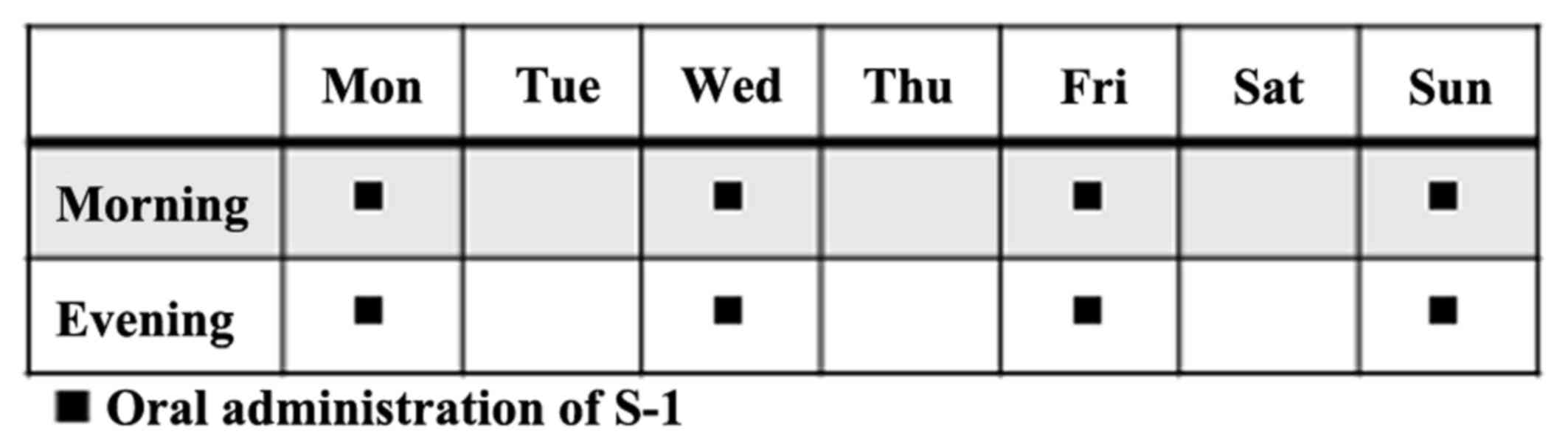
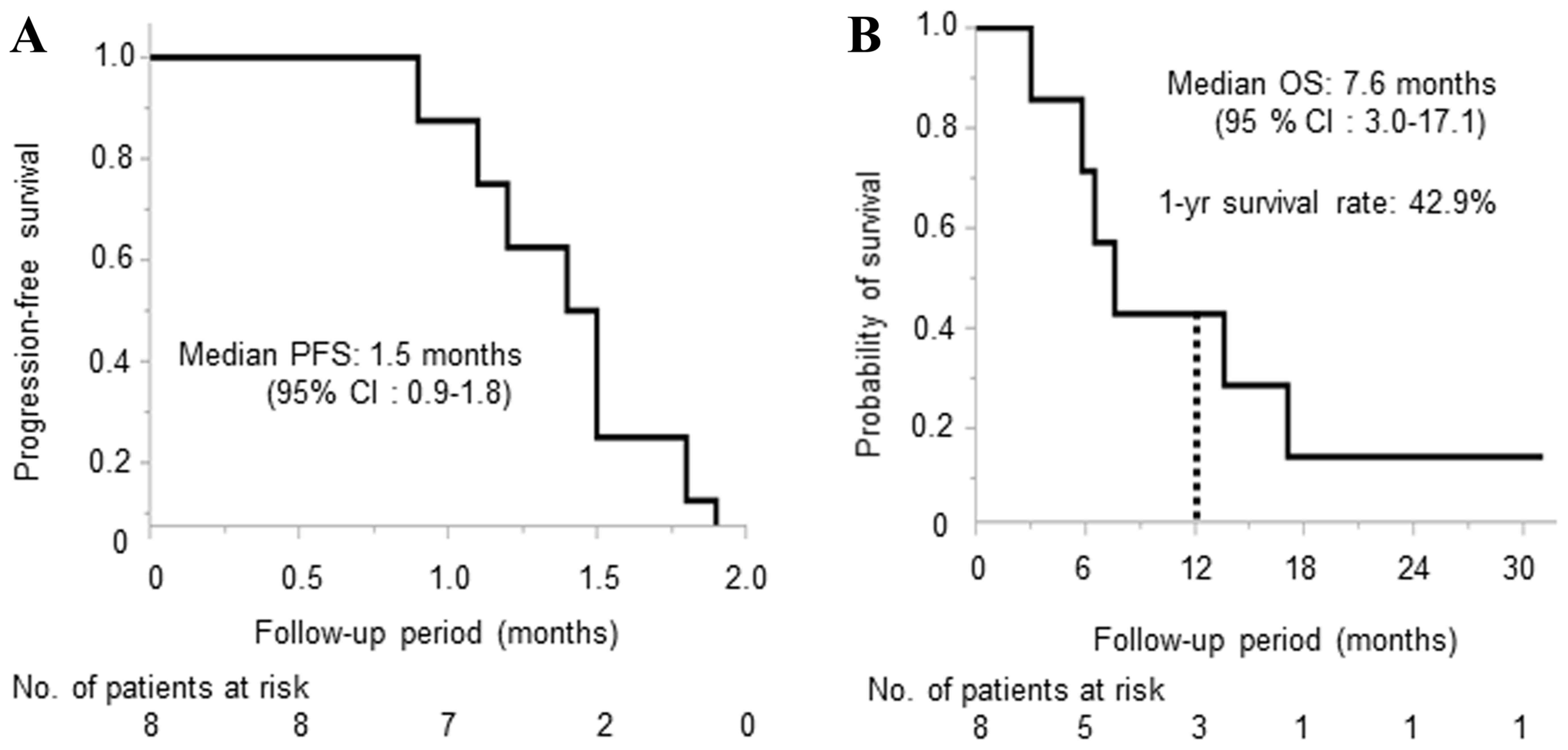
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quoix E, Zalcman G, Oster JP, Westeel V,
Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L,
et al: Carboplatin and weekly paclitaxel doublet chemotherapy
compared with monotherapy in elderly patients with advanced
non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial.
