surgery. S-1 monotherapy was resumed in this patient
after the ileus had improved, and no recurrence was observed.
Therefore, we considered that the ileus was not related with S-1
administration. The 2 patients who discontinued S-1 due to grade 2
kidney dysfunction had low CCr before S-1 administration. We
considered that S-1 administration may be associated with renal
dysfunction. However, no severe renal dysfunction was reported in a
previous study in which alternate-day or standard daily S-1 was
administered to NSCLC patients including 14.8% elderly patients
(10). In addition, another study of
standard S-1 administration as first-line treatment for the elderly
NSCLC patients also did not report any severe renal dysfunction
(18). These results show that
alternate-day S-1 administration does not induce severe renal
dysfunction in elderly patients with NSCLC.
In the present study, severe adverse events were not
observed. However, we should recognize that even mild diarrhea and
stomatitis due to S-1 would induce kidney dysfunction, which
increases the toxicity of S-1 in elderly patients. The renal
function of elderly patients might be overestimated because their
serum creatinine is decreased due to muscle atrophy.
While this study design was not for investigating
the efficacy of alternate-day S-1 administration, the median PFS
period was 1.5 months, indicating poor outcomes. However, a
previous study reported a median PFS of 2.1 months in the
alternate-day S-1 monotherapy for previously treated NSCLC patients
including approximately 15% elderly participants (10). The present study only included
elderly patients, and 6 of the 8 patients had PS score of 1. Taking
these into consideration, the efficacy of the treatment regimen in
this study may have been reasonable.
In conclusion, the alternate-day administration of
S-1 can be safer than the standard daily administration and would
be a possible treatment regimen for elderly patients with NSCLC.
However, the current study was a pilot feasibility study, and the
number of patients was inadequate to draw conclusive results; thus,
the findings should be interpreted cautiously. In the future, the
therapeutic safety and efficacy of alternate-day S-1 administration
in elderly NSCLC patients should be compared against that of the
standard S-1 administration in a large-scale study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS, KF and NH were involved in the study conception
and design. TM, MW, KF, KH, NI, MD, SK, YH, SM, TN, HI and NH were
involved in the acquisition of data. TM, MW, KF, KY, SS and NH
analyzed and interpreted the data. TM, MW, KF, KH, NI, MD, SK, KY,
SS, YH, SM, TN, TS, HI, HH and NH drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
ethics committee of each hospital prior to implementation. Written
informed consent was obtained from all patients prior to
enrollment. All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional research committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small-cell lung cancer
|
|
PFS
|
progression-free survival
|
|
DTX
|
docetaxel
|
|
OS
|
overall survival
|
|
ECOG PS
|
Eastern Cooperative Oncology Group
performance status
|
|
EGFR
|
epidermal growth factor receptor
|
|
CCr
|
creatinine clearance
|
|
PD
|
progressive disease
|
|
DCR
|
disease control rate
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quoix E, Zalcman G, Oster JP, Westeel V,
Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L,
et al: Carboplatin and weekly paclitaxel doublet chemotherapy
compared with monotherapy in elderly patients with advanced
non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial.
Lancet. 378:1079–1088. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santos FN, de Castria TB, Cruz MR and
Riera R: Chemotherapy for advanced non-small cell lung cancer in
the elderly population. Cochrane Database Syst Rev: CD010463. 2015.
View Article : Google Scholar
|
|
4
|
National Coprehensive Cancer Network, .