Lancet. 378:1079–1088. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santos FN, de Castria TB, Cruz MR and
Riera R: Chemotherapy for advanced non-small cell lung cancer in
the elderly population. Cochrane Database Syst Rev: CD010463. 2015.
View Article : Google Scholar
|
|
4
|
National Coprehensive Cancer Network, .
Non-small cell lung cancer. NCCN Guidelines for clinical practice
in oncology. Version 2. 2018.https://www.nccn.org/professionals/physician_gls/default.aspxMarch
1–2018
|
|
5
|
Gridelli C, Perrone F, Gallo C, Cigolari
S, Rossi A, Piantedosi F, Barbera S, Ferraù F, Piazza E, Rosetti F,
et al: Chemotherapy for elderly patients with advanced
non-small-cell lung cancer: The multicenter italian lung cancer in
the elderly study (MILES) phase III randomized trial. J Natl Cancer
Inst. 95:362–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Effects of vinorelbine on quality of life
and survival of elderly patients with advanced non-small-cell lung
cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J
Natl Cancer Inst. 91:66–72. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudoh S, Takeda K, Nakagawa K, Takada M,
Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, et al:
Phase III study of docetaxel compared with vinorelbine in elderly
patients with advanced non-small-cell lung cancer: Results of the
west Japan thoracic oncology group trial (WJTOG 9904). J Clin
Oncol. 24:3657–3663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishio M, Mok TSK, Nakagawa K, et al:
EAST-LC: Randomized controlled phase III trial of S-1 versus
docetaxel in patients with non-small-cell lung cancer who had
received a platinum-based treatment. Ann Oncol. 28:x124–x143.
2017.
|
|
9
|
Yoshioka H, Okamoto I, Morita S, Ando M,
Takeda K, Seto T, Yamamoto N, Saka H, Atagi S, Hirashima T, et al:
Efficacy and safety analysis according to histology for S-1 in
combination with carboplatin as first-line chemotherapy in patients
with advanced non-small-cell lung cancer: Updated results of the
west Japan oncology group LETS study. Ann Oncol. 24:1326–1331.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki A, Maemondo M, Sugawara S, Nakagawa
T, Taima K, Inoue A, Matsuno K, Usui K, Yokoi T, Kanbe M, et al:
Randomized phase II trial of daily administration versus
alternate-day administration of S-1 in patients with advanced
non-small cell lung cancer. Cancer Treat Res Commun. 12:56–61.
2017. View Article : Google Scholar
|
|
11
|
Yamaue H, Shimizu A, Hagiwara Y, Sho M,
Yanagimoto H, Nakamori S, Ueno H, Ishii H, Kitano M, Sugimori K, et
al: Multicenter, randomized, open-label Phase II study comparing
S-1 alternate-day oral therapy with the standard daily regimen as a
first-line treatment in patients with unresectable advanced
pancreatic cancer. Cancer Chemother Pharmacol. 79:813–823. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions, : The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Cancer institute, . Common
terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmMarch
1–2018
|
|
14
|
Lipkin M, Sherlock P and Bell B: CELL
proliferation kinetics in the gastrointestinal tract of man. II.
Cell renewal in stomach, ileum, colon and rectum. Gastroenterology.
45:721–729. 1963.PubMed/NCBI
|
|
15
|
Clarkson B, Ota K, Ohkita T and O'Connor
A: Kinetics of proliferation of cancer cells in neoplastic
effusions in man. Cancer. 18:1189–1213. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arai W, Hosoya Y, Haruta H, Kurashina K,
Saito S, Hirashima Y, Yokoyama T, Zuiki T, Sakuma K, Hyodo M, et
al: Comparison of alternate-day versus consecutive-day treatment
with S-1: Assessment of tumor growth inhibition and toxicity
reduction in gastric cancer cell lines in vitro and in vivo. Int J
Clin Oncol. 13:515–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakuma K, Hosoya Y, Arai W, Haruta H, Ui
T, Kurashina K, Saito S, Hirashima Y, Yokoyama T, Zuiki T, et al:
Alternate-day treatment with S-1 in patients with gastric cancer: A
retrospective study of strategies for reducing toxicity. Int J Clin
Oncol. 15:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiroyama T, Kijima T, Komuta K, Yamamoto
S, Minami S, Ogata Y, Okafuji K, Imamura F, Hirashima T, Tachibana
I, et al: Phase II tailored S-1 regimen study of first-line
chemotherapy in elderly patients with advanced and recurrent
non-small cell lung cancer. Cancer Chemother Pharmacol. 70:783–789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goto H, Okano Y, Machida H, Hatakeyama N,
Ogushi F, Haku T, Kanematsu T, Urata T, Kakiuchi S, Hanibuchi M, et
al: Phase II study of tailored S-1 monotherapy with a 1-week
interval after a 2-week dosing period in elderly patients with
advanced non-small cell lung cancer. Respir Investig. 56:80–86.
2018. View Article : Google Scholar : PubMed/NCBI
|