Non-small cell lung cancer. NCCN Guidelines for clinical practice
in oncology. Version 2. 2018.https://www.nccn.org/professionals/physician_gls/default.aspxMarch
1–2018
|
|
5
|
Gridelli C, Perrone F, Gallo C, Cigolari
S, Rossi A, Piantedosi F, Barbera S, Ferraù F, Piazza E, Rosetti F,
et al: Chemotherapy for elderly patients with advanced
non-small-cell lung cancer: The multicenter italian lung cancer in
the elderly study (MILES) phase III randomized trial. J Natl Cancer
Inst. 95:362–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Effects of vinorelbine on quality of life
and survival of elderly patients with advanced non-small-cell lung
cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J
Natl Cancer Inst. 91:66–72. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudoh S, Takeda K, Nakagawa K, Takada M,
Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, et al:
Phase III study of docetaxel compared with vinorelbine in elderly
patients with advanced non-small-cell lung cancer: Results of the
west Japan thoracic oncology group trial (WJTOG 9904). J Clin
Oncol. 24:3657–3663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishio M, Mok TSK, Nakagawa K, et al:
EAST-LC: Randomized controlled phase III trial of S-1 versus
docetaxel in patients with non-small-cell lung cancer who had
received a platinum-based treatment. Ann Oncol. 28:x124–x143.
2017.
|
|
9
|
Yoshioka H, Okamoto I, Morita S, Ando M,
Takeda K, Seto T, Yamamoto N, Saka H, Atagi S, Hirashima T, et al:
Efficacy and safety analysis according to histology for S-1 in
combination with carboplatin as first-line chemotherapy in patients
with advanced non-small-cell lung cancer: Updated results of the
west Japan oncology group LETS study. Ann Oncol. 24:1326–1331.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki A, Maemondo M, Sugawara S, Nakagawa
T, Taima K, Inoue A, Matsuno K, Usui K, Yokoi T, Kanbe M, et al:
Randomized phase II trial of daily administration versus
alternate-day administration of S-1 in patients with advanced
non-small cell lung cancer. Cancer Treat Res Commun. 12:56–61.
2017. View Article : Google Scholar
|
|
11
|
Yamaue H, Shimizu A, Hagiwara Y, Sho M,
Yanagimoto H, Nakamori S, Ueno H, Ishii H, Kitano M, Sugimori K, et
al: Multicenter, randomized, open-label Phase II study comparing
S-1 alternate-day oral therapy with the standard daily regimen as a
first-line treatment in patients with unresectable advanced
pancreatic cancer. Cancer Chemother Pharmacol. 79:813–823. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions, : The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Cancer institute, . Common
terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmMarch
1–2018
|
|
14
|
Lipkin M, Sherlock P and Bell B: CELL
proliferation kinetics in the gastrointestinal tract of man. II.
Cell renewal in stomach, ileum, colon and rectum. Gastroenterology.
45:721–729. 1963.PubMed/NCBI
|
|
15
|
Clarkson B, Ota K, Ohkita T and O'Connor
A: Kinetics of proliferation of cancer cells in neoplastic
effusions in man. Cancer. 18:1189–1213. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arai W, Hosoya Y, Haruta H, Kurashina K,
Saito S, Hirashima Y, Yokoyama T, Zuiki T, Sakuma K, Hyodo M, et
al: Comparison of alternate-day versus consecutive-day treatment
with S-1: Assessment of tumor growth inhibition and toxicity
reduction in gastric cancer cell lines in vitro and in vivo. Int J
Clin Oncol. 13:515–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakuma K, Hosoya Y, Arai W, Haruta H, Ui
T, Kurashina K, Saito S, Hirashima Y, Yokoyama T, Zuiki T, et al:
Alternate-day treatment with S-1 in patients with gastric cancer: A
retrospective study of strategies for reducing toxicity. Int J Clin
Oncol. 15:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiroyama T, Kijima T, Komuta K, Yamamoto
S, Minami S, Ogata Y, Okafuji K, Imamura F, Hirashima T, Tachibana
I, et al: Phase II tailored S-1 regimen study of first-line
chemotherapy in elderly patients with advanced and recurrent
non-small cell lung cancer. Cancer Chemother Pharmacol. 70:783–789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goto H, Okano Y, Machida H, Hatakeyama N,
Ogushi F, Haku T, Kanematsu T, Urata T, Kakiuchi S, Hanibuchi M, et
al: Phase II study of tailored S-1 monotherapy with a 1-week
interval after a 2-week dosing period in elderly patients with
advanced non-small cell lung cancer. Respir Investig. 56:80–86.
2018. View Article : Google Scholar : PubMed/NCBI
